Fabrication of biologically active surface-modified Taxol nanowires using anodic aluminum oxide templates
Mohamed H.
Abumaree
a,
Lingyan
Zhu
b,
Christopher J.
Bardeen
b,
Salem D.
Al-Suwaidan
c and
Rabih O.
Al-Kaysi
*a
aCollege of Basic Sciences, King Saud bin Abdulaziz University for Health Sciences, National Guard Health Affairs, Riyadh 11426, Saudi Arabia. E-mail: kaysir@ksau-hs.edu.sa; rabihalkaysi@gmail.com
bDepartment of Chemistry, University of California Riverside, Riverside, CA 92521, USA
cKing Abdullah International Medical Research Center, National Guard Health Affairs, Riyadh 11426, Saudi Arabia
First published on 9th September 2011
Abstract
Nanowires composed of the anti-cancer drug Paclitaxel (Taxol) were fabricated using solvent annealing method inside Anodic Aluminum Oxide (AAO) templates. Surface modification of the AAO template with n-octadecyltrichlorsilane (OTS), prior to loading with Taxol, prevented the nanowires from agglomerating after dissolving the template in 20% phosphoric acid. Incorporating 0.4% sodium dodecyl sulfate (SDS) with the phosphoric acid solution helped disperse the nanowires. Using this method we were able to fabricate nanowires with tunable diameters (200, 80, 55, 35 and 18 nm). Nanowires with a diameter < 80 nm formed a clear dispersion in a phosphate buffer saline (PBS) solution. These nanowires were characterized using optical and electron microscopy. The amount of Taxol in the nanowires was confirmed gravimetrically and spectroscopically using UV-Vis. Nanowires with different diameters were incubated with human monocytic U937 cells and these cells undergo morphology changes consistent with Taxol exposure. Templated nanowire growth can provide a novel strategy for the preparation of biologically active nanostructures composed of low solubility drug molecules.
1. Introduction
Organic nanostructures have shown very interesting properties and uses1–3 from photomechanical actuation4,5 to wave guiding6 and chemical sensing.7 Another promising application of organic nanostructures is in medicine and drug delivery.8,9Nanostructures containing drug molecules can enhance the efficacy, solubility, deliverability and biodistribution of the drug.10,11 This mode of drug delivery can be used to overcome problems associated with low aqueous solubility that tend to limit bioavailability.12 An example is Taxol (Fig. 1), a diterpenoid alkaloid extracted from the inner bark of the Pacific yew tree13 or synthesized in the lab,14 that has an extremely low water solubility of around 2 μg/mL.15–18 Nonetheless the potency of this drug in fighting breast, lung, ovarian, head and neck cancer19,20 has compelled researchers to find ways to solubilize the drug by encapsulating Taxol nanoparticles in liposomes or emulsions. Formulations containing pharmaceutically acceptable solubilizing agents such as Cremophor EL (polyoxyethoxylated castor oil),21DMSO,22ethanol or polymers23 have been used to encapsulate Taxol nanoparticles, which tend to agglomerate otherwise, in order to improve in vivo delivery. Unfortunately, serious side effects from these delivery “vehicles” have limited the use of this drug in some patients.24 These side effects have led to the design and testing of more biologically benign “vehicles”.25 These formulations often consist of surfactant or polymer micelle stabilized Taxol nanoparticles made by reprecipitation23,26,27 or femtosecond laser ablation.28 Both methods result in polymorphic drug particles with broad size distributions, random particle shapes, and a large micelle to drug ratio.27 In general, the resulting suspensions are unstable and the particles tend to aggregate and precipitate over the course of hours in aqueous solution.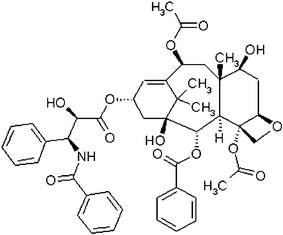 | ||
| Fig. 1 Molecular structure of Taxol. | ||
Anodic Aluminum Oxide (AAO) template assisted synthesis of organic nanowires has proven to be a reliable and general method for making one-dimensional (1D) nanostructures with accurate and precise diameters.1–3 We have developed and optimized the conditions necessary to grow variable diameter 1D nanostructures made from simple organic molecules that can crystallize when solvent annealed inside an AAO template.29 The goal of the present work is to determine whether this template-based approach can be extended to fabricate nanowires composed of more complicated drug molecules like Taxol. We found that the low solubility of Taxol presented unique challenges that required the development of new surface functionalization chemistry for the AAO templates. By using n-octadecyltrichlorosilane (OTS) to chemically coat the surface of the AAO pores prior growing Taxol nanowires in them, we generated n-octadecylsiloxane (ODS) surface modified Taxol nanowires (SM-TNs). These nanowires can be released from the AAO template to yield well-dispersed nanowires. By varying the pore size of the AAO template used, we were able to fabricate nanowires with diameters ranging from 18 nm to 200 nm. The length of the SM-TNs can be shortened from the standard length of 50 μm to sub-micron lengths by ultrasonication, without affecting the degree of dispersion or chemical composition of the drug. After characterizing the composition, morphology and stability of the nanowires using spectroscopy and microscopy experiments, we added a nanowire suspension to live human fibroblast cells and found that the cytotoxic properties of Taxol are retained in the SM-TN form. These results demonstrate that the templated growth methods used to make nanowires of conjugated organic materials can be adapted for the preparation of uniform size and shape nanostructures composed of complex drug molecules.
