A one-pot microwave-assisted non-aqueous sol–gel approach to metal oxide/graphene nanocomposites for Li-ion batteries†
Seunghwan
Baek
a,
Seung-Ho
Yu
a,
Seung-Keun
Park
c,
Andrea
Pucci
b,
Catherine
Marichy
b,
Dong-Chan
Lee
a,
Yung-Eun
Sung
a,
Yuanzhe
Piao
c and
Nicola
Pinna
*ab
aWorld Class University (WCU) program of Chemical Convergence for Energy & Environment (C2E2), School of Chemical and Biological Engineering, College of Engineering, Seoul National University (SNU), Seoul 151-744, Korea. E-mail: pinna@snu.ac.kr
bDepartment of Chemistry, CICECO, University of Aveiro, 3810-193, Aveiro, Portugal. E-mail: pinna@ua.pt
cDepartment of Nano Science and Technology, Seoul National University, Suwon, 443-270, Korea
First published on 27th October 2011
Abstract
A one-pot non-aqueous synthesis of crystalline SnO2- and Fe3O4-based graphene heterostructures in just a few minutes is introduced. The combined properties of the microwave heating and the “benzyl alcohol route” allow the selective growth of metal oxide nanoparticles at the surface of graphene oxide, which is reduced during synthesis. The as-fabricated nanostructures show good lithium intercalation–deintercalation performances at high rate and good cycling stability compared to the separated nano-building blocks.
There is a clear need for novel nanomaterials for rechargeable lithium batteries in order to “achieve the increase in energy and power density essential to meet the future challenges of energy storage”.1 On this basis, the development of rational and versatile syntheses through controlled and well understood protocols, which allow control of the size, crystallinity, homogeneity and assembly behavior is of primary importance. Only well defined materials and homogeneous nanostructures will permit the establishment of a clear structure–functionality relationship to go beyond the present state of the art. Recently, graphene turned out to be particularly attractive as a support for the stabilization of metal oxide nanoparticles for Li-ion battery applications.2–18 However, simple approaches that can also be generalized to a large variety of metal oxides have not yet been reported. Among metal oxides, tin oxide (SnO2) and magnetite (Fe3O4) are attractive materials for the fabrication of negative electrodes for lithium ion batteries because of their high theoretical reversible capacity of 782 and 926 mAh g−1, respectively, which are more than twice that of graphite (i.e. 372 mAh g−1).
Non-aqueous sol–gel chemistry was successfully applied for the synthesis of various metal oxide nanoparticles,19–21 hybrid materials22 and also for the growth of metal oxide thin films by atomic layer deposition.23 In particular the syntheses of SnO2 and Fe3O4 by the “benzyl alcohol route” were reported to be very robust and versatile.24–26 Microwave synthesis was recently shown to bring important advantages compared to traditional heating for the synthesis of inorganic nanomaterials by soft chemistry.27 In particular, the “benzyl alcohol route” greatly benefits from microwave heating by decreasing the reaction time to just minutes for nanoparticle formation.28,29 In this work, we use another advantage of microwave radiation which is based on the high absorption of the graphene oxide (GO) compared to the solvent and metal oxide precursors. It will be shown that GO acts as the principal microwave absorber and can therefore be selectively heated leading to the nucleation of the metal oxide onto its surface. The fabrication of well controlled metal oxide–graphene composites in one pot in just a few minutes is demonstrated and their structural characterization and properties in lithium ion batteries are described. Finally, a clear structure–property relationship can be addressed due to the ability to easily control the surface particle density.
