Synthesis of 2-alkyl substituted benzimidazoles under microwave irradiation: Anti-proliferative effect of some representative compounds on human histiocytic lymphoma cell U937†
Chhanda
Mukhopadhyay
*a,
Sabari
Ghosh
a,
Sumita
Sengupta (Bandyopadhyay)
b and
Soumasree
De
b
aDepartment of Chemistry, University of Calcutta, 92 APC Road, Kolkata, 700009, India. E-mail: cmukhop@yahoo.co.in; Tel: +91 9433019610
bDepartment of Biophysics, Molecular Biology and Bioinformatics, University of Calcutta, 92 APC Road, Kolkata, 700009, India
First published on 7th September 2011
Abstract
The objective of this research was the synthesis of 2-alkyl substituted benzimidazoles under microwave irradiation using 62% aqueous hexafluorophosphoric acid (10 mol%) as catalyst. Some of the newly synthesised compounds were tested for their potential to inhibit proliferation of cancer cells (histiocytic lymphoma cell U937) by cell viability assay.
Introduction
Benzimidazoles have some claim to technological importance (through its use in corrosion inhibition), biochemical importance (through its ability to bind to metals, as in vitamin B12 and are related to DNA base purine and the stimulant caffeine), pharmaceutical importance (antihistamine Astemizole,1 the anti-ulcerative Esomeprazole2 and Albendazole,3 which is used to treat parasitic diseases) and can act as ligands to transition metals for modeling of biological systems.4 Our interest stems from the arena of the 2-alkyl substituted benzimidazoles whose synthesis from aliphatic acids require drastic conditions and the yields are very poor compared to the 2-aryl benzimidazoles.Conventional synthesis of benzimidazoles involves heating the reactants in refluxing aqueous hydrochloric acid5 or in slurry of the dehydrating agent polyphosphoric acid.6 The reaction mixture must then be neutralized with a base, such as aqueous ammonia. The pure product is obtained after recrystallisation from an organic solvent. A cleaner route to benzimidazoles in solid phase synthesis7 and silica gel as a solid acid catalyst,8 are two approaches that have been reported. High temperature and pressure autoclave reaction (which is not easily available in a laboratory) have also been reported.9 These previous works mainly report the synthesis of the 2-aryl benzimidazoles. Renard et al. described the supported enzymatic catalyst activated mild synthesis of benzimidazoles; however the enzyme lipozyme used was very selective towards the long chain fatty acids.10
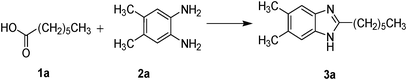
We report a general viable method for high-throughput synthesis of the 2-alkyl substituted benzimidazoles which can be of immense value in drug discovery process. This is a simple one step protocol where the aliphatic acid (1) reacts with ortho-phenylenediamines (2) in the presence of aqueous hexafluorophosphoric acid as catalyst under microwave heating to produce 2-alkyl substituted benzimidazoles (3). Some of these synthesized benzimidazoles act as potent inducer of cell death for human histiocytic lymphoma cell U937. Leukemia is one of the leading cause of death throughout the world. U937, human histiocytic lymphoma cell-line has been chosen to study the anti-proliferative activities of these newly synthesized compounds so that it can be extended further for the development of drugs which could be more effective for treatment of leukemia. Development of new anticancer agents with novel structures and mode of action remains the primary goal of scientists for the solution of the human epidemic that cancer has become. There is a dearth of target-specific drugs in cancer cells and is an active area of current research. Previously, benzimidazoles were recognized to display significant activity against several viruses. Antiviral activity was demonstrated for 2-alkyl substituted benzimidazoles.11