2. Results and Discussion
Our first attempt to fabricate Taxol nanowires involved using the same methods that have worked previously for a variety of conjugated organic molecules.29 A solution of the compound in dichloroethane (DCE) was deposited onto an AAO template and then annealed under DCE solvent vapor for a variable period of time. After carefully polishing the template surface, gravimetric analysis indicated that roughly 1.1 mg of Taxol had been deposited inside a standard 200 nm pore diameter template (Anodisc-13). Taxol is most stable in low pH aqueous solutions17 which made it safe to remove the template using 20% phosphoric acid.† Unfortunately, the released nanowires clumped up in micron-sized bundles and formed a visible precipitate. An SEM scan of the precipitate revealed that the nanowires had fused into large bundles taking the shape of the template (Fig. 2). Attempts to break up the bundles by ultrasonication led to the formation of even larger aggregates that quickly fell out of solution, similar to what happens when an ethanol solution of Taxol is reprecipitated in water.30 Upon closer inspection of the precipitate it was evident that individual nanowires had fused to generate large micron sized aggregates (Fig. 2 inset). This phenomenon was independent of the type of annealing solvent used such as ethylacetate, dichloroethane or tetrahydrofuran or the AAO template pore diameter. In an attempt to prevent nanowire aggregation, we tried using a surfactant to coat the surface of the nanowires while being released from the AAO template. We dissolved the template in 20% phosphoric acid containing 0.8% SDS, but again the nanowires aggregated to form large micron-scale structures along with a tiny fraction of scattered nanowires with variable diameter. Increasing the concentration of SDS to 4% caused dissolution of the Taxol nanowires. Using a cationic surfactant such as 1% cetyltrimethylammonium bromide (CTAB) gave similar results. Dissolving the template using 1 molar NaOH solution with 1% SDS caused instant decomposition of the Taxol31 with the formation of brownish oily byproducts.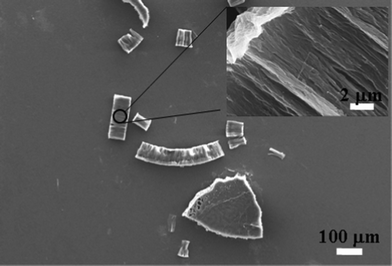 | ||
| Fig. 2 Taxol nanowires without surface modification (200 nm diameter) fused together to form large micron sized chunks. Inset: A close-up view of the surface showing 200 nm diameter nanowires fused together. | ||
From our initial results, it appeared that a more effective chemical coating would be required to disperse the Taxol nanowires in aqueous solution. We decided to covalently functionalize the surface of the AAO template with a hydrophobic layer32–34 that can be incorporated inside the Taxol nanowire during the solvent annealing process. We chose n-octadecyltrichlorosilane because it can covalently bond to the surface of the Alumina surface and crosslink to form a thin hydrophobic coating.35Fig. 3 shows the general reaction scheme and expected products. During the solvent annealing process, the Taxol molecules can incorporate the n-octadecyl hydrocarbon chains in the nanowire structure due to the high lipophilic nature of Taxol (as illustrated in Fig. 3b). The siloxy end of the ODS layer will not be incorporated in the Taxol molecule due to its inorganic and lipophobic nature. Hydrolyzing unreacted Si–Cl bonds36 with ethanol/1 molar HCl solution and then drying in an oven ensured the consumption of sites where additional ODS molecules could covalently attach by promoting acid catalyzed crosslinking between neighboring Si–OH units to form a Si–O–Si bond network.37 After loading the SF–AAO template with Taxol using the previous solvent annealing conditions, it is dissolved in H3PO4. Acid hydrolysis of the Al–O–Si bond should yield Si–OH groups38 on the surface of the Taxol nanowire (as seen in Fig. 3c). Under acidic conditions, these surface Si–OH groups have a tendency to condense with neighboring Si–OH to eliminate water and form a Si–O–Si network. It is likely that the surface of a SM–TN in the phosphoric acid solution is in a state of dynamic equilibrium between Si–OH and Si–O–Si bond formation.39 When the nanowires are released, by dissolving the AAO template in 20% H3PO4, the n-octadecyl pendent groups remain embedded inside the nanowire, while the hydrophilic Si–O(–Si, –H) end groups act like a pseudo surfactant coating the surface of the Taxol nanowire (as seen in Fig. 3c). The surrounding monolayer of siloxane can act as a chemically inert sheath protecting the Taxol from hydrolysis, similar to the effect cyclodextrins have on slowing down the hydrolysis of Taxol molecules in alkaline or acidic solutions.17,40 We should note that SM–TN’s with 200 nm pore diameter can be easily liberated as a stable dispersion from the SF–AAO template using only 20% H3PO4. This was not the case for smaller diameter nanowires. Although the surface modified AAO template slowed down the rate of agglomeration, it was nonetheless observed that smaller nanowires merged to form larger diameter micro-wires in SDS-free 20% H3PO4 (as seen in Fig. 4). To prevent such inter-nanowire cross-linking, we incorporated 0.4% SDS with the phosphoric acid solution as a “capping agent” to enhance the dispersion of the nanowires. The SDS concentration was too small to cause dissolution to the Taxol nanowires, since it only increased the solubility of Taxol in water to 35 μg/mL.‡
