Peapod-like nickel@mesoporous carbon core-shell nanowires: a novel electrode material for supercapacitors†
Hao
Jiang
ab,
Ting
Sun
c,
Chunzhong
Li
*a and
Jan
Ma
*bc
aKey Laboratory for Ultrafine Materials of Ministry of Education, School of Materials Science and Engineering, East China University of Science & Technology, Shanghai 200237, China. E-mail: czli@ecust.edu.cn; Fax: 86-21-6425-0624; Tel: 86-21-6425-0949
bTemasek Laboratories, Nanyang Technological University, Singapore 637553, Singapore. E-mail: asjma@ntu.edu.sg
cSchool of Materials Science and Engineering, Nanyang Technological University, Singapore 639798, Singapore
First published on 5th September 2011
Abstract
We demonstrate a rational and facile strategy for synthesizing a novel peapod-like Ni@mesoporous carbon core-shell nanowire, which exhibit a higher specific capacitance with enhanced rate capability and cycling stability in 1 M KOH aqueous solution.
Supercapacitors, also called electrochemical capacitors, have recently attracted considerable interest because they can instantaneously provide higher power density than batteries and higher energy density than conventional dielectric capacitors, which make them probably the most promising candidates for next generation energy storage device.1–3 They have extensive applications, such as consumer electronics, energy management, memory back-up systems, industrial power and mobile electrical systems.4,5 Many materials have been investigated as the electrode materials in supercapacitors, including transition metal oxides,6,7 carbonaceous materials8,9 and conducting polymers10,11. Among them, NiO has been considered as one of the most promising electrode materials due to its good pseudo-capacitive behavior, low cost, environmental benignity and practical availability.12–14 However, like other transition metal oxide materials, the poor cycling stability and rate capability of NiO have limited its high specific capacitance potential in practical applications.
It is well known that the high rate capability of an electrode material is controlled by the ion diffusion resistance within the crystal structure, which can be mitigated by shortening the diffusion path.15 One-dimensional nanostructure materials have been intensely investigated to achieve faster redox reactions, higher specific surface areas and shorter diffusion paths for electrons and ions.7,16–18 On the other hand, the nano-sized particles may greatly enhance the utilization of active materials and lead to a higher specific capacitance because only a thin layer of the oxide material participates in the charge storage process. Therefore, a rational design of electrode materials at the nanoscale can be used to synthesize core-shell structures consisting of mesoporous carbon shells located outside and active materials embedded inside.8,19–21 Thus far, incorporation of metal oxides with carbon nanotubes,9graphene22 or conductive polymers23 to form hybrid composites has been reported to improve the electrochemical performance. However, there are few reports on the synthesis of such hybrid core-shell structures from other active materials except for Mn3O48 and Co3O4.21
In this communication, we demonstrated for the first time a facile strategy to realize the synthesis of peapod-like Ni@mesoporous carbon core-shell nanowires, labeled as Ni@MPC. The unique structure, when applied as a supercapacitor electrode, possesses three obvious advantages. (a) The outer mesoporous carbon shells not only construct the conducting pathway for the system’s electron transfer but also prevent the inner nanoparticles from aggregation and pulverization. (b) The mesopore feature of carbon shells ensures the interaction between electrolyte and inner active materials. On the other hand, it’s also reckoned to digest the possible volume changes while cycling. (c) The nano size of the particles will increase the contact areas between electrolyte and active materials, hence enhancing the effective utilization of active materials. More significantly, we found that the present Ni@MPC exhibited a higher specific capacitance with enhanced rate capability and cycle stability in 1 M KOH aqueous solution.
All the reagents used in the experiment were analytical grade (Sigma-Aldrich) and used without further purification. Ni(OH)2 nanowires have first been synthesized according to the method reported by Chu et al.24 For the synthesis of the Ni@MPC, dopamine, which can form a layer of coating on the surface of the as-synthesized Ni(OH)2 nanowires, was used as carbon source.8 For the generating of the mesoporous structures, triblock copolymer PEO-PPO-PEO (P123) was used. In a typical procedure, 100 mg of the as-obtained Ni(OH)2 nanowires (with average diameters of ∼ 25 nm, see the corresponding SEM image in the ESI), 200 mg of P123 and 122 mg of 2-amino-2-hydroxymethyl-propane-1,3-dio (Tris) were dissolved and dispersed in 100 mL distilled water. Subsequently, 100 mg of dopamine was added to the mixture with continuous stirring at room temperature for about 5 h. After that, the precipitates, i.e. polydopamine/Ni(OH)2, were collected by filtration, washed several times with absolute ethanol and dried at 60 °C for 6 h. Finally, the resulting products were carbonized and reduced into the Ni@MPC in flowing argon at 900 °C for 2 h.