Microwave-assisted organic synthesis (MAOS) has been known since 1986.12 This “non-conventional” synthetic method has shown broad applications as a very efficient way to accelerate the course of many organic reactions, producing high yields and higher selectivity, lower quantities of side products and, consequently, easier work-up and purification of the products. In MAOS many organic reactions can be carried out under solvent-free conditions and is an important tool for combinatorial chemistry.13,14,15 Microwave heating (dielectric heating) is a very efficient process since the microwave couple directly with the molecules that are present in the reaction mixture, leading to a fast rise in temperature, faster reactions and cleaner chemistry. The two fundamental mechanisms for transferring energy from microwaves to the substance are dipole rotation and ionic conduction. When the temperature increases, the transfer of energy becomes more efficient. The MW theory and how the MW increase reactions rate has been discussed in details by several authors.14,15,16 The increased interest in microwave technology has raised the number of companies supporting new microwave ovens for laboratory use,17 such as mono-mode microwave, also called single-mode microwave.18
Results and discussion
During the screening of effective catalysts and reaction conditions, we chose heptanoic acid (1a) and 4,5-dimethyl-benzene-1,2-diamine (2a) as the model substrates. It can be seen from Table 1 that some usual Brønsted acids such as HNO3, TfOH, HClO4 and H2SO4 were all ineffective for the reactions of 1a and 2a, except that HCl, HBF4 and HF gave 10%, 15% and 35% isolable yields (Table 1, entries 1–7). HPF6 proved to be an effective catalyst for the addition of 1a to 2a to generate 3a. In this HPF6-catalyzed addition, only 10 mol% of catalyst loading was required. The reactions of 1a and 2a catalyzed by HPF6 could be conveniently conducted in air in the absence of solvent, in water or in commercial grade solvents such as 1,2-dichloroethane (DCE), chloroform or methanol; the solvent-free reaction gave the best result (Table 1, entry 12). Since the reaction goes very well with only catalytic amount of HPF6, it cannot act as a dehydrating agent but only as a catalyst. To the best of our knowledge, the synthesis of 2-alkyl substituted benzimidazoles requiring only 5 min of reaction time is not a very frequent report in organic synthesis. In the previous microwave assisted synthesis of 2-alkyl benzimidazoles by Stadler et al.,12g the 2-alkyl chain had been restricted to 2-H, 2-Me and 2-Et only. In this publication, we report a wide variety of 2-alkyl substituted benzimidazoles 3a–3r (Table 2, entries 1–18). 62% Hexafluorophosphoric has been used in catalytic amount thereby reducing the reaction temperature from 145 °C to 115 °C. This surely helps sustain a wide variety of functional groups. The reaction time is also reduced to 5 min from 20 min. Also, the carboxylic acid (Reactant 1, Scheme 1) is not required in excess; an equivalent amount is sufficient for this methodology.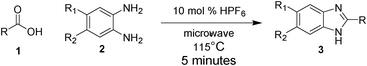 | ||
| Scheme 1 A general scheme showing the formation of the 2-alkyl substituted benzimidazole. | ||
|
|
||||
|---|---|---|---|---|