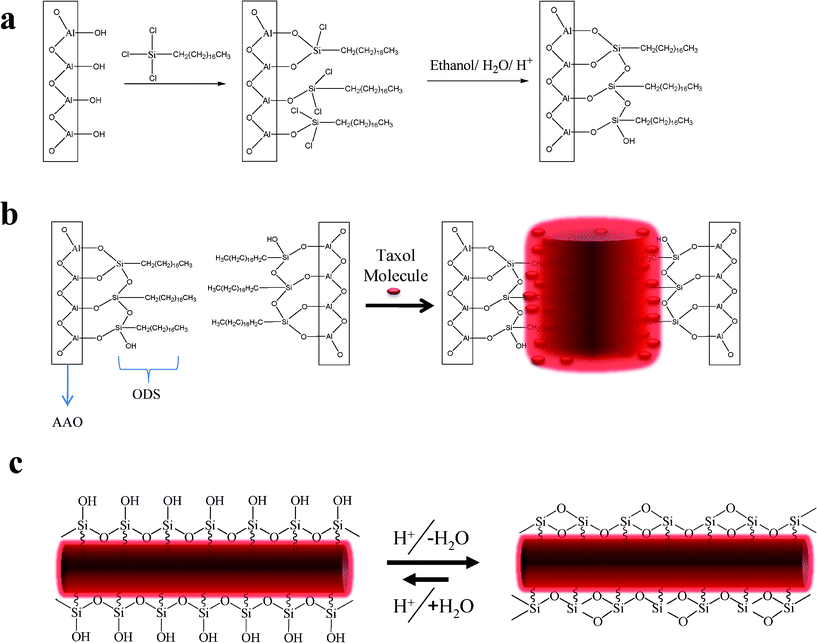 | ||
| Fig. 3 a) Reaction of the AAO template surface –OH groups with OTS to yield a covalently decorated SF-AAO template. b) Taxol molecules solvent annealed inside SF-AAO template. The lipophilic nature of the molecule will incorporate the n-Octadecyl chain in the structure of the nanowire. c) After removing the template the ODS will remain tethered to the Taxol nanowire with the formation of Si–OH surface in equilibrium with Si–O–Si. | ||
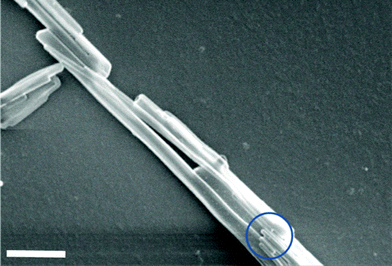 | ||
| Fig. 4 Fused 55 nm SM-TNs. Bottom circle reveals the presence of individual 55 nm nanowires before fusing to generate larger diameter nanowires. Scale bar = 1 μm. | ||
The general reaction scheme outlined in Fig. 3 was confirmed by using IR spectroscopy to detect the different chemical species at different points during the preparation. The IR spectrum of the bare AAO template is shown in Fig. 5a. After the template is exposed to the OTS treatment, the spectrum in Fig. 5b shows the appearance of C–H stretching bands between νmax/cm−1 3000–2800 corresponding to the n-octadecyl hydrocarbon chain.39 After the Taxol was deposited in the templates, its signature carbonyl bands were also observed in the IR absorption as shown in Fig. 5c, confirming its presence in the template. To confirm that the ODS layer remains attached to the liberated Taxol nanowires, a suspension of a 200 nm diameter SM-TNs in 20% H3PO4 was filtered, dissolved in methanol and centrifuged. After dissolution of the Taxol interior, the residual n-octadecylsiloxane layer was collected and deposited on a KBr disk. The FTIR spectrum confirmed the presence of n-octadecylsiloxane as shown in Fig. 5d, which can be compared to the spectrum in Fig. 5b. Both spectra show the signature C–H stretching bands of the alkyl chain around 3000 cm−1. The spectrum in Fig. 5d can also be compared to the FTIR spectrum of an unreacted OTS film shown in Fig. 5e. The SiCl bond stretch located between νmax/cm−1 570 and 590 in the unreacted OTS is completely absent in Fig. 5d, confirming that these bonds in the OTS are completely consumed in the process.
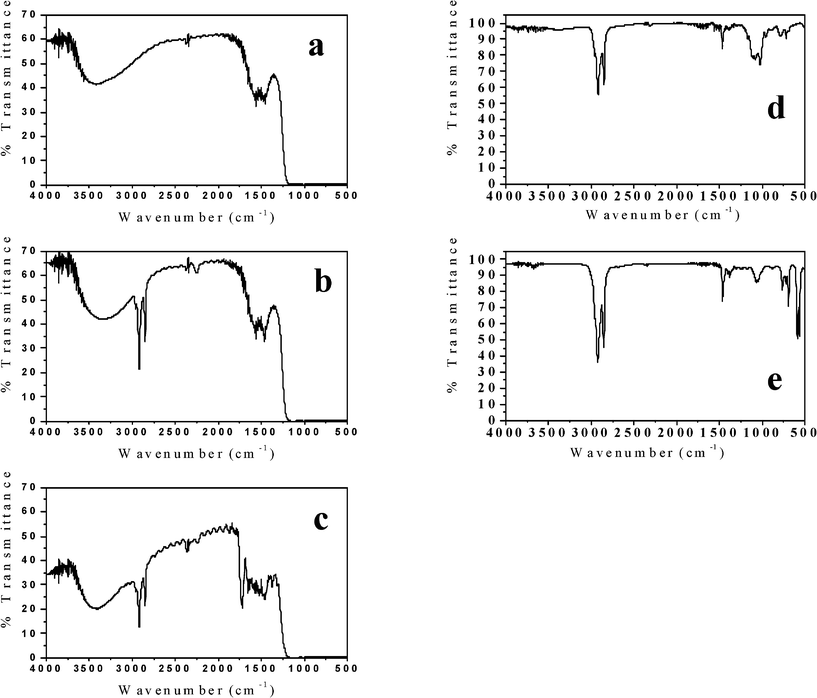 | ||
| Fig. 5 FTIR of AAO template. a) Before treatment with OTS. b) After OTS treatment, where the signature C–H stretches now appear between νmax/cm−1 3000–2800. c) After OTS treatment and Taxol loading. The stretch at νmax/ cm−1 ∼ 1750 indicates the presence of the carbonyl groups on the Taxol. d) n-Octadecylsiloxane shells after separating them from SF-TN. Note the absence of the large Si–Cl stretching peak around νmax/cm−1 580. e) A thin layer of unreacted OTS over KBr disk showing the presence of Si–Cl stretch at νmax/cm−1 580. | ||
The aqueous suspension of the different nanowires ranged from cloudy to clear, depending on their diameter. SM-TNs with a diameter smaller than 80 nm showed superb dispersion yielding a clear suspension (as seen in Fig. 6 a, b, c). Fig. 6d shows a suspension of unmodified Taxol nanowires, which have clumped up into large aggregates. The acid dispersed SM-TN solution could be frozen and stored at −150 °C. The structure and morphology of individual nanowires was studied using electron microscopy. The scanning electron microscopy (SEM) images in Fig. 7 show that the use of AAO templates with different pore diameters resulted in nanowires with varying diameters, as expected. Typically, the length varied due to breakage of the wires during processing, but the diameters were quite uniform, with less than a 10% variation within a single sample. The nanowire diameter tended to be slightly larger than the nominal AAO pore diameter, probably due to coating effects from the layer of Platinum. Direct evidence for the presence of the Si coating on the nanowires was obtained from the transmission electron microscopy (TEM) and energy dispersive X-ray (EDX) spectroscopy of single SM-TN. A TEM scan of a SM-TN was not sensitive enough to resolve the atomically thick layer of Si atoms on the surface of the nanowire but an EDX spectrum of the nanowire confirmed the presence of Si on the nanowire surface. Fig. 8a shows the image of 200 nm diameter SM-TNs scattered on a TEM sample holder, after separation from the phosphoric and SDS solution. Fig. 8b shows the close-up image of an isolated 200 nm diameter SM-TN. Most of the nanowires yield Si signals on the order of 0.3% Si by weight. If we assume that the Si atoms are uniformly distributed across the surface, this mass factor results in a calculated surface density of 3 Si atoms/nm2. This value is very close to that calculated for a monolayer of ODS, 3.8 Si atoms/nm2. Dark patches on the surface correspond to regions of higher Si concentration, typically on the order of 0.6% by weight. These regions most likely have a double layer of ODS, but they are clearly a minority component. The EDX data also suggests that the ODS layer is truly anchored inside the Taxol nanowire and was not washed out by the SDS or during the sonication process. The absence of a sulfur signal from the EDX shows that the SDS was not absorbed inside the Taxol nanowire and was completely removed during the washing process.