The synthesis of the metal oxide nanoparticles involves the solvothermal reaction of tin(IV) chloride or iron(III) acetylacetonate in benzyl alcohol.24,26 It leads to the formation of highly crystalline and almost monodisperse nanoparticles when traditional heating is applied for 1 or 2 days, respectively. Instead, in this work microwave irradiation was used in order to tremendously decrease the reaction time. The nanoparticles were synthesized at 185 °C for SnO2 and at 200 °C for Fe3O4 in only 5 to 10 min (cf.Table 1 and detailed experimental section in the supplementary information†). The synthesis of metal oxide/graphene composites was performed in a one-pot reaction keeping the quantity of metal precursor with respect to the amount of solvent constant and only varying the amount of GO. Benzyl alcohol acts as a reactant for the synthesis of the metal oxides and as a reducing agent leading to the partial reduction of GO. Therefore, commonly used post-synthesis treatments for the GO reduction were not needed (e.g.annealing under argon Ar3,30,31 or chemical treatments with NaBH45 or N2H46,7). It should also be pointed out that the approach permits the synthesis of a relatively large amount of composites in just a few minutes. A typical synthesis in 20 mL of benzyl alcohol produces between 0.5 and 1 g of nanocomposites depending on the reaction conditions (cf.Table 1). The carbon content and the yield of the reaction linearly increase with the amount of GO added. The metal oxide content was estimated from TGA analysis by subtracting the weight loss between 200 and 800 °C (Table 1). The as-synthesized composites, GO and reduced graphene oxide in benzyl alcohol under microwave heating (graphene nano-sheets, GNS2) were characterized by X-ray diffraction (Fig. SI1†). The characteristic reflections of GO and GNS were situated around 2θ = 12 and 24°, respectively, and are in good agreement with previous reports.6
| Sample name | Metal oxide precursor (mmol) | GO (g) | Benzyl alcohol (ml) | Reaction time (min) | Reaction temperature (°C) | Yield (g) | Weight loss (%)a |
|---|---|---|---|---|---|---|---|
| a Between 200 and 800 °C estimated from TGA. | |||||||
| SnO2 | 4 | 0 | 20 | 10 | 185 | 0.50 | 6.0 |
| TGC1 | 4 | 0.05 | 20 | 10 | 185 | 0.65 | 10.9 |
| TGC2 | 4 | 0.30 | 20 | 10 | 185 | 0.89 | 29.7 |
| TGC3 | 4 | 0.40 | 20 | 10 | 185 | 0.96 | 34.7 |
| Fe3O4 | 15 | 0 | 20 | 10 | 180 | 0.89 | 6.1 |
| IGC1 | 1 | 0.05 | 5 | 5 | 200 | 0.088 | — |
| IGC2 | 3 | 0.20 | 20 | 5 | 190 | 0.39 | 24.6 |
| IGC3 | 3 | 0.30 | 20 | 5 | 200 | 0.48 | 52.6 |
The XRD patterns of the composites present typical reflections of the cassiterite (SnO2, JCPDS 41–1445) and magnetite (Fe3O4, JCPDS 19–629) structures. The broad character of the reflections is due to the small crystalline size of the oxides. The average particle sizes, extracted from the width of the peaks applying the Scherrer equation, were 5 nm for tin oxide (considering the 110 reflection) and 6 nm for magnetite (considering the 311 reflection). It should be pointed out that the crystalline sizes of the magnetite and tin oxide nanoparticles were not influenced by the synthesis conditions making this system particularly attractive for the study of the influence of the amount of graphene on the final properties of the composite. No peaks corresponding to GO were observed for both composite materials, suggesting that GO was reduced during the synthesis. As a matter of fact, the main broad reflection of the GNS centered around 2θ = 24° is clearly visible on the patterns of the magnetite/graphene composites. In the case of tin oxide, due to the overlapping of the main GNS reflection with the most intense 110 reflection of the cassiterite structure, its presence can be detected only because the latter become asymmetric.
The SEM images of tin oxide/graphene composites (Fig. 1a,b) show that the GNS (darker contrast) are covered by a continuous and homogeneous layer of nanoparticles (TGC1) or by clusters of nanoparticles (TGC2) as the amount of graphene added during the synthesis decreases. TEM images of the TGC1 prove that the GNS are fully covered by SnO2 nanoparticles (Fig. 1c), on the other hand free nanoparticles are also present. The TEM images of the uncoated GNS1 and GNS2 (Fig. SI4†) clearly highlight the different contrast between the fully coated and the uncoated GNS. The high resolution TEM (HRTEM) image (Fig. 1d) of an edge of the composite shows the dense packing of nanoparticles onto the GNS, which is indicated by an arrow. The power spectrum (i.e. square of the Fourier transform) of this image (inset) shows the reflections characteristic of the cassiterite structure, proving that the GNS are indeed fully covered by randomly oriented SnO2 nanoparticles. When the concentration of GO added during the synthesis increases, the particle density linearly decreases (Fig. 1e,f) and simultaneously free particles are no longer observed on the carbon coated TEM grid. Similarly, the SEM and overview TEM images of a magnetite-based composite (Fig. 2a,b) show that the GNS are fully coated with nanoparticles and that no free particles are present.