| Entry | Acid | Solvent | temp (°C) | Yield (%)b |
| a Reaction conditions: acid (0.1 mmol), 1a (1.0 mmol), 2a (1.0 mmol), 18 h, 2.0 mL of solvent was added where noted. The HPF6 catalysed reactions were carried out for 5 min in a microwave oven at 600 Watt. The solvent-free reactions with the other Brønsted acids were carried out for 15 min in a microwave oven at 600 Watt. The reaction time was 2.5 h when thermal heating was used. b Based on 2a except for entry 16 where 1.0 mmol of 1a and 2.0 mmol of 2a were used. c Thermal heating was used. d 0.05 mmol of HPF6 was used. e The reaction time was 1 h. All reactions were carried out under aerobic conditions. | ||||
| 1 | HCl | — | 115 | 10 |
| 2 | HBF4 | — | 115 | 15 |
| 3 | HNO3 | — | 115 | 0 |
| 4 | TfOH | — | 115 | 0 |
| 5 | HClO4 | — | 115 | 0 |
| 6 | HF | — | 115 | 35 |
| 7 | H2SO4 | — | 115 | 0 |
| 8 | HPF6 | water | 115 | 65 |
| 9 | HPF6 | DCE | 70 | 30 |
| 10 | HPF6 | CHCl3 | 70 | 35 |
| 11 | HPF6 | CH3OH | 70 | 45 |
| 12 | HPF6 | — | 115 | 98 |
| 13 | HPF6 | — | 115 | 90c |
| 14 | HPF6 | — | 70 | 68 |
| 15 | HPF6 | — | 90 | 75 |
| 16 | HPF6 | — | 115 | 95 |
| 17 | HPF6 | — | 115 | 75d |
| 18 | HPF6 | — | 115 | 72e |
| Entry | R1 | R2 | R | Producta |
|---|---|---|---|---|
| a Yield is given within parenthesis. b This yield did not improve even after an hour of prolonged reaction time (the other reactions were completed within 5 min only). | ||||
| 1 | CH3 | CH3 | (CH2)5CH3 |

|
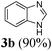
| 220 | H | H | H |
|
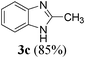
| 39 | H | H | CH3 |
|
| 412g | H | CH3 | CH3 |
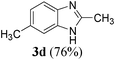
|
| 512g | CH3 | CH3 | CH3 |

|
| 620 | Cl | Cl | CH3 |
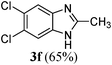
|
| 712f | H | H | (CH2)2CH3 |

|
| 821 | H | CH3 | (CH2)2CH3 |
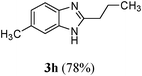
|
| 920 | CH3 | CH3 | (CH2)2CH3 |
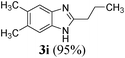
|
| 10 | H | CH3 | CH(CH2CH3)Ph |
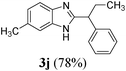
|
| 1122 | H | H | CH2(α-naphthyl) |
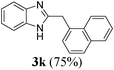
|
| 12 | CH3 | CH3 | CH2(α-naphthyl) |
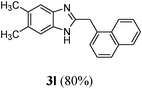
|
| 1322 | H | H | CH2(3-indolyl) |
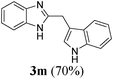
|
| 14 | H | CH3 | CH2(3-indolyl) |

|
| 15 | CH3 | CH3 | CH2(3-indolyl) |
|
| 16 | CH3 | CH3 | (CH2)3(3-indolyl) |
|
| 17 | CH3 | CH3 | cyclopropyl |
|
| 1820 | CH3 | CH3 | CH2Ph |
|
Subsequently, the addition of aliphatic acids to various o-phenylenediamines was examined under solvent-free conditions, at 115 °C (microwave heating), using 10 mol% of HPF6 as the catalyst (Table 2). Strictly speaking, the reaction is not totally solvent-free since the catalyst hexafluorophosphoric acid comes as a 62% solution in water. Whilst this might not mean there is much solvent, there is still some. The reaction of the o-phenylenediamines with the various types of aliphatic acids were highly efficient in most cases. When an electron-withdrawing group like Cl was present in the aromatic diamine part (Table 2, entry 6), the yield suffered and this yield could not be enhanced even when the reaction time was prolonged. The molecular structure of compounds 3l and 3p were established in details on the basis of elemental analysis and spectroscopic data [IR, 1H and 13C NMR including 2D NMR (H–H COSY, HMBC = 1H detected heteronuclear multiple bond connectivity, HSQC = 1H detected heteronuclear single quantum coherence and NOESY = 1H detected the nuclear Overhauser effect correlation), see ESI† (Supplementary Parts 2 and 3)].
