Thermochromic behavior of chloro-nickel(II) in deep eutectic solvents and their application in thermochromic composite films
Chang-Dong
Gu
* and
Jiang-Ping
Tu
State Key Laboratory of Silicon Materials and Department of Materials Science and Engineering, Zhejiang University, Hangzhou, 310027, China. E-mail: changdong_gu@hotmail.com; cdgu@zju.edu.cn; Fax: +86 571 7952573; Tel: +86 571 87952573
First published on 16th September 2011
Abstract
Novel non-aqueous thermochromic solutions were facilely prepared by dissolving transition metal chlorides, such as NiCl2·6H2O, CrCl3·6H2O, FeCl3, and CoCl2·6H2O, in an inexpensive, widely available ionic liquid analogous deep eutectic solvent (DES). It was found that only the NiCl2·6H2O dissolved choline chloride-based DES exhibited a stable and significant thermochromic behavior over a wide working temperature ranging from room temperature to about 150 °C. Typically, the colour of the 0.1 M NiCl2·6H2O dissolved DES solution changed in this order from pale green (room temperature), spring green (∼70 °C), to blue (∼120 °C). An effective strategy was proposed to produce more thermochromic solutions by mixing Ni(II) with other metal ions in the choline chloride-based DES. The thermochromic PVDF composite film was demonstrated by incorporating DES-based Ni(II) solution into the microporous PVDF film. The preliminary results are expected to stimulate interest of the development of high performance thermochromic materials for the facile fabrications from DESs.
1. Introduction
Thermochromism is defined as the phenomenon of reversible colour changes of a substance or system in response to heating and cooling, which is seen as one of the most beautiful and exciting aspects of chemistry.1,2 If responsive to solar heat, thermochromic materials can contribute to improving the energy efficiency of the built environment and hence to the reduction of energy consumption and CO2 emission.3 Considering the more conveniently accessible solar heat is usually low temperature (<100 °C) in nature, it would be very much desirable to develop new thermochromic systems that can utilize the readily available low temperature solar heat.2 Many transition metal complexes, e.g. nickel halides and copper halides, in certain solvents can undergo the tetrahedral-octahedral configuration changes assisted by the complex interacting with the solvent molecules (i.e.solvolysis) during repeated heating-cooling cycles, leading to significant changes in the d-d transitions and to a strong change in the colour of the complexes in solution.1,2,4–6 However, irreversible (aqueous or organic) solvent evaporation upon heating would occur, which is a detriment to the cycle life of the thermochromic systems or devices.2 Therefore, room temperature ionic liquids (ILs) may be the ideal candidate solvents for the stabilisation of metal complex based thermochromism due to their intrinsic negligible vapour pressure and high thermal stability.2,3,7,8Recently, Chen's group proposed a novel thermochromic system by dissolving [bmim]2NiCl4 (bmim: 1-butyl-3-methylimidazolium) in CnOHmim+ (CnOHmim: 1-hydroxyalkyl-3-methylimidazolium, n = 2 or 3) based ILs. This work shows that the IL-based thermochromic system are more sensitive and energy effective to temperature changes, much wider in working temperatures and more stable in repeated heating-cooling cycle operations in comparison with previously reported systems based on conventional solvents.2 Furthermore, for the first time, the same group had demonstrated the successful combination of such thermochromic ILs and poly(vinylidene fluoride) (PVDF) into stable solar-thermochromic composite films.3 However, the synthetic route of the thermochromic CnOHmim+ based ILs seems to be complicated, which involves the indispensable operation in a dry glove-box and a necessary procedure of the introduction of donor groups, e.g.alcohol, to the cation of the IL for the thermochromism.2 The complicated procedure may bring a negative impact on the popularization and application of the IL based thermochromic systems. Based on this thought, this work demonstrated a facile synthetic route of IL-based thermochromic system. An inexpensive, widely available IL analogous deep eutectic solvent (DES) is chosen as the base solvent to enable the thermochromism. Moreover, a preliminary application of the novel DES-based solution was also demonstrated to produce the thermochromic PVDF composite films by using a facile thermal drying procedure as shown in ref. 3.
Unlike the conventional ILs, DESs invented by Abbott and co-workers9,10 can be produced using quaternary ammonium salts R1R2R3R4N+X− complexed with hydrogen bond donors such as acids, amides and alcohols, where X− is generally a halide anion (usually Cl−). Most studies have concentrated on choline chloride (ChCl) as the quaternary ammonium salt because it is non-toxic, biodegradable, and easily accessible. Hence it can be applied economically to large-scale processes. DESs formed with ChCl and either urea or ethylene glycol (EG) have successfully been employed for metal deposition.11–15 Owing to the high thermal stability, low vapor pressure, especially the natural hydroxyl donor group and abundant halide anion in DESs, DESs are definitely promising candidate solvents in the solvolysis of the metal complex and hence enable thermochromism.
2. Experimental
Chemicals and materials
Chemicals used in this study, such as NiCl2·6H2O, CrCl3·6H2O, CoCl2·6H2O, FeCl3, and N,N-dimethylformamide (DMF), are all analytical grade (A.R.) and purchased from Sinopharm Chemical Reagent Co., Ltd. PVDF powder (KYNAR(R) HSV 900 PWD) was purchased from Arkema Inc. ChCl (A.R., Aladdin), EG (A.R., Sinopharm) and Urea (A.R., Aladdin) were used as received. The DESs were prepared according to ref. 12,16; it was formed by stirring the mixture of the two components in a mol ratio of 1 ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 EG or 1 ChCl
2 EG or 1 ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 Urea, at 80 °C until a homogeneous colourless liquid was formed. For convenience, 1 ChCl
2 Urea, at 80 °C until a homogeneous colourless liquid was formed. For convenience, 1 ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 EG and 1 ChCl
2 EG and 1 ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 Urea will hereafter be denoted as CE and CU, respectively.