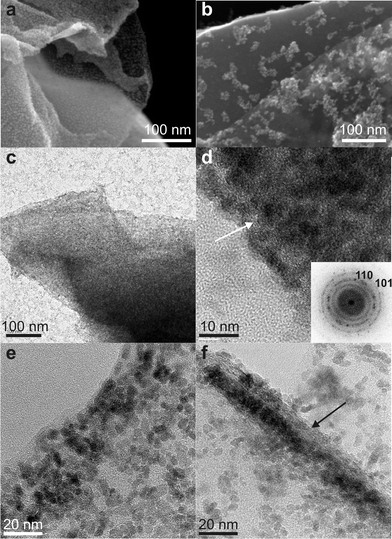 | ||
| Fig. 1 Electron microscopy studies of tin oxide/graphene nanocomposites. a,b) FE-SEM images of TGC1 and TGC2. c,d) Overview and high resolution TEM images of TGC1, inset shows the power spectrum. e,f) High resolution TEM images of TGC2 and TGC3. In d) and f) the arrows point out the stacking of GNS. | ||
 | ||
| Fig. 2 Electron microscopy images of magnetite/graphene nanocomposites. a) FE-SEM, b) TEM and c) HRTEM of IGC1. | ||
The HRTEM image of the edge of a GNS proves that the particles homogeneously and fully coat the substrates (Fig. 2c). These findings suggest that microwave heating is a particularly effective method for the selective coating of materials that highly absorb microwave radiation. Hot spots acting as nucleation centers can be selectively created at the surface of GO. Indeed, GO absorbs microwave radiation more efficiently compared to the solvent and metal oxide precursors. This is attributed to the low dipolar moment of benzyl alcohol, which makes it a bad microwave absorber. This can be indirectly proved by the fact that the synthesis of tin oxide and iron oxide in the absence of GO requires a much larger power in order to reach the same temperature. As an example, under the same reaction conditions, the synthesis of pure SnO2 requires a power of at least 200 W in order to maintain a temperature of 185 °C. On the other hand, in the case of TGC2 and TGC3 the presence of GO reduces the required power for maintaining the same temperature to 100 W or less. Moreover, the presence of functional groups acting as anchoring sites for the deposition of metal oxides onto GO is also a prerequisite for the selective deposition of nanoparticles. The coexistence of free SnO2 nanoparticles when the synthesis is performed with a particularly low amount of GO (sample TGC1) can be explained by the fact that, under the applied reaction conditions, the nucleation of SnO2 can also take place in the homogeneous phase. Although, as demonstrated above, the deposition of SnO2 onto GO is favored (cf. samples TGC2 and TGC3), once GO is fully covered by nanoparticles free nanoparticles can coexist. Moreover, the role of the microwave heating is highlighted by transmission electron microscopy images of composites synthesized by traditional heating (Fig. SI3†). In this case the particle density is sensibly lower and the coverage less homogeneous.
In order to assess the chemical modification of GO, during the solvothermal treatment assisted by microwave heating in benzyl alcohol, Fourier transform infrared (FT-IR) spectra of the nanocomposites were recorded (Fig. SI6†).