Fig. 1 shows the XRD pattern of the as-synthesized Ni@MPC. All of the diffraction peaks can be indexed to the cubic phase of Nickel with lattice constants a = 0.354 nm, which is consistent with the literature value of a = 0.354 nm (JCPDS 65-0380). No other characteristic peaks are detected in the spectrum.
 | ||
| Fig. 1 XRD pattern of the as-obtained Ni@MPC. | ||
Detailed structural information of the Ni@MPC was analyzed using TEM at different magnifications. From a low magnification TEM image (Fig. 2a), it is clear that the monodispered nanoparticles with around 20 nm in diameter are uniformly encapsulated in the mesoporous carbon along the nanowires’ axial direction with periodic intervals. The mesopore feature of carbon can be confirmed by high magnification TEM images (Fig. 2(b, c), the white arrows). A representative peapod-like nanostructure with a diameter of about 25 nm was shown in Fig. 2b. The average thickness of carbon layer on the surface of nanoparticles was measured to be around 2.5 nm, as shown in Fig. 2c. Furthermore, the obvious graphitic layers indicate the carbon coating has a highly crystalline structure. These features are anticipated to be very beneficial for electrochemical properties. The high resolution TEM (HRTEM, Fig. 2d) reveals that the d-spacing of adjacent fringes for the nanoparticles is 0.18 nm, corresponding to the (200) planes of cubic Ni, which indicates that the Ni(OH)2 has been reduced into metal Ni completely during the carbonization process.
 | ||
| Fig. 2 (a) Low magnification and (b) a representative TEM images of the Ni@MPC; (c) high magnification and (d) high resolution TEM images of the Ni@MPC. | ||
The Ni@MPC also possess a higher BET surface area of 178 m2 g−1 with a proe volume of 0.49 m3 g−1. Nitrogen adsorption and desorption isotherm and the pore size distribution curves are shown in Fig. 3. The isotherm exhibits the type IV characteristics with a distinct hysteresis loop in the range of ca. 0.45–1.0 P/P0, which suggests the presence of mesoporous structures.8,25 The pore size distribution, calculated from desorption data using the BJH modal, was evaluated to have an average of ∼3.9 nm. It is reckoned that the Ni@MPC, with the unique structures of high BET surface area and uniform mesoporous size, will provide the possibility of efficient transport of electrons and ions in electrodes, hence leading to high electrochemical performance.
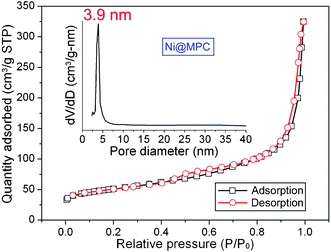 | ||
| Fig. 3 Nitrogen adsorption and desorption isotherm and its corresponding pore size distribution curves of the Ni@MPC. | ||
It is well known that electrochemical oxidation of nano-sized Ni to its corresponding NiO can be carried out by potential scan at the Ni oxide region.26,27 Additionally, inspired by the unique core-shell nanostructures, we evaluated the electrochemical performances of the as-synthesized Ni@MPC as electrode materials for supercapacitors using cycle voltammetry (CV) and galvanostatic charge-discharge (CD) measurements in 1 M KOH aqueous solution. Fig. 4(a) shows the CV curves of the Ni@MPC and substrate at a scan rate of 5 mV s−1. It can be observed that the CV curve of the Ni@MPC consists of a pair of strong redox peaks, which indicates that the capacitance characteristics are mainly governed by Faradaic reactions baed on the surface redox mechanism of Ni2+ to Ni3+. The equation can be expressed as follows:
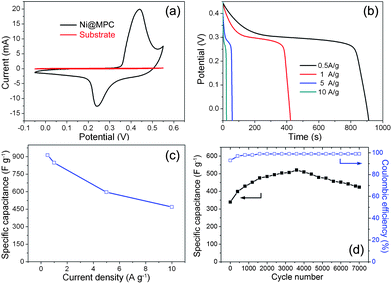 | ||
| Fig. 4 (a) CV curves of the Ni@MPC at a scan rate of 5 mV s−1, (b) discharge curves of the Ni@MPC at different current densities, (c) the specific capacitance as function of the current densities and (d) variations of specific capacitance and Coulombic efficiency with cycle number at a scan rate of 20 mV s−1 of the Ni@MPC. | ||
It is well accepted that galvanostatic charge/discharge examination is an established method for estimating the supercapacitive performance.28 The discharge curves of the present sample at different current densities within a potential range of −0.05 to 0.45 V were shown in Fig. 4(b). The nonlinear discharge curves further verify the pseudo-capacitance behavior. The specific capacitance as a function of current density for the Ni@MPC was illustrated in Fig. 4(c). It can be seen that the specific capacitance can achieve a maximum of 912 F g−1 at a low current density of 0.5 A g−1, which can still retain 468 F g−1 (about 51.2% retention) even at a current density as high as 10 A g−1. To the best of our knowledge, such high specific capacitance and good rate