Electrochemically induced cascade Knoevenagel–Michael reactions of tetronic acid and aldehydes: synthesis of methylenebistetronic acids
Zheng-Zheng
Zhang
a,
Ni-Tao
Zhang
a,
Li-Ming
Hu
a,
Zhong-Qing
Wei
b,
Cheng-Chu
Zeng
*a,
Ru-Gang
Zhong
*a and
Yuan-Bin
She
c
aCollege of Life Science & Bioengineering, Beijing University of Technology, Beijing, 100124, China. E-mail: zengcc@bjut.edu.cn; lifesci@bjut.edu.cn; Fax: 0086-10-67392001
bCAS Key Lab for Biomedical Effects of Nanomaterials and Nanosafety, Institute of High Energy Physics, The Chinese Academy of Sciences (CAS), Beijing, 100049, China
cCollege of Environmental & Energy Engineering, Beijing University of Technology, Beijing, 100124, China
First published on 6th October 2011
Abstract
The results of cascade Knoevenagel–Michael reactions of tetronic acid and various aldehydes induced by electrochemically-generated base are described. It has been observed that electrochemical method at 0 °C gave higher yields of the corresponding methylenebistetronic acids. Also, a wide spectra of products could generate from electrolysis at room temperature, including seven-membered cyclic acetals, dehydrodimer of tetronic acid and the desired methylenebistetronic acids. The electrochemical results were also evaluated and compared with those obtained by using a conventional chemical method.
1. Introduction
Knoevenagel condensation and Michael addition are well-known reactions. Their combination into a cascade reaction has emerged as a powerful strategy in organic synthesis due to a fact that this approach allows molecular complexity and diversity to be created by assembling two or more reactions into a simple one-pot transformation. In addition, tedious isolation of intermediates in each step is avoided.1 In this context, most of this type of cascade reactions start from the initial formation of a corresponding carbanion of compound involving an active methylene group (e.g.malononitrile, acetylacetone, nitro methane, etc.), followed by a nucleophilic addition to a carbonyl compound and an elimination of a water molecule to generate polarized alkene (Knoevenagel reaction). The formed Knoevenagel condensation product serving as a Michael-addition acceptor further reacts with a nucleophile to complete the cascade reaction. Undoubtedly, the base plays an important role in promoting the cascade reaction.Cathodic reduction of some organic compounds possessing acidic H atom may lead to a formation of an intermediate that behaves as a base (electrochemically generated base, EGB). When this type of process is performed in alcohol/alkaline metal halides, alkoxide anion is generated at the cathode and, in principle, may induce reactions that need a base. The EGBs induced reactions possess the following advantages: (1) if the EGB is generated in situ in an undivided cell, the whole electrolyte is still neutral, and therefore, some base-sensitive functional groups can tolerate and survive such conditions, and (2) the electrolysis of the alcohol/alkali metal halides supporting system could generate a chemical oxidant at the anode, such as a halogen molecule, which may oxidize substrates and leads to a different reaction pathway. Consequently, molecules of structural diversity may produce. For these reasons, EGB has been paid much attention in organic synthesis.2