The FT-IR spectrum of GO shows the presence of various oxygen-containing groups, such as O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (νO–C
O (νO–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O at 840 cm−1), C–O (νC–O at 1050 cm−1), C–O–C (νC–O–C at 1250 cm−1), OH (νOH at 1620 cm−1) and carboxyl functional moieties at 1730 cm−1. After solvothermal synthesis, most of the contributions due to oxygen-containing groups decrease and only the peak attributed to C–O–C centered at 1250 cm−1 and C
O at 840 cm−1), C–O (νC–O at 1050 cm−1), C–O–C (νC–O–C at 1250 cm−1), OH (νOH at 1620 cm−1) and carboxyl functional moieties at 1730 cm−1. After solvothermal synthesis, most of the contributions due to oxygen-containing groups decrease and only the peak attributed to C–O–C centered at 1250 cm−1 and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C centered at 1560 cm−1 remain. On the tin oxide-based nanocomposite the two peaks of SnO2 centered at 560 and 670 cm−1 are clearly observable together with the peaks of reduced graphene, proving that the GO is effectively reduced during the microwave assisted solvothermal treatment. These data are also in agreement with X-ray photoelectron spectroscopy analysis of the C1s edge (cf. Fig SI7†) and a recent report on the reduction of GO by alcohols.32 The C1s peak of graphene oxide consists of C–C (sp2carbon in the basal plan, 284.6 eV), C–O (286.5 eV), C
C centered at 1560 cm−1 remain. On the tin oxide-based nanocomposite the two peaks of SnO2 centered at 560 and 670 cm−1 are clearly observable together with the peaks of reduced graphene, proving that the GO is effectively reduced during the microwave assisted solvothermal treatment. These data are also in agreement with X-ray photoelectron spectroscopy analysis of the C1s edge (cf. Fig SI7†) and a recent report on the reduction of GO by alcohols.32 The C1s peak of graphene oxide consists of C–C (sp2carbon in the basal plan, 284.6 eV), C–O (286.5 eV), C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (288.1 eV) and O–C
O (288.1 eV) and O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (289 eV). The contribution of C–O is particularly high. After microwave treatment of the GO in benzyl alcohol, the O/C ratio decreases notably due to the reduction of GO to GNS, suggesting that the large majority of the oxygenated species are removed. Finally, a broad and weak peak centered above 290.0 eV is identified as a shake-up satellite due to π–π* transitions. Therefore, the treatment in benzyl alcohol induces the reduction of GO to GNS also during nanoparticle deposition. As a matter of fact, the C1s edge of the sample TGC2 shows mainly a contribution due to the C–C bond of graphene and a small contribution due to C–O bonds, which very likely are the anchoring sites of the SnO2 nanoparticles.
O (289 eV). The contribution of C–O is particularly high. After microwave treatment of the GO in benzyl alcohol, the O/C ratio decreases notably due to the reduction of GO to GNS, suggesting that the large majority of the oxygenated species are removed. Finally, a broad and weak peak centered above 290.0 eV is identified as a shake-up satellite due to π–π* transitions. Therefore, the treatment in benzyl alcohol induces the reduction of GO to GNS also during nanoparticle deposition. As a matter of fact, the C1s edge of the sample TGC2 shows mainly a contribution due to the C–C bond of graphene and a small contribution due to C–O bonds, which very likely are the anchoring sites of the SnO2 nanoparticles.
The capacity versus cycle number plots for the GNS, pure SnO2 nanoparticles and TGC composites are shown in Fig. 3 and SI8.† The coulombic efficiency in the first cycle of the as-synthesized sample is generally low, which is attributed to two contributions: i) The irreversible formation of amorphous lithium oxide and the reduction of SnO2 to metallic Sn following the reaction:
| SnO2 + 4Li+ + 4e− → Sn + 2Li2O |
 | ||
| Fig. 3 Cycling performance of GNS (triangles, red), SnO2 (squares, magenta), TGC1 (circles, light blue), TGC2 (diamonds, green) and TGC3 (stars, blue) at various current densities. Empty symbols indicate discharge and full symbols charge. | ||
Fig. 3 presents the cycling performance of TGC evaluated at various current densities for up to 60 cycles compared to the performances of GNS and pure SnO2 nanoparticles. It is evident that the graphene framework has greatly improved the electron transfer and the stability of the electrode compared to pure SnO2 allowing cycling at a high rate without suffering from high capacity loss. As a matter of fact, at 1600 mA g−1 the capacity drops to around 100 mAh g−1 for the pure SnO2 and GNS and stays above 300 mAh g−1 for the SnO2/graphene composites. Finally, the initial reversible capacity of the nanocomposite samples was recovered at 100 mA g−1, suggesting that the nanostructure is not dramatically affected by the high rate charge/discharge cycles. Improvements in capacity retention and high rate cycling are even better observed for the Fe3O4/graphene composites (Fig. 4). During the first cycle at a current density of 100 mA g−1 the pure magnetite sample presents the highest capacity although the reversibility is rather poor. On the other hand, the nanocomposite samples show a much higher capacity retention. Moreover, at higher current density the effect of graphene stabilization becomes evident. At the highest current density (1600 mA g−1) the capacities of the GNS and pure magnetite samples drop to 100 mAh g−1 whereas that of IGC2 stays at 250 mAh g−1 and that of IGC3 lies above 400 mAh g−1. These findings confirm that the larger the graphene/metal oxide ratio is, the better the high rate capacity of the composites.