Compounds 3d, 3h, 3j and 3n did not exist as tautomeric mixtures at room-temperature. In case a tautomeric mixture were present, there would have been two sets of signals in 1H NMR for each tautomer.19 The predominant tautomer should be the one with the aromatic methyl group meta to NH as established from previous DFT calculations from our laboratory.19
Cell viability assay
Number of viable cells exposed to the newly synthesized benzimidazole derivatives mentioned above was enumerated by Trypan blue exclusion assay (Fig. 1). 5 × 104 cells were seeded in each well in 24-well micro titer plates and were incubated for 2 h prior to addition of various concentrations of the test compounds for 24 h. Cells treated with equivalent amount of methanol were taken as control. After 24 h, the cells were counted under microscope using trypan blue dye (in presence of trypan blue dye, viable cells were found bright and were counted where dead cells appear as blue in color). Percentages of surviving cells to untreated controls were calculated by using the formula as % viability = [(Ct/Cs) × 100]%, where Ct and Cs indicate the cell count of the sample and solvent control respectively. All of the experiments were done in triplicate, and the results were presented as means ± S.E.M. | ||
| Fig. 1 Cell viability assay for compound 3l. (A) 0 h. (B) After 3 h. (C) After 20 h. (D) After 24 h of treatment of U937 cells with compound 3l visualised under inverted microscope. | ||
Anti-proliferative effect of the compounds (3l, 3o, 3p, 3q and 3j) on human histiocytic lymphoma cell-line U937
To determine the effects of the compounds synthesized, on the proliferation of human histiocytic lymphoma cell-line U937, Trypan blue dye exclusion assay was performed. Cells were exposed to different concentrations of the compounds for 24 h. Fig. 2 shows the dose dependent cytotoxicity of the compounds # 3l, 3o, 3p, 3q and 3j in a U937 cell-line. It was found that with increasing concentrations, the cell viability decreases as compared to untreated U937 cells for 3l, 3o, 3p and 3j, whereas, 3q has almost no effect on cytotoxicity towards concentration. This result suggests that 3l, 3o, 3p and 3j act as anti-proliferative agent, whereas, 3q is not as good as the other compounds mentioned above in human histiocytic lymphoma cell U937 after 24 h of treatment. The IC50 values are mentioned in Table 3 for comparison.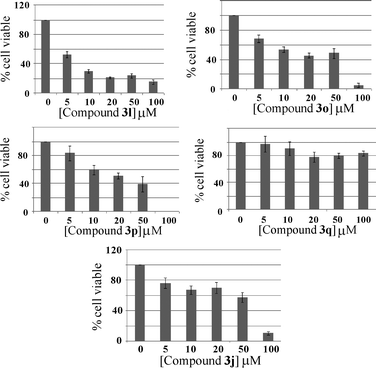 | ||
| Fig. 2 Dose dependent cytotoxicity of the compounds 3l, 3o, 3p, 3q and 3j. | ||
| Compounds | IC50 (μM) |
|---|---|
| 3l | 11.7 |
| 3o | 35.6 |
| 3p | 36.8 |
| 3q | 298.9 |
| 3j | 54.9 |
The IC50 value of Compound 3l is the lowest and it is the most effective. The viability decreases with increasing concentrations of drug in a bi-phasic manner. 50% loss in viability is found at a low concentration of 5 μM, whereas, for higher concentration of drugs there is almost no change in the % viability at drug concentrations of 10–100 μM (Fig. 2). Compound 3o and 3p have almost same IC50 values and are less effective compared to 3l (Table 3). Compound 3q is not much effective at the concentration range we have studied (Fig. 2). Compound 3j is less effective compared to 3l, 3o and 3p, but in presence of this drug at higher concentration (100 μM) the viability of cells is very low (Fig. 2). Thus we can say that 3l is the most effective one, 3o, 3p and 3j are of intermediate effectiveness, whereas, 3q is not very effective drug at the concentration range we have studied (Table 3).