Since most of the cascade Knoevenagel–Michael reactions are initiated by chemical base, it is reasonable to envision that EGBs may replace the chemical base and also induce such a cascade reaction. Previously Elinson and coworkers have developed an electrocatalytic chain transformation procedure of organic compounds and applied the electrogenerated alkoxide anion in an undivided cell to the synthesis of a number of heterocyclic compounds of medicinally relation.3 On the other hand, Inesi et al. reported the electrochemical generation of cyanomethyl anion and its utility to synthesize carbamates4 and β-hydroxynitriles,5 and to selectively activate amides and C–H bonds in cyclization reactions, leading to the formation of pyrrolin-2-ones6 and indoles.7 In this paper, tetronic acid (also named dihydrofuran-2,4-diones) was elected to undergo tandem Knoevenagel–Michael reactions with aldehydes induced by electrochemically generated ethoxy anion to form methylenebistetronic acid derivatives. In addition, chemical approach was also performed to compare the advantage and limitation of the electrochemical process. To the best of our knowledge, only one example involved the preparation of 3,3′-(2-nitrobenzylidene)bistetronic acid by a two-component condensation using diethylamine as a base and ethanol as a solvent.8
2. Results and discussion
2.1. EGB and chemical base induced cascade Knoevenagel–Michael reactions of tetronic acid and aldehydes
Ethoxy anion (EGB) could be generated in situ by constant current electrolysis (CCE) of ethanol/alkali metal halides at the cathode in an undivided cell.9 Instead of using an external base to induce the Knoevenagel–Michael reactions of tetronic acid and aldehydes, all the experiments were performed in a simple electrochemically undivided cell equipped with a graphite anode and iron cathode using catalytic amount of NaBr in ethanol solution as the supporting electrolyte. Initial studies were performed by constant current electrolysis (10 mA, current density: ∼2 mA cm−2) at room temperature. With the progress of electrolysis, the colorless solution slowly turned brown and hydrogen evolution was observed at the iron cathode.As shown in Scheme 1 and Table 1 (Entry 1), when benzaldehyde 2a and 2 equiv. of tetronic acid were subjected to electrolysis, the expected 3a was isolated in 18% yield, along with 4a (38%) and dehydrodimer 5 (19%). Compound 4a is a seven-membered cyclic acetal stemmed from the base-induced reaction of the initially formed dehydrodimer 5 and benzaldehyde. Upon running the electrolysis at two different temperatures, we observed that the reaction temperature plays an important role. At room temperature the electrochemically induced cascade Knoevenagel–Michael reaction of tetronic acid and aldehydes was less selective and seven-membered acetal 4 is the main product. This trend was further confirmed when other benzaldehydes were subjected to the cascade reaction under identical conditions. For example, the reaction of 2b and tetronic acid gave a mixture of 3b (15%), 4b (25%) and 5 (5%) (Table 1, Entry 2). As for 2e, the three compounds 3e, 4e and 5 were isolated in 14%, 39% and 10% yields, respectively (Table 1, Entry 3). However, a selective formation of the corresponding methylenebistetronic acid 3 could be achieved at 0 °C. For example, electrolysis of the mixture of 2a and tetronic acid at 0 °C gave exclusively 3a in 81% yield (Table 1, Entry 4).
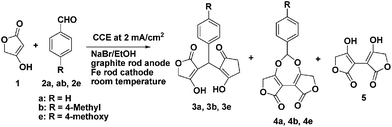 | ||
| Scheme 1 Electrochemical condensation of tetronic acid and aldehydes at room temperature. | ||
| Entry | Aldehyde | T/°C | Passed charge (F/mol) | Yield of 3 (%) | Yield of 4 (%) | Yield of 5 (%) |
|---|---|---|---|---|---|---|
| 1 | 2a | 20–25 | 1.6 | 18 | 38 | 19 |
| 2 | 2b | 20–25 | 1.6 | 15 | 25 | 5 |
| 3 | 2e | 20–25 | 1.6 | 14 | 39 | 10 |
| 4 | 2a | 0 | 1.6 | 81 | — | — |
To further investigate the generality and scope of the EGBs induced cascade Knoevenagel–Michael reactions of tetronic acid and aldehydes, a series of aldehydes were subjected to electrolysis in the presence of tetronic acid at 0 °C. As shown in Scheme 2 and Table 2, after passing 1.6 F/mol of charge, 4-methylbenzaldehydes 2b gave the corresponding methylenebistetronic acid 3b in 63% yield, whereas 3c was obtained in 76% yield from the electrolysis of a mixture of 2c and tetronic acid (Table 2, Entries 2 and 3).