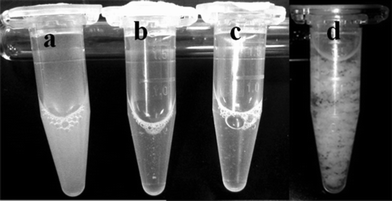 | ||
| Fig. 6 SM-TN dispersed in PBS solution. a) 200 nm, b) 80 nm, c) 35 nm. All the solutions have similar concentration of Taxol (~400 μg/ mL). The 200 nm nanowires scatter light thus appearing turbid. d) Unmodified 200 nm Taxol nanowires suspended in water. | ||
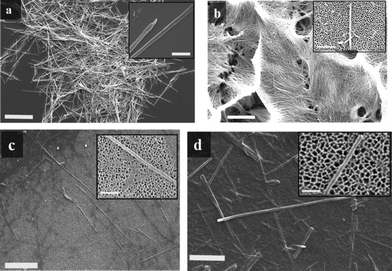 | ||
| Fig. 7 a) SEM image of SM-TN bundles with 200 nm diameter. Scale bar = 20 μm. Inset: close up image of 200 nm SM-TN, scale bar = 1 μm . b) SEM image of 80 nm SM-TNs bundles from a partially etched AAO template. Scale bar = 10 μm. Inset: close up image of 80 nm SM-TN over an AAO template surface, scale bar = 1 μm. c) SEM image of 55 nm SM-TNs over AAO template. Scale bar = 2 μm. Inset: close up image of 55 nm SM-TN, scale bar = 500 nm. d) SEM image of 35 nm SM-TNs over AAO template. Scale bar = 500 nm. Inset: close up image of 35 nm SM-TN, scale bar = 200 nm. | ||
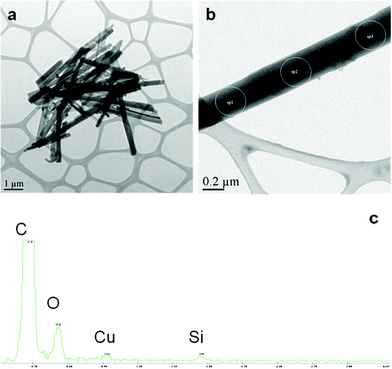 | ||
| Fig. 8 TEM and EDX spectra of SM-TN. a) TEM of 200 nm diameter SM-TNs cluster after cleaning from H3PO4 and SDS residue. b) TEM of a single 200 nm diameter SM-TN. c) EDX spectrum of sp1, 2 and 3 of the nanowire in figure (b). The overwhelming signal of the C relative to the Si is consistent with the proposed structure of the SM-TN. The % Si along the nanowire was uniform. The Cu peak is from the fine coating on the TEM grid. | ||
Gravimetric analysis of the templates before and after coating with OTS and loading with Taxol confirmed the presence of the surface coating and allowed us to quantify the yield of nanowires. On average, we assumed that one OTS molecule reacts with a single OH group on the alumina surface,41 thus leaving two unreacted Si–Cl bonds which are then hydrolyzed and crosslinked using ethanol/1 molar HCl to form a chain of Si–O–Si bonds.42 The ODS layer thickness T can be calculated using gravimetric analysis and eqn (1)
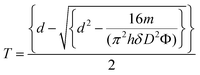 | (1) |
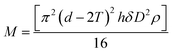 | (2) |
| d: AAO pore diameter (nm) | 200 ± 30 | 80 ± 8 | 55 ± 6 | 35 ± 3 | 18 ± 3 |
| δ: AAO pore density/cm2 | 7.1 × 108 | 2 × 109 | 5 × 109 | 1 × 1010 | 5 × 1010 |
| D: AAO template diameter (cm) | 1.3 | 1.3 | 1.3 | 1.3 | 1.3 |
| T: Thickness of ODS layer (nm) | 4 | 2.9 | 2.3 | 3.1 | 2.7 |
| M: Mass of deposited Taxol/mg (Theoretical) | 1.08 (1.90) | 0.41 (0.70) | 0.46 (0.82) | 0.53 (0.55) | 0.22 (0.51) |
| [Taxol] in the form of SM-TN in 20% H3PO4/0.4% SDS solution (μg/mL) | 1440 | 547 | 613 | 707 | 293 |
| % Taxol in SM-TN | 89% | 84% | 77% | 73% | 31% |
As a final check on the Taxol content of the nanowires, we used UV-Vis spectroscopy. A three-week-old sample of 200 nm SM-TNs in 20% H3PO4 and 0.4% SDS was thawed from −150 °C, centrifuged and washed with distilled water. The precipitated nanowires were extracted into methanol and a UV-Vis absorption spectrum was collected. The shape of the absorption spectrum obtained from the extracted nanowires was indistinguishable from that of pure Taxol in the same solvent (Fig. 9 a, b).45,46 Knowing the total volume, the mass of nanowires dissolved, and the absorption coefficient of Taxol at maximum wavelength (lit.,47λmax (MeOH)/ nm = 227 (ε/dm−3 mol−1 cm−1 29 800)), we calculated a peak absorption value at 227 nm that was within 8% of the value calculated using gravimetric methods.