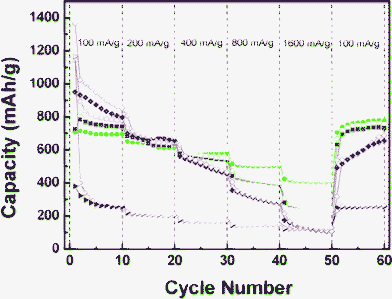 | ||
| Fig. 4 Cycling performance of GNS (triangles, red), pure Fe3O4 (diamonds, magenta), IGC2 (squares, blue), IGC3 (circles, green) and at various current densities. Empty symbols indicate discharge and full symbols charge. | ||
In conclusion, a simple and rapid one-pot microwave-assisted solvothermal route for the preparation of metal oxide/graphene nanocomposites has been introduced. It permits the selective growth of metal oxide nanoparticles at the surface of graphene oxide, which is simultaneously reduced during synthesis.
Acknowledgements
This work was partially supported by the WCU (World Class University) program through the National Research Foundation (NRF) of Korea funded by the Ministry of Education, Science and Technology (R31-10013) and FCT projects PTDC/CTM/098361/2008, PTDC/CTM-NAN/110776/2009, SFRH/BD/71453/2010, and SFRH/BD/45177/2008.References
- P. G. Bruce, B. Scrosati and J.-M. Tarascon, Angew. Chem., Int. Ed., 2008, 47, 2930–2946 CrossRef CAS.
- D. Wang, D. Choi, J. Li, Z. Yang, Z. Nie, R. K. Hu, C. Wang, L. V. Saraf, J. Zhang, I. A. Aksay and J. Liu, ACS Nano, 2009, 3, 907–914 CrossRef CAS.
- G. Zhou, D.-W. Wang, F. Li, L. Zhang, N. Li, Z.-S. Wu, L. Wen, G. Q. Lu and H.-M. Cheng, Chem. Mater., 2010, 22, 5306–5313 CrossRef CAS.
- H. Wang, L.-F. Cui, Y. Yang, H. S. Casalongue, J. T. Robinson, Y. Liang, Y. Cui and H. Dai, J. Am. Chem. Soc., 2010, 132, 13978–13980 CrossRef CAS.
- J. Yao, X. Shen, B. Wang, H. Liu and G. Wang, Electrochem. Commun., 2009, 11, 1849–1852 CrossRef CAS.
- S.-M. Paek, E. Yoo and I. Honma, Nano Lett., 2009, 9, 72–75 CrossRef CAS.
- X. Zhu, Y. Zhu, S. Murali, M. D. Stoller and R. S. Ruoff, ACS Nano, 2011, 5, 3333–3338 CrossRef CAS.
- Z.-S. Wu, W. Ren, L. Wen, L. Gao, J. Zhao, Z. Chen, G. Zhou, F. Li and H.-M. Cheng, ACS Nano, 2010, 4, 3187–3194 CrossRef CAS.
- S. Q. Chen and Y. Wang, J. Mater. Chem., 2010, 20, 9735–9739 RSC.