 | ||
| Scheme 2 Electrochemical condensation of tetronic acid and aldehydes at 0 °C. | ||
| Entry | Compound | Passed charge (F/mol)a | Yield (%)a | Reaction timeb | Yieldb (%) |
|---|---|---|---|---|---|
| a Under electrochemical conditions at 0 °C. b Under chemical conditions. c Along with the formation of the Knoevenagel condensation product in 68% yield. d No corresponding 3e was detected and isolated. e Only catalytic amount of charge (0.3 F/mol) was required when the reaction was repeated at room temperature and 3f and 3h were afforded in 87% and 85% yields, respectively. | |||||
| 1 | 3a | 1.6 | 81 | 5 h | 67 |
| 2 | 3b | 1.6 | 63 | 5 h | 59 |
| 3 | 3c | 1.6 | 76 | 5 h | 52 |
| 4 | 3d | 1.6 | 48 | 10 h | 5c |
| 5 | 3e | 1.6 | 43 | 5 h | —d |
| 6 | 3f | 1.1e | 85 | 3 h | 82 |
| 7 | 3g | 1.1 | 83 | 5 h | 63 |
| 8 | 3h | 1.1e | 84 | 2 h | 80 |
| 9 | 3i | 1.6 | 69 | 5 h | 72 |
| 10 | 3j | 1.6 | 45 | 5 h | 43 |
It was also observed that the yields of methylenebistetronic acids 3 are sensitive to the nature of the substituents of the benzaldehydes. Benzaldehydes possessing electro-donating substituent give moderate yields of products. For example, 4-hydroxy-3-methoxy- and 4-methoxybenzoaldehyde yielded 3d and 3e in 48% and 43% yields, respectively (Table 2, Entries 4 and 5). In contrast, when electro-withdrawing groups were present, good to excellent yields of 3 were obtained. For example, 3f was obtained in 85% yield when 3-nitrobenzaldehyde was used (Table 2, Entry 6). In the cases of 4-nitro- and 2-nitrobenzaldehydes, 3g and 3h were isolated in 83% and 84% yields, respectively (Table 2, Entries 7 and 8). Delightedly, when this reaction was repeated at room temperature, only catalytic amount of charge (0.3 F/mol) was required and 3f and 3h were afforded in 87% and 85% yields, respectively. In addition, 4-bromobenzaldehyde gave 69% yield of 3i (Table 2, Entry 9). The fact that the cascade Knoevenagel–Michael reaction prefers the electron-withdrawing group substituted benzaldehydes is logical because electron-withdrawing substituents promote the electrophilicity of the carbonyl group and facilitate the Knoevenagel condensation.
Finally, it was observed that the cascade Knoevenagel–Michael reactions induced by electrochemically generated ethoxy anion is also compatible to aliphatic aldehyde, although with a bit lower yields. For example, the corresponding methylenebistetronic acids 3j was obtained in 45% yield when a solution containing butanal and tetronic acid was electrolyzed at 0 °C (Table 2, Entry 10).
To compare the advantage and limitation of EGB-induced cascade Knoevenagel–Michael with that induced by conventional chemical synthesis, we investigated the base induced tandem reaction (Scheme 3), following the experimental conditions of a known example of obtaining 3,3′-(2-nitrobenzylidene)bistetronic acid. As shown in Table 2 (column 6), the cascade reaction of benzaldehyde 2a and tetronic acid in the presence of diethylamine as base and after refluxing for 5 h afforded the corresponding benzylidenebistetronic acid (3a) in 67% yield, a bit lower yield compared with that from the electrochemical approach. Other aldehydes were also tested and the results are summarized in Table 2. 4-Methyl- and 2-methylbenzoaldehyde yielded 3b and 3c in 59% and 52% yields, respectively (Table 2, Entries 2 and 3). However, in the case of 4-hydroxy-3-methoxybenzoaldehyde, only 5% yield of 3d was obtained after refluxing for 10 h, along with compound 6, a direct Knoevenagel condensation product, isolated in 68% yield (Scheme 3 and Table 2, Entry 4). Moreover, no 3e was detected when 2e was refluxed with tetronic acid in the presence of diethylamine (Table 2, Entry 5).
 | ||
| Scheme 3 The reaction between tetronic acid and 2 in the presence of diethylamine | ||
A similar tendency was also observed with electron-withdrawing substituents. For example, when 3-nitrobenzaldehyde was subjected to cascade reactions with tetronic acid in the presence of diethylamine, 3f was smoothly obtained in 82% yield (Table 2, Entry 6). In the cases of 4-nitro- and 2-nitrobenzaldehydes, 3g and 3h were achieved in 63% and 80% yields, respectively (Table 2, Entries 7 and 8). In addition, 4-bromobenzaldehyde and butanal give 3i and 3j in 72% and 43% yields. (Table 2, column 6, Entries 9 and 10).
On the basis of the above experimental results, we can conclude that most of the aldehydes used gave higher yields of the corresponding methylenebistetronic acids upon employing the electrochemical method. In addition, the use of more than stoichiometric amount of diethylamine and heating for extended period of reaction time were avoided. Therefore, the EGB-induced procedure is superior to the chemical approach.