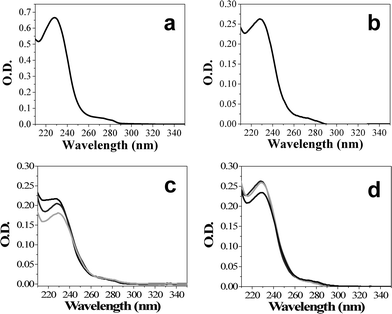 | ||
| Fig. 9 UV absorption spectra of Taxol in methanol. a) Standard Taxol. b) Taxol extracted from SM-TNs after isolation from phosphoric acid solution. c) 200 nm SM-TN dispersed in PBS solution, ultrasonicated then incubated at 37 °C. The absorption at λmax = 227 nm decreases from an initial value of 0.262 to 0.233 over the course of 16 h. d) 35 nm SM-TN dispersed in PBS solution and incubated at 37 °C. The absorption at λ = 227 nm decreases from an initial value of 0.217 to 0.176 over the course of 16 h. | ||
Having established the composition of the SM-TNs, the next step was to determine their compatibility with biological buffer systems, in preparation for observing their effects on live cells. A sample of SM-TN (200 nm diameter, 55 μm long) with a concentration of 1.08 mg/ml in 20% H3PO4/0.4% SDS was diluted 10 times with phosphate buffer saline (PBS) solution (pH = 7.4). The diluted solution was ultrasonicated for 15 min while maintaining room temperature conditions, resulting in a semi clear dispersion of SM-TN with much shorter lengths and an SDS concentration of 400 ppm (0.04%) which is below the accepted biological toxicity limit of 660 ppm.48,49 Note that the SM-TNs have both ends of the wire unmodified by the ODS layer, thus offering a way to expose raw Taxol to the cells. Ultrasonication of the nanowires snaps them into smaller segments thus increasing the exposed area. The length of the nanowires was reduced to submicron dimensions, without the formation of aggregates. After suspending the 200 nm or 35 nm diameter SM-TNs in PBS solution at 37 °C for 16 h, the solutions were extracted with methanol to measure the Taxol absorption spectrum. Both samples showed an average of 14% degradation of Taxol during this period (as seen in Fig. 9c, d). There was no measurable decomposition at room temperature (21 °C) during a 24-hour period.
With the stability of Taxol in nanowire form confirmed, we then tested the effect of the SM-TNs on U937 monocytic cells. The concentration of SDS was further reduced to 0.4 ppm (1.2 μM) after the SM-TNs were diluted in vitro with the U937 cells. The microscope images in Fig. 10 confirm that the SM-TNs and microcrystalline Taxol had similar effect on the cell morphology.50,51 Untreated cells are shown in Fig. 10a, with irregular edges and clearly defined nuclei. To rule out any cytotoxic effect of the ODS layer, U937 monocytes were treated with empty ODS shells extracted from 200 and 35 nm SF-AAO templates using 20% H3PO4 in 0.4% SDS. After 24 h of incubation, the ODS-treated cells in Fig. 10b looked identical to the untreated cells in Fig. 10a with a similar rate of natural cell death of less than 3%. When Taxol microcrystals are added to the cell culture (the standard way that Taxol is released into biological systems), decreased cell proliferation rate, pronounced swelling and vacuolization are observed, as shown in Fig. 10c. These effects are all consistent with Taxol exposure.52–54 When the SM-TNs are used instead, the same changes in cell morphology are observed. Both 200 nm and 35 nm diameter nanowires give rise to swelling and the appearance of vacuoles in the cell interior, as shown in Fig. 10d and 10e. In Fig. 10d, a 200 nm diameter nanowire is clearly resolved, confirming the presence of Taxol in nanowire form. One question that cannot be answered at this time is whether the Taxol is released from the nanowires and then diffuses through the buffer solution to individual cells, or whether actual physical contact of the cells with the nanowire is required to transfer the drug and induce a toxic effect. It is doubtful that SDS plays any role in solubilizing Taxol from SM-TNs since its final concentration in the U937 cell culture plate was on the order of 1.2 μM, roughly 6,500 times lower than the critical micelle concentration.55 In any case, the results in Fig. 10 show that the effects of Taxol exposure can be seen when the nanowire delivery mechanism is used. Preliminary results show that for the same concentration, SM-TNs were more effective than microcrystalline Taxol in killing cancer cells and slowing down their proliferation over a 24-hour incubation period. This effect was enhanced for smaller diameter SM-TNs. This subject is pending investigation and will be published in a separate paper.