Conclusions
In conclusion, we have synthesised a range of very useful benzimidazoles which show antiproliferative effect on the human histiocytic lymphoma cell-line U937. The microwave synthesis of these benzimidazoles using catalytic amount (10 mol%) of 62% aqueous hexafluorophosphoric acid was also very convenient and an extremely rapid methodology (employing only 5 min of reaction time).Acknowledgements
One of the authors (SG) thanks the University Grants Commission, New Delhi, for her fellowship (SRF). We thank Professor Amarendranath Patra of Department of Chemistry, University of Calcutta, for 2D-NMR spectra. We thank the CAS Instrumentation Facility, University of Calcutta for spectral data. We also acknowledge grant received from UGC funded Major project, F. No. 37-398/2009 (SR) dated 11-01-2010.References
- H. Al Muhaimeed, J. Int. Med. Res., 1997, 25, 175 CAS.
- L. J. Scott, C. J. Dunn, G. Mallarkey and M. Sharpe, Drugs, 2002, 62, 1503 CrossRef CAS.
- P. Venkatesan and J. Antimicrob, Chemother., 1998, 41, 145 CAS.
- (a) E. Bouwman, W. L. Driessen and J. Reedijk, Coord. Chem. Rev., 1990, 104, 143 CrossRef CAS; (b) M. A. Pujar and T. D. Bharamgoudar, Transition Met. Chem., 1988, 13, 423 CrossRef CAS.
- M. A. Phillips, J. Chem. Soc., 1928, 2393 RSC.
- D. W. Hein, R. J. Alheim and J. J. Leavitt, J. Am. Chem. Soc., 1957, 79, 427 CrossRef CAS.
- B. Raju, N. Nguyen and G. W. Holland, J. Comb. Chem., 2002, 4, 320 CrossRef CAS.
- Y. Njoya, N. Boufatah, A. Gellis, P. Rathelot, M. P. Crozet and P. Vanelle, Heterocycles, 2002, 57, 1423 CrossRef CAS.
- There is a report of synthesis of this molecule from 1894. (a) O. Hinsberg and F. Funcke, Ber. Dtsch. Chem. Ges., 1894, 27, 2187 CrossRef; (b) L. M. Dudd, E. Venardou, E. Garcia-Verdugo, P. Licence, A. J. Blake, C. Wilson and M. Poliakoff, Green Chem., 2003, 5, 187 RSC.
- G. Renard and D. A. Lerner, New J. Chem., 2007, 31, 1417 RSC.
- J. Valdez, R. Cedillo, A. Hernandez-Campos, L. Yepez, F. Hernandez-Luis, G. Navarrete-Vazquez, A. Tapia, R. Cortes, M. Hernandez and R. Castillo, Bioorg. Med. Chem. Lett., 2002, 12, 2221 CrossRef CAS.
- (a) R. J. Giguere, T. L. Bray, S. M. Duncan and G. Majetich, Tetrahedron Lett., 1986, 27, 4945 CrossRef CAS; (b) R. Gedye, F. Smith, K. Westaway, H. Ali, L. Baldisera, L. Laberge and J. Rousell, Tetrahedron Lett., 1986, 27, 279 CrossRef CAS; (c) S. Jubie, R. Rajeshkumar, B. Yellareddy, G. Siddhartha, M. Sandeep, K. Surendrareddy, H. S. Dushyanth and K. Elango, J. Pharm. Sci. & Res., 2010, 2, 69 CAS; (d) K. Niknam and A. Fatehi-Raviz, J. Iran. Chem. Soc., 2007, 4, 438 CAS; (e) S. Kaul, A. Kumar, B. Sain and A. K. Bhatnagar, Synth. Commun., 2007, 37, 2457 CrossRef CAS; (f) S. Y. Lin, Y. Isome, E. Stewart, J. F. Liu, D. Yohannes and L. Yu, Tetrahedron Lett., 2006, 47, 2883 CrossRef CAS; (g) M. Treu, T. Karner, R. Kousek, H. Berger, M. Mayer, D. B. McConnell and A. Stadler, J. Comb. Chem., 2008, 10, 863 CrossRef CAS; (h) B. Gutmann, D. Obermayer, B. Reichart, B. Prekodravac, M. Irfan, J. M. Kremsner and C. O. Kappe, Chem.–Eur. J., 2010, 16, 12182 CrossRef CAS; (i) M. Damm, T. N. Glasnov and C. O. Kappe, Org. Process Res. Dev., 2010, 14, 215 CrossRef CAS.