It is noteworthy that tetronic acid and methylenebistetronic acids 3 are useful and versatile synthetic intermediates for the synthesis of 4H-pyranes or 4H-pyridines with potential anti-cancer, antiviral and anti-inflammatory activities. In addition, methylenebistetronic acids 3 can be used as building block for the synthesis of substituted xanthenes which are structural key-units in several natural products.10
The structures of all compounds were identified by NMR, IR and ESI-MS methods, where, compounds 3 and 4 can be distinguished from spectral data. Taking 3b and 4b as an example, ESI-MS data (m/z: 302 for 3b and m/z: 300 for 4b) of them show that each compound is a double adduct of 4-methylbenzaldehyde with two tetronic acid molecules. Both 1H NMR spectra indicates that the two tetronic acids are in an identical chemical environment. The only discrimination is the chemical shift of the methylidyne unit. As for 4b, the methylidyne is connected directly by two oxygen atoms which allow for its signal situating at 6.60 ppm, whereas, the corresponding signal of compound 3b is at 4.68 ppm (Fig. 1). Therefore, we can conclude that compounds 4 are seven-membered cyclic acetal.
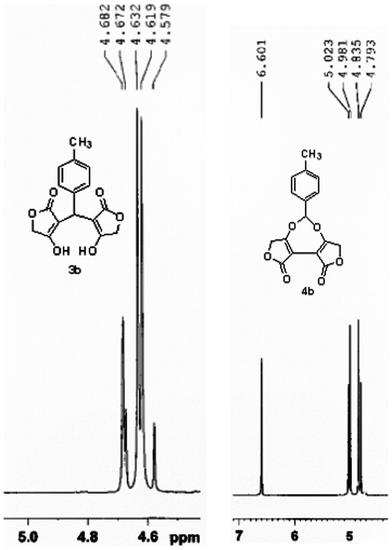 | ||
| Fig. 1 Partial 1H NMR spectra of compounds 3b and 4b showing the chemical shifts of the methylidyne unit | ||
As for compound 6, its 1H NMR show characteristic signals of two similar compounds. The two singlets at 4.65 ppm and 4.77 ppm are not exchangeable with D2O, which implies that compound 6 is not involved in a keto-enol equilibrium but to a mixture of E- and Z-isomers, consistent with that reported in reference.11 The ratio of the two isomers is estimate to be 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 based on the integration.
7 based on the integration.
2.2. Reaction mechanism of electrochemical process
To understand the mechanism for the formation of all compounds, a controlled experiment was performed, where, the tetronic acid in the absence of aldehydes was electrolyzed. It was observed that 3-bromo-4-hydroxyfuran-2(5H)-one 7 and dehydrodimer 5 were formed when 0.5 F/mol charge was passed through the cell.Based on the above results, we can propose a plausible electrochemically induced mechanism. As shown in Scheme 4, the outlined mechanism involves an initial formation of an ethoxy anion on the cathode and bromine on the anode. Then, the ethoxy anion functions as a base to deprotonate the tetronic acid and generate tetronic acid anion. Once the key specie is formed, at least two different pathways may involve, in parallel or in competitive, depending on the nature of the aldehydes and the reaction conditions. One pathway is that the tetronic acid anion undergoes Knoevenagel condensation with aldehydes to form ylidenetetronic acid derivatives, followed by Michael addition to produce methylenebistetronic acid derivatives 3. The second pathway is the bromination of tetronic acid anion to form brominated tetronic acid, followed by nucleophilic substitution to form dehydrodimer, which further condense with aldehydes and generate seven-membered acetals 4. This proposal is demonstrated by the isolation of dehydrodimer of tetronic acid 5. In addition, paralleled to the reaction that involving brominated tetronic acid 7, direct oxidation of tetronic acid anion at the anode is also possible. Then the formed radical species undergoes C–C coupling and yields compound 5.