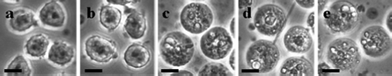 | ||
| Fig. 10 Microscope image (40×) magnification of U937 cells after exposure to SM-TNs. a) Untreated U937 cells. b) U937 cells treated with 200 nm, and 35 nm ODS empty shells. c) U937 cells treated with microcrystalline (free) Taxol prepared by sonicating Taxol in 20%H3PO4/0.8% SDS solution. d) U937 cells treated with 200 nm SM-TN. A SM-TN is seen in the middle of the picture. e) U937 cells treated with 35 nm SM-TN. The nanowires are too small to see at this magnification, but their effect on the cells is clearly visible. Scale bar = 10 μm. | ||
3. Experimental
3.1 Materials and Equipment
AAO templates were purchased from two separate sources. Templates with a nominal pore diameter of 200 nm (Anodisc-13) were purchased from Whatman Inc., while precision fabricated templates with a narrower pore size distribution (80, 55 and 35 and 18 nm) were acquired from Synkera Technologies. All the templates had the same diameter of 1.3 cm and approximate template thickness of 55μm. n-Octadecyltrichlorosilane (95%) was purchased from Acros Organics. Taxol (99%) was acquired from the Selleck Chemical Company and used without further purification. All organic solvents were distilled and stored over activated molecular sieves (3Å). IR measurements were performed using IR Affinity-1 FTIR from Shimadzu. SEM measurements were performed using JEOL JSM-6510LV scanning electron microscope. Samples were sputter coated with Pt prior to scanning. TEM measurements were done on a PHILIPS TECNAI 12. Gravimetric analysis was performed using Sartorius SE2 Ultra Micro Balance with 0.1 μg precision. UV-Ozone cleaning was done using PSD-UV-Benchtop UV-Ozone Cleaner from Novascan. UV-Vis measurements were done using Spectro UV-Vis Double beam (UVD-2950) spectrophotometer from Labomed. The human monocytic cell line (U937) was obtained from Health Protection Agency (HPA), UK.3.2 n-Octadecyltrichlorosilane surface functionalized AAO templates (SF-AAO)
An AAO template was ultrasonicated in acetone then hexane. The template was air dried before ultrasonicating with hot 30% H2O243 followed by washing with distilled water and drying in an oven set at 120 °C for 30 min. Organic contaminants on the AAO template surface were removed by placing it under a UV-Ozone cleaner for 45 min thus activating the surface of the template by exposing more OH bonds. The template was further oven dried at 120 °C before the hot template was quickly submerged in a solution of OTS in Benzene 5% v/v.56Ultrasonication for 30 min eliminated trapped air from inside the AAO pores and exposed them to OTS. The template/OTS solution was gently agitated at 78 °C for 24 h in a sealed vial before the solution was drained and the template ultrasonicated twice with 5 ml of distilled Benzene. After a briefly drying the template in the oven, a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of ethanol in 1 molar HCl was added to the surface modified template and ultrasonicated for 30 s before gently stirring for 30 min. The template was washed with distilled ethanol and oven dried at 120 °C. AAO template surface functionalization was confirmed gravimetrically and spectroscopically using an ultra micro balance and FTIR respectively.
1 mixture of ethanol in 1 molar HCl was added to the surface modified template and ultrasonicated for 30 s before gently stirring for 30 min. The template was washed with distilled ethanol and oven dried at 120 °C. AAO template surface functionalization was confirmed gravimetrically and spectroscopically using an ultra micro balance and FTIR respectively.
3.3 Fabrication of surface modified Taxol nanowires (SM-TN) using SF-AAO templates
We describe a general method for making 200, 80, 55, 35 and 18 nm diameter nanowires. Taxol (3 mg) was dissolved in 50 μl of dichloroethane (DCE). The SF-AAO template was placed on top of a Teflon holder with a slightly smaller diameter. The Taxol solution (25 μl) was deposited on the template and the solvent evaporated using a gentle stream of Argon gas. The template was then flipped and the remaining solution deposited on the surface. Gentle swirling of the solution over the template ensured an even spread. A small beaker containing a filter paper soaked with DCE was tipped over the template holder forming a DCE saturated atmosphere which redissolved the deposited Taxol. Slow evaporation of the solvent (solvent annealing) over a period of 30 min at 45 °C yielded a crust of excess Taxol over the template, which was scrapped off using a surgical blade after which the template surface was polished with 2000 grit sandpaper. The polished template was placed under vacuum (20 mbar) for 3 h to remove any residual annealing solvent. The SM-TNs were released by dissolving the AAO template in 0.75 mL of 20% H3PO4 /0.4% SDS aqueous solution at 21 °C. Complete dissolution of the template was achieved in less than 6 h with gentle agitation. We found that changing the annealing solvent to THF, when fabricating smaller diameter nanowires, deposited more nanowires per template.3.4 Imaging Taxol nanowires using Electron Microscopy
a) Scanning Electron Microscopy (SEM):The hydrophilic layer surrounding the SM-TN made it exceptionally hard to separate fully dispersed smaller diameter nanowires (< 80 nm) from the acid/SDS solution using a centrifuge in order to visualize them viaSEM without the presence of residual phosphoric acid or SDS. Thus 50 μl of a SM-TN suspension in 20% H3PO4/0.4% SDS (55, 35 and 18 nm) was diluted with 0.2 mL of water and deposited over an AAO template with 20 nm surface pore diameter. The template was placed over a glass frit attached to a suction filtration system. The liquid slowly drained through the AAO template before rinsing the residue with 0.2 mL of DI water. The AAO template/SM-TN residue was fixed on an SEM stub via conducting carbon tape and sputter coated with Pt (∼10 nm thickness).
For SM-TNs with 200 or 80 nm diameter, separating them from the acid/SDS solution and washing with deionized water was done using a centrifuge and multiple wash cycles with distilled water. These nanowires were spread over a sheet of freshly cleaved mica before SEM imaging.
b) Transmission Electron Microscopy (TEM):
A 50 μl suspension of a 200 nm diameter SM-TNs in distilled water was diluted with 1 ml of distilled water. The suspension was sonicated briefly before placing a drop of the suspension on a TEM grid. The sample was allowed to air dry leaving behind scattered SM-TNs.