- D. Choi, D. Wang, V. V. Viswanathan, I.-T. Bae, W. Wang, Z. Nie, J.-G. Zhang, G. L. Graff, J. Liu, Z. Yang and T. Duong, Electrochem. Commun., 2010, 12, 378–381 CrossRef CAS.
- S.-L. Chou, J.-Z. Wang, M. Choucair, H.-K. Liu, J. A. Stride and S.-X. Dou, Electrochem. Commun., 2010, 12, 303–306 CrossRef CAS.
- Y. Ding, Y. Jiang, F. Xu, J. Yin, H. Ren, Q. Zhuo, Z. Long and P. Zhang, Electrochem. Commun., 2010, 12, 10–13 CrossRef CAS.
- J. K. Lee, K. B. Smith, C. M. Hayner and H. H. Kung, Chem. Commun., 2010, 46, 2025–2027 RSC.
- L. Shen, C. Yuan, H. Luo, X. Zhang, S. Yang and X. Lu, Nanoscale, 2011, 3, 572–574 RSC.
- X. Wang, X. Zhou, K. Yao, J. Zhang and Z. Liu, Carbon, 2011, 49, 133–139 CrossRef CAS.
- L.-S. Zhang, L.-Y. Jiang, H.-J. Yan, W. D. Wang, W. Wang, W.-G. Song, Y.-G. Guo and L.-J. Wan, J. Mater. Chem., 2010, 20, 5462–5467 RSC.
- M. Zhang, D. Lei, X. Yin, L. Chen, Q. Li, Y. Wang and T. Wang, J. Mater. Chem., 2010, 20, 5538–5543 RSC.
- J. Zhu, T. Zhu, X. Zhou, Y. Zhang, X. W. Lou, X. Chen, H. Zhang, H. H. Hng and Q. Yan, Nanoscale, 2011, 3, 1084–1089 RSC.
- N. Pinna and M. Niederberger, Angew. Chem., Int. Ed., 2008, 47, 5292–5304 CrossRef CAS.
- P. H. Mutin and A. Vioux, Chem. Mater., 2009, 21, 582–596 CrossRef CAS.
- M. Niederberger, Acc. Chem. Res., 2007, 40, 793–800 CrossRef CAS.
- N. Pinna, J. Mater. Chem., 2007, 17, 2769–2774 RSC.
- G. Clavel, E. Rauwel, M.-G. Willinger and N. Pinna, J. Mater. Chem., 2009, 19, 454–462 RSC.
- N. Pinna, S. Grancharov, P. Beato, P. Bonville, M. Antonietti and M. Niederberger, Chem. Mater., 2005, 17, 3044–3049 CrossRef CAS.
- N. Pinna, G. Neri, M. Antonietti and M. Niederberger, Angew. Chem., Int. Ed., 2004, 43, 4345–4349 CrossRef CAS.
- J. Ba, J. Polleux, M. Antonietti and M. Niederberger, Adv. Mater., 2005, 17, 2509–2512 CrossRef CAS.
- I. Bilecka and M. Niederberger, Nanoscale, 2010, 2, 1358–1374 RSC.
- I. Bilecka, I. Djerdj and M. Niederberger, Chem. Commun., 2008, 886–888 RSC.
- I. Bilecka, P. Elser and M. Niederberger, ACS Nano, 2009, 3, 467–477 CrossRef CAS.
- G. Wang, B. Wang, X. Wang, J. Park, S. Dou, H. Ahn and K. Kim, J. Mater. Chem., 2009, 19, 8378–8384 RSC.
- L.-S. Zhang, L.-Y. Jiang, H.-J. Yan, W. D. Wang, W. Wang, W.-G. Song, Y.-G. Guo and L.-J. Wan, J. Mater. Chem., 2010, 20, 5462–5467 RSC.
- D. R. Dreyer, S. Murali, Y. Zhu, R. S. Ruoff and C. W. Bielawski, J. Mater. Chem., 2011, 21, 3443–3447 RSC.
Footnote |
| † Electronic Supplementary Information (ESI) available: experimental section, high resolution and additional TEM and SEM images, FT-IR and XPS analysis. See DOI: 10.1039/c1ra00797a/ |
| This journal is © The Royal Society of Chemistry 2011 |