- (a) A. Loupy, A. Petit, J. Hamelin, F. Texier-Boullet, P. Jacquault and D. Mathé, Synthesis, 1998, 1213 CrossRef CAS; (b) R. S. Varma, Green Chem., 1999, 43 RSC; (c) M. Kidwai, Pure Appl. Chem., 2001, 73, 147 CrossRef CAS; (d) R. S. Varma, Pure Appl. Chem., 2001, 73, 193 CrossRef CAS.
- (a) R. Martínez-Palou, Química en Microondas, CEM Publishing, Mattews, NC 2006 Search PubMed; (b) P. Lidstöm and J. P. Tierney, Microwave-Assisted Organic Synthesis, Blackwell Scientific, 2005 Search PubMed; (c) C. O. Kappe and A. Stadler, Microwaves in Organic and Medicinal Chemistry, Wiley-VCH, Weinheim, 2005 Search PubMed; (d) A. Loupy, Microwaves in Organic Synthesis, Wiley-VCH, Weinheim, 2002 Search PubMed; (e) B. L. Hayes, Microwave Synthesis: Chemistry at the Speed of Light, CEM Publishing, Matthews, NC, 2002 Search PubMed.
- (a) R. Martínez-Palou, Mol. Diversity, 2006, 10, 435 CrossRef; (b) C. O. Kappe and D. Dallinger, Nat. Rev. Drug Discovery, 2006, 5, 51 CrossRef CAS; (c) C. O. Kappe, Chimia, 2006, 60, 308 CrossRef CAS; (d) T. N. Glasnov and C. O. Kappe, Macromol. Rapid Commun., 2007, 28, 395 CrossRef CAS; (e) M. Matloobi and C. O. Kappe, Chim. Oggi, 2007, 25, 26 CAS; (f) D. Dallinger and C. O. Kappe, Chem. Rev., 2007, 107, 2563 CrossRef CAS.
- (a) P. Lindstrom, J. Westman and A. Lewis, Comb. Chem. High Throughput Screen, 2002, 5, 441 Search PubMed; (b) H. E. Blackwell, Org. Biomol. Chem., 2003, 1, 1251 RSC; (c) V. Santagada, F. Frecentese, E. Perissutti, L. Favretto and G. Caliendo, QSAR Comb. Sci., 2004, 23, 919 CrossRef CAS.
- (a) Microwave oven for laboratory use suppliers; (b) Biotage AB, (www.biotage.com); (c) Milestone, (www.milestonesci.com); (d) Anton Paar, (www.anton-paar.com); (e) Lambda Technologies, (www.microcure.com).
- J. Alcázar, G. Diels and B. Schoentjes, QSAR Comb. Sci., 2004, 23, 906 Search PubMed.
- C. Mukhopadhyay, S. Ghosh and R. J. Butcher, Arkivoc, 2010, ix, 75 Search PubMed.
- M. Murray, A. J. Ryan and P. J. Little, J. Med. Chem., 1982, 25, 887 CrossRef CAS.
- Y. Yamamoto, H. Mizuno, T. Tsuritani and T. Mase, J. Org. Chem., 2009, 74, 1394 CrossRef CAS.
- J. Charton, S. Girault-Mizzi, M. Debreu-Fontaine, F. Foufelle, I. Hainault, J. Bizot-Espiard, D. Caignard and C. Sergheraert, Bioorg. Med. Chem., 2006, 14, 4490 CrossRef CAS.
Footnote |
| † Electronic Supplementary Information (ESI) available (Supplementary Parts 1 to 5): Synthesis of the benzimidazoles, characterisation data, copies of all 1H-NMR and 2D-NMR spectra. See DOI: 10.1039/c1ra00470k/ |
| This journal is © The Royal Society of Chemistry 2011 |