3.5 UV-Vis measurement of Taxol concentration
A 75 μl sample of SM-TN dispersion in 20% H3PO4/0.4% SDS was added to 900 μl of PBS solution. The mixture was ultrasonicated for 15 min while maintaining room temperature conditions before incubating at 37 °C for a total of 16 h. A 200 μl sample in the PBS solution was added to 2 mL of methanol in order to precipitate the buffer salt and solubilize the Taxol. The solution was centrifuged at 5000 rpm to remove the precipitated buffer salts before measuring the absorption spectrum. To determine absolute concentrations from the absorption spectrum, the value of the absorption coefficient at the peak of the absorption was taken to be (ε/dm−3 mol−1 cm−1 29 800)47 at λmax (MeOH)/nm = 227. Three samples were measured at times = 0, 4, and 16 h.3.6 Cell culture
Human monocytic U937 cancer cells (5×105/well) were cultured in a 6-well culture plate with 2 mL of RPMI-1640 medium containing 10% fetal bovine serum (FBS), 100 mg/mL of L-glutamate, 100 mg/mL streptomycin and 100 U/l penicillin. A 200 nm or 35 nm diameter SM-TN suspension in PBS solution was each added in a separate well to give a 50 nM concentration of Taxol in the form of 4 × 104 SM-TNs with 200 nm diameter/55 μm long or 1.5 × 106nanowires with 35 nm diameter/49 μm long and a final SDS concentration around 1.2 μM. The cells were incubated at 37 °C for a period of 24 h in a humidified atmosphere containing 5% CO2 and 95% air. For comparison we incubated U937 cells with microcrystalline Taxol. A stock sample of microcrystalline Taxol was prepared by sonicating 1 mg of Taxol in 1 mL solution of 20% H3PO4/0.4% SDS. The microcrystalline suspension of Taxol was added to PBS solution and sonicated prior to adding to the cell medium. Another sample of microcrystalline Taxol was prepared by directly adding a stock solution of Taxol solution in DMSO to the cell medium. The final concentration of Taxol microcrystals in the U937 cell culture was 50 nM. The mixture was incubated under the same conditions for 24 h. Both microcrystalline samples gave similar results.4. Conclusion
We were able to fabricate SM-TNs with different diameters using surface modified AAO templates. The liberated nanowires were stable in water and aqueous buffered solutions and did not cluster to form micron sized particulates or decompose. Incubating SM-TNs with U937 cells showed a significant effect on the cell morphology consistent with Taxol exposure. The n-octadecylsiloxan sheath around the Taxol nanowires may offer a covalent handle on to which antibodies can be attached for specific targeting of cancer cells. It should be possible to extend this method to other water insoluble drugs or small molecules that are amenable to nanowire formation.Acknowledgements
R. O. Al-Kaysi acknowledges the support of KSAU-HS/KAIMRC through grants RC08/093 and RC10/104 and King Abdulaziz City for Science and Technology (KACST) through grant AT-435-30. C. J. Bardeen acknowledges the support of the National Science Foundation, grant DMR-0907310. The electron microscopy measurements were done at the Center for Advanced Materials and Microscopy (CFAMM) at UC Riverside.References
- R. O. Al-Kaysi, C. J. Bardeen, in Nanomaterials for the Life Sciences: Polymeric Nanomaterials, ed. C. S. S. R. Kumar, Wiley-VCH, Germany, 2011, vol. 10, p. p. 429 Search PubMed.
- R. O. Al-Kaysi, C. J. Bardeen, in Advances in Nanotechnology, ed. Z. Bartul, J. Trenor, NOVA, 2011, vol. 5 Search PubMed.
- R. O. Al-Kaysi, T. H. Ghaddar and G. Guirado, Journal of Nanomaterials, 2009, 2009 Search PubMed.
- R. O. Al-Kaysi, A. M. Muller and C. J. Bardeen, J. Am. Chem. Soc., 2006, 128, 15938 CrossRef CAS.
- R. O. Al-Kaysi and C. J. Bardeen, Adv. Mater., 2007, 19, 1276 CrossRef CAS.
- Y. S. Zhao, P. Zhan, J. Kim, C. Sun and J. Huang, ACS Nano, 2010, 4, 1630 CrossRef CAS.
- Y. S. Zhao, J. Wu and J. Huang, J. Am. Chem. Soc., 2009, 131, 3158 CrossRef CAS.
- S. Singh, J. Nanosci. Nanotechnol., 2010, 10, 7906 CrossRef CAS.
- M. M. d. Villiers,P. Aramwit,G. S. Kwon, ed., Nanotechnology in drug delivery, Springer, New York, 2009 Search PubMed.
- R. Aston, R. Saffie-Siebert, L. Canham and J. Ogden, Pharmaceutical Technology Europe, 2005, 17, 21 Search PubMed.
- G. A. Hughes, Nanomed.: Nanotechnol., Biol. Med., 2005, 1, 22 CrossRef CAS.
- Y. N. Usha and T. T. Angel, N. Udupa, Pharma Review, 2010, 8, 59 CAS.
- M. C. Wani, H. L. Taylor, M. E. Wall, P. Coggon and A. McPhail, J. Am. Chem. Soc., 1971, 93, 2325 CrossRef CAS.
- K. C. Nicolaou, Z. Yang, J. J. Liu, H. Ueno, P. G. Nantermet, R. K. Guy, C. F. Claiborne, J. Renaud, E. A. Couladouros, K. Paulvannan and E. J. Sorensen, Nature, 1994, 367, 630 CrossRef CAS.
- R. T. Liggins, W. L. Hunter and H. M. Burt, J. Pharm. Sci., 1997, 86, 1458 CrossRef CAS.
- A. E. Mathew, M. R. Mejillano, J. P. Nath, R. H. Himes and V. J. Stella, J. Med. Chem., 1992, 35, 145 CrossRef CAS.
- S. K. Dordunoo and H. M. Burt, Int. J. Pharm., 1996, 133, 191 CrossRef CAS.
- F. M. V. Bockxmeer, C. E. Martin, D. E. Thompson and I. J. Constable, Investigative Ophthalmology & Visual Science, 1985, 26, 1140 Search PubMed.
- C. M. Spencer and D. Faulds, Drugs, 1994, 48, 794 CrossRef CAS.
- M. A. Jordan and W. Leslie, Nat. Rev. Cancer, 2004, 4, 253 CrossRef CAS.
- A. Sparreboom, L. v. Zuylen, E. Brouwer, W. J. Loos, P. d. Bruijn, H. Gelderblom, M. Pillay, K. Nooter, G. Stoter and J. Verweij, Cancer Res., 1999, 59, 1454 CAS.
- D. Chen, D. Song, M. G. Wientjes and J. L.-S. Au, Clin. Cancer Res., 2003, 9, 363 CAS.
- J. Wang, D. Mongayt and V. P. Torchilin, J. Drug Targeting, 2005, 13, 73 CrossRef CAS.