 | ||
| Scheme 4 A proposed mechanism for the electrochemically induced condensation between tetronic acid and aldehydes | ||
3. Conclusions
The tandem Knoevenagel condensation/Michael addition of tetronic acid and aldehydes induced by both electrochemical-generated base and conventional base has been carried out and compared. It was observed that higher yields of the corresponding methylenebistetronic acids could be achieved for most of the aldehydes studied by using electrochemical method at 0 °C in comparison to the use of a conventional chemical method. Therefore, from an environmental and safety point view, this method is superior to the chemical approach. In addition, electrolysis at room temperature was also performed and was founded to be less selective. Seven-membered cyclic acetals and dehydrodimer of tetronic acid were isolated, in addition to the corresponding methylenebistetronic acids in more cases.4. Experimental section
4.1. Instruments and reagents
All melting points were measured with a XT4A Electrothermal melting point apparatus and are uncorrected. IR spectra were recorded as KBr pellets. 1H and 13C NMR spectra were recorded with an AV 400M Bruker spectrometer (400 MHz 1H frequency, 100 MHz 13C frequency). Chemical shifts are given as δ values (internal standard: TMS). The MS spectra (ESI) were recorded on a Bruker Esquire 6000 mass spectrometer. Tetronic acid 1 was reagent-grade and obtained from Alfa Aesar China (Tianjin Co., Ltd.), other chemicals and solvents were from Beijing Chemicals Co. and used without further purification. The graphite rod anodic and iron rod cathodical electrodes (each Ø: ∼0.5 cm) were obtained from Tianjin Aidahengchen Co., Ltd4.2. General procedure for the synthesis of methylenebistetronic acids
Acknowledgements
This work was supported by Grants from the National Natural Science Foundation of China (No. 20772010), Beijing Natural Science Foundation (No. 7112008), the National Basic Research Program of China (No. 2009CB930200, 2011CB933101) and funding to Zeng, C. C. from Beijing City Education Committee (KM201010005009).References
- (a) K. C. Nicolaou, D. J. Edmonds and P. G. Bulger, Angew. Chem., Int. Ed., 2006, 45, 7134–7186 CrossRef CAS; (b) L. F. Tietze, Chem. Rev., 1996, 96, 115–136 CrossRef CAS.
- (a) Y. N. Ogibin, M. N. Elinson and G. I. Nikishin, Russ. Chem. Rev., 2009, 78, 89–140 CrossRef CAS; (b) M. E. Niyazymbetov and D. H. Evans, Tetrahedron, 1994, 49, 9627–9688 CrossRef.
- (a) M. N. Elinson, A. S. Dorofeev, R. F. Nasybullin and G. I. Nikishin, Synthesis, 2008, 1933–1937 CrossRef CAS; (b) M. N. Elinson, A. S. Dorofeev, F. M. Miloserdov, A. I. Ilovaisky, S. K. Feducovich, P. A. Belyakov and G. I. Nikishin, Adv. Synth. Catal., 2008, 350, 591–601 CrossRef CAS.
- M. Feroci, M. Orsini, L. Rossi, G. Sotgiu and A. Inesi, J. Org. Chem., 2007, 72, 200–203 CrossRef CAS.
- G. Bianchi, M. Feroci and L. Rossi, Eur. J. Org. Chem., 2009, 23, 3863–3866 CrossRef.
- A. Arcadi, A. Inesi, F. Marinelli, L. Rossi and M. Verdecchia, Eur. J. Org. Chem., 2007, 15, 2430–2437 CrossRef.
- A. Arcadi, G. Bianchi, A. Inesi, F. Marinelli and L. Rossi, Eur. J. Org. Chem., 2008, 5, 783–787 CrossRef.
- K. Goerlitzer, J. Trittmacher and U. Bartke, Pharmazie., 2002, 57, 606–609 CAS.
- (a) M. N. Elinson, A. S. Dorofeev, S. K. Feducovich, S. V. Gorbunov, R. F Nasybullin, N. O. Stepanov and G. I. Nikishin, Tetrahedron Lett., 2006, 47, 7629–7633 CrossRef CAS; (b) M. N. Elinson, A. I. Ilovaisky, V. M. Merkulova, F. Barba and B. Batanero, Tetrahedron, 2008, 64, 5915–5919 CrossRef CAS.
- (a) R. Schobert and A. Schlenk, Bioorg. Med. Chem., 2008, 16, 4203–4221 CrossRef CAS; (b) I. V. Magedov, M. Manpadi, S. Van slambrouck, W. F. Steelant, A. E. Rozhkova, N. M. Przheval'skii, S. Rogelj and A. Kornienko, J. Med. Chem., 2007, 50, 5183–5192 CrossRef CAS; (c) J. P. Poupelin, G. Saint-Ruf, O. Foussard-Blanpin, G. Narcisse, G. Uchida-Ernouf and R. Lacroix, Eur. J. Med. Chem., 1978, 13, 67–71 CAS.
- H. Zimmer, W. W. Hillstrom, J. C. Schmidt and P. D. Seemuth, J. Org. Chem., 1978, 43, 1541–1544 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2011 |