capability are comparable or superior to the best results reported for NiO/carbon composites in the literature. In a previous study, it was reported that NiO/CNTs films delivered a high specific capacitance of 1000 F g−1 by electrochemical precipitation.29 Such investigations intend to balance the cost and the performance of supercapacitors. However, the introduction of CNTs increases the fabrication cost, in addition to their complicated method, hence limiting its commercial potential. Furthermore, the specific capacitance of our materials is also higher than that of pure NiO with various morphologies reported in the literature, such as flowerlike NiO hollow nanospheres (770 F g−1)30, porous NiO nano/micro spherical superstructures (710 F g−1)14 and mesoporous NiO nanotubes (405 F g−1)31. Very recently, Kong et al. reported the synthesis of loose-packed NiO nano-flakes, showing a highest specific capacitance as high as 942 F g−1 under a low annealing temperature (at 250 °C).32 However, they showed a poor stability (18% loss after only 1000 cycles) and a low potential window (0.4 V) due to their low crystallinity. In our case, the unique core-shell nanostructure not only delivered a higher specific capability, but significantly possessed enhanced rate capability and cycling stability due to its obvious advantages, as discussed in the introduction section. Furthermore, The energy density and power density of the Ni@MPC nanocomposites electrode are also calculated according to the discharge curves at different current densities (see Fig. S4†). The specific energy densities decreases from 36.8 to 16.7 Wh kg−1, while the specific power densities increases from 145 to 2569 W kg−1.
The long-term cycle stability of electrode materials is another critical requirement for practical applications. Fig. 4(d) depicts the specific capacitance and Coulombic efficiency as a function of cycle number plots at a scan rate of 20 mV s−1 up to 7000 cycles. It can be observed that the specific capacitance increases rapidly in the first 1500 cycles and then reaches a maximum value at around 3500 cycles. The reason can be attributed to the fact that metal Ni in the core gradually undergoes an electrochemical oxidation into NiO in 1 M KOH aqueous solution during the cycles.26 In detail, in the first hundreds of CV cycles, metal Ni nanoparticles in the core were easy to form a very thin oxide layer, which resulted in the rapid growth of the specific capacitance of the Ni@MPC. However, the further oxidation of metal Ni became more and more difficult due to the presence of oxide layer. The corresponding specific capacitance of Ni@MPC also increased slowly in the subsequent thousands of CV cycles, as shown in Fig. 4(d). When the electrochemical oxidation of metal Ni reached a certain extent, a maximum value was finally obtained. More significantly, the specific capacitance of the composite electrode can still remain at 82% of the maximum capacitance in the subsequent 3500 cycles with stable Coulombic efficiencies (∼99%). The results indicated that the Ni@MPC also possessed outstanding electrochemical stability.
In conclusion, we have demonstrated a rational and facile strategy to synthesize a novel peapod-like Ni@mesoporous carbon core-shell nanowire. As predicted, the as-prepared Ni@MPC, when applied as supercapacitor electrode, exhibited a higher specific capacitance of 912 F g−1 with enhanced rate capability and cycle stability. Such excellent capacitive behaviors are attributed to the unique core-shell nanostructures, which confirm the feasibility of rational design of electrode materials for supercapacitor applications. The findings in the present work are important not only for the synthesis of the unique peapod-like core-shell nanostructure, but also the development of advanced electrode materials based on transition metal oxides for the enhancement of capacitive performance. Furthermore, it is also reckoned that the as-synthesized unique core-shell nanostructures can be employed in other important areas, such as heterogeneous catalysis, magnetic drug delivery, and so forth.
Notes and references
- A. S. Arico, P. Bruce, B. Scrosati, J. -M. Tarascon and W. V. Schalkwijk, Nat. Mater., 2005, 4, 366 CrossRef CAS.
- M. Winter and R. J. Brodd, Chem. Rev., 2004, 104, 4245 CrossRef CAS.
- P. J. Hall, M. Mirzaeian, S. I. Fletcher, F. B. Sillars, A. J. R. Rennie, G. O. Shitta-Bey, G. Wilson, A. Cruden and R. Carer, Energy Environ. Sci., 2010, 3, 1238 CAS.
- J. R. Miller and P. Simon, Science, 2008, 321, 651 CrossRef CAS.