- H. Gelderblom, J. Verweij, K. Nooter and A. Sparreboom, Eur. J. Cancer, 2001, 37, 1590 CrossRef CAS.
- J. M. Terwogt, B. Nuijen, W. W. Huinink and J. H. Beijnen, Cancer Treat. Rev., 1997, 23, 87 CrossRef CAS.
- S. C. Lee, C. Kim, I. C. Kwon, H. Chung and S. Y. Jeong, J. Controlled Release, 2003, 89, 437 CrossRef CAS.
- J. M. Koziara, P. R. Lockman, D. D. Allen and R. J. Mumper, J. Controlled Release: Official Journal of the Controlled Release Society, 2004, 99, 259 CrossRef CAS.
- S. Kenth, J. -P. Sylvestre, K. Fuhrmann, M. Meunier and J. -C. Leroux, J. Pharm. Sci., 2011, 100, 1022 CrossRef CAS.
- R. O. Al-Kaysi and C. J. Bardeen, Chem. Commun., 2006, 1224 Search PubMed.
- J.-H. Kim, I.-S. Kang, H.-K. Choi, S.-S. Hong and H.-S. Lee, Biotechnol. Lett., 2000, 22, 1753 CrossRef CAS.
- S. L. Richheimer, D. M. Tinnermeier and D. W. Timmons, Anal. Chem., 1992, 64, 2323 CrossRef CAS.
- L. Zhang, C. Pan, J. Zhu and C. Wang, Nanotechnology, 2005, 16, 2242 CrossRef CAS.
- V. Smuleac, D. A. Butterfield, S. K. Sikdar, R. S. Varma and D. Bhattacharyy, J. Membr. Sci., 2005, 251, 169 CrossRef CAS.
- A. Sah, H. L. Castricum, A. Bliek, D. H. A. Blank and J. E. t. Elshof, J. Membr. Sci., 2004, 243, 125 CrossRef CAS.
- S. Sugawara, M. Konno and S. Saito, J. Membr. Sci., 1989, 43, 313 CrossRef CAS.
- J. W. Klaus, A. W. Ott, J. M. Johnson and S. M. George, Appl. Phys. Lett., 1997, 70, 1092 CrossRef CAS.
- L. L. Hench and J. K. West, Chem. Rev., 1990, 90, 33 CrossRef CAS.
- W. A. Deer,R. A. Howie,W. S. Wise,J. Zussman, in Rock-forming minerals: Framework silicates: feldspars, Geological Society, London, 2001, vol. 4, p. p. 322 Search PubMed.
- K. Kojio, A. Takahara and T. Kajiyama, Colloids Surf., A, 2000, 169, 295 CrossRef CAS.
- A. Rasheed, C. K. A. Kumar and V. V. N. S. S. Sravanthi, Sci. Pharm., 2008, 76, 567 CrossRef CAS.
- Y. Shimizu, J. Ceram. Soc. Jap., 1987, 95, 1067 CrossRef CAS.
- L. V. Ng and A. V. McCormick, J. Phys. Chem., 1996, 100, 12517 CrossRef CAS.
- L. N. Mitchon and J. M. White, Langmuir, 2006, 22, 6549 CrossRef CAS.
- Q. Gao, J. M. Wei and S. H. Chen, Pharm. Res., 1995, 12, 337 CrossRef CAS.
- V. Gangadevi and J. Muthumary, Mycologia Balcanica, 2008, 5, 1 Search PubMed.
- B. Chakravarthi, P. Das, K. Surendranath, A. A. Karande and C. Jayabaskaran, J. Biosci., 2008, 33, 259 CrossRef CAS.
- In Merck index, 12 edn, 1996, p. p. 7117 Search PubMed.
- Inactive Ingredient Guide (Redacted), U.S. Food and Drug Administration, Center for Drug Evaluation and Research, 1996 Search PubMed.
- S. S. Kwon, Y. S. Nam, J. S. Lee, B. S. Ku, S. H. Han, J. Y. Lee and I. S. Chang, Colloids Surf., A, 2002, 210, 95 CrossRef CAS.
- A. Vantieghem, Y. Xu, Z. Assefa, J. Piette, J. R. Vandenheede, W. Merlevede, P. A. M. d. Witte and P. Agostinis, J. Biol. Chem., 2002, 277, 37718 CrossRef CAS.
- X.-P. Wang, T.-S. Chen, L. Sun, J.-Y. Cai, M.-Q. Wu and M. Mok, Micron, 2008, 39, 1216 CrossRef CAS.
- I. Androic, A. Krämer, R. Yan, F. Rödel, R. Gätje, M. Kaufmann and K. Strebhardt, J. Yuan, BMC Cancer, 2008, 8, 1 Search PubMed.
- M. A. Tichomirowa, M. Theodoropoulou, A. F. Daly, A. Yassouridis, S. Hansen, J. Lu, M. Lange, R. H. Goldbrunner, G. K. Stalla and U. Renner, Int. J. Cancer, 2008, 123, 1956 CrossRef CAS.
- L. Vicari, T. Musumeci, I. Giannone, L. Adamo, C. Conticello, R. De Maria, R. Pignatello, G. Puglisi and M. Gulisano, BMC Cancer, 2008, 8, 212 CrossRef.
- A. P. Romani, A. E. d. H. Machado, N. Hioka, D. Severino, M. S. Baptista, L. Codognoto, M. R. Rodrigues and H. P. M. d. Oliveira, J. Fluoresc., 2008, 19, 327 CrossRef.
- Y. Y. Chen, B. Y. Yu, J. H. Wang, R. E. Cochran and J. J. Shyue, Inorg. Chem., 2009, 48, 681 CrossRef CAS.
Footnotes |
| † The AAO template can be dissolved in 5% H3PO4 at the expense of a longer dissolution time. |
| ‡ Taxol powder was stirred in 20% H3PO4/0.4% SDS for 6 h at room temperature till saturation. The suspension was centrifuged and the concentration of soluble Taxol was calculated from the OD at λmax(227 nm) of the UV absorption spectrum. |
| This journal is © The Royal Society of Chemistry 2011 |