- E. Faggioli, P. Rena, V. Danel, X. Andrieu, R. Mallant and H. Kahlen, J. Power Sources, 1999, 84, 261 CrossRef CAS.
- H. Jiang, T. Zhao, C. Z. Li and J. Ma, J. Mater. Chem., 2011, 21, 3818 RSC.
- H. Jiang, T. Zhao, J. Ma, C. Y. Yan and C. Z. Li, Chem. Commun., 2011, 47, 1264 RSC.
- H. Jiang, L. P. Yang, C. Z. Li, C. Y. Yan, P. S. Lee and J. Ma, Energy Environ. Sci., 2011, 4, 1813 CAS.
- H. Zhang, G. P. Cao, Z. Y. Wang, Y. S. Yang, Z. J. Shi and Z. N. Gu, Nano Lett., 2008, 8, 2664 CrossRef CAS.
- K. Wang, J. Y. Huang and Z. X. Wei, J. Phys. Chem. C, 2010, 114, 8062 CAS.
- P. Fonseca, J. E. Benedetti and S. Neves, J. Power Sources, 2006, 158, 789 CrossRef.
- P. Lin, Q. J. She, B. L. Hong, X. J. Liu, Y. N. Shi, Z. Shi, M. S. Zheng and Q. F. Dong, J. Electrochem. Soc., 2010, 157, A818 CrossRef CAS.
- J. H. Kim, K. Zhu, Y. F. Yan, C. L. Perkins and A. J. Frank, Nano Lett., 2010, 10, 4099 CrossRef CAS.
- Z. Yuan, X. G. Zhang, L. H. Su, B. Gao and L. F. Shen, J. Mater. Chem., 2009, 19, 5772 RSC.
- M. S. Wu and M. J. Wang, Chem. Commun., 2010, 46, 6968 RSC.
- H. B. Zeng, W. P. Cai, Y. Li, J. L. Hu and P. S. Liu, J. Phys. Chem. B, 2005, 109, 18260 CrossRef CAS.
- Y. G. Sun, J. Q. Hu, Z. G. Chen, Y. Bando and D. Golberg, J. Mater. Chem., 2009, 19, 7592 RSC.
- S. J. Guo, S. J. Dong and E. K. Wang, Energy Environ. Sci., 2010, 3, 1307 CAS.
- K. T. Lee, Y. S. Jung and S. M. Oh, J. Am. Chem. Soc., 2003, 125, 5652 CrossRef CAS.
- W. M. Zhang, J. S. Hu, Y. G. Guo, S. F. Zheng, L. S. Zhong, W. G. Song and L. J. Wan, Adv. Mater., 2008, 20, 1160 CrossRef CAS.
- Y. Wang, H. J. Zhang, L. Lu, L. P. Stubbs, C. C. Wong and J. Y. Lin, ACS Nano, 2010, 4, 4753 CrossRef CAS.
- X. R. Wang, S. M. Tabakman and H. J. Dai, J. Am. Chem. Soc., 2008, 130, 8152 CrossRef CAS.
- R. Liu and S. B. Lee, J. Am. Chem. Soc., 2008, 130, 2942 CrossRef CAS.
- L. H. Dong, Y. Chu and W. D. Sun, Chem.–Eur. J., 2008, 14, 5064 CrossRef CAS.
- A. Vinu, D. P. Sawant, K. Ariga, M. Hartmann and S. B. Halligudi, Microporous Mesoporous Mater., 2005, 80, 195 CrossRef CAS.
- V. Ganesh, V. Lakshminarayanan and S. Pitchumani, Electrochem. Solid-State Lett., 2005, 8, A308 CrossRef CAS.
- K. W. Nam and K. B. Kim, J. Electrochem. Soc., 2002, 149, A346 CrossRef CAS.
- M. D. Stoller and R. S. Ruoff, Energy Environ. Sci., 2010, 3, 1294 CAS.
- K. W. Nam, E. S.Lee, J. H. Kim, Y. H. Lee and K. B. Kim, J. Electrochem. Soc., 2005, 152, A2123 CrossRef.
- Y. Cao, W. Guo, Z. M. Cui, W. G. Song and W. Cai, J. Mater. Chem., 2011, 21, 3204 RSC.
- S. L. Xiong, C. Z. Yuan, X. G. Zhang and Y. T. Qian, CrystEngComm, 2011, 13, 626 RSC.
- J. W. Liang, L. B. Kong, W. J. Wu, Y. C. Luo and L. Kang, Chem. Commun., 2008, 4213 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Materials characterization and electrochemical measurements, SEM image of Ni(OH)2 NWs, CV curves of Ni@MPC. See DOI: 10.1039/c1ra00458a |
| This journal is © The Royal Society of Chemistry 2011 |