2 Urea will hereafter be denoted as CE and CU, respectively.
Fabrication of composite film
1 g PVDF powder was added to DMF (12 ml) under 60 °C and supersonic stirring until complete dissolution. 1 ml 0.5 M NiCl2·6H2O in CE was then added dropwise into the PVDF/DMF solution. After complete mixing, the obtained clear blue solution was heated at about 70 °C and then dropped onto a glass slide. The glass slide was vertically placed in a vacuum oven for drying at 90 °C. After drying, the polymer film could be readily removed from the glass slide and attached to other substrate surfaces. The film thickness was measured in the range from 0.010 to 0.015 mm.Equipment
Visible spectra were recorded using a Varian Cary 5000 Spectrophotometer (USA). The DES and the air were the references, respectively. The morphology of the gold-coated pure PVDF and composite films were characterized by a field emission scanning electron microscopy (FE-SEM, Hitachi S-4800). Chemical compositions were analyzed with an X-ray energy dispersive spectroscope (EDS, Bruker AXS) attached to the SEM. Crystalline structure of the films were studied by X-ray diffractometer (XRD, Rigaku D/max-2550, Japan) with a Cu target (λ = 1.54056 Å) and a monochronmator at 40 kV and 250 mA with the scanning rate and step being 4°/min and 0.02°, respectively.3. Results and discussion
Interestingly, when the 0.05 M NiCl2·6H2O was dissolved in CE to form the Ni(II)-CE solution, a reversible thermochromic behavior (pale green at room temperature; spring green at ∼70 °C) occurred in this solution and was confirmed by visual observation and spectral measurements, respectively (see Fig. 1a). However, the NiCl2·6H2O dissolved CU solution (Fig. 1c) did not exhibit a pronounced thermochromic behavior in the temperature range of 27–72 °C, which implied that a synergistic effect from ChCl and EG might be responsible for the thermochromism of the Ni(II)-CE solution. The absence of the thermochromic behavior of the Ni(II)-CU solution also indicated that the influence of EG on the absorptions might be resulted from the solvolysis of the hydroxyl group coordinating with Ni(II).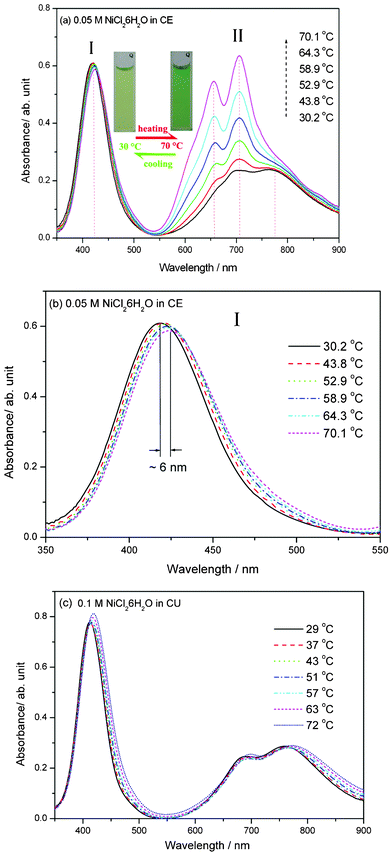 | ||
| Fig. 1 Visible absorption spectral changes of the Ni(II) solutions upon increasing the temperature from room temperature to about 70 °C. (a) 0.05 M NiCl2·6H2O dissolved CE solution; (b) the enlarged view of (a) at the wavelength range of 350–550 nm; (c) 0.1 M NiCl2·6H2O dissolved CU solution. | ||
Fig. 1a shows the absorption spectra of the Ni(II)-CE solution at various temperatures. The spectra exhibit two absorption bands, I and II. Band I is represented by an obvious absorption peak at around 420 nm, which was expected from the 3A2g(F)→3T1g(P) transition of the octahedral complex [Ni(H2O)6]2+.2,5 With increasing the temperature from 30 °C to 73 °C, the absorbance of Band I only gradually decreases about 0.1 and the peak position shifts to the higher wavelength by about 6 nm, as shown in Fig. 1(b). However, Band II ranging from 600 to 800 nm is significantly temperature dependent as shown in Fig. 1a. At the lower temperature range of 30–47 °C, two peaks centered at 705 and 773 nm and a shoulder at 654 nm can be distinguished. However, further increasing the temperature to 73 °C, the center of Band II shifts to the lower wavelength range and two significant peaks at around 654 nm and 705 nm appeared. Moreover, a shoulder at 610 nm can also be detected. It should be noted that in the visible spectra measurements, the temperature is only increased to about 73 °C because of the limitation of our equipment. However, the thermochromic behavior of the Ni(II)-CE solution is not only restricted to this temperature range. As shown in Fig. 1a, the increase trend of the intensities of peaks in Band II becomes more obviously and do not intend to be ceased at higher temperature ranges. These adsorptions shown in Band II should be attributed to the blue-coloured NiCl42− complex in the regular tetrahedral symmetry with the transition of 3T1(F)→3T1(P).2,5 However, the appeared colour of the Ni(II)-CE solution at about 73 °C (spring green) indicates that the transition of 3T1(F)→3T1(P) should not be completed. As shown in Fig. 2, when the Ni(II)-CE solutions (labeled by C and F) were heated to about 120–150 °C in the oven, the colour of blue of the Ni(II)-CE solution was visually observed, which agrees with the blue-coloured NiCl42− complex in the regular tetrahedral symmetry as discussed above.2,5 Further spectral measurements on the Ni(II)-CE solutions at the temperatures of above 100 °C should be done to verify the assumption.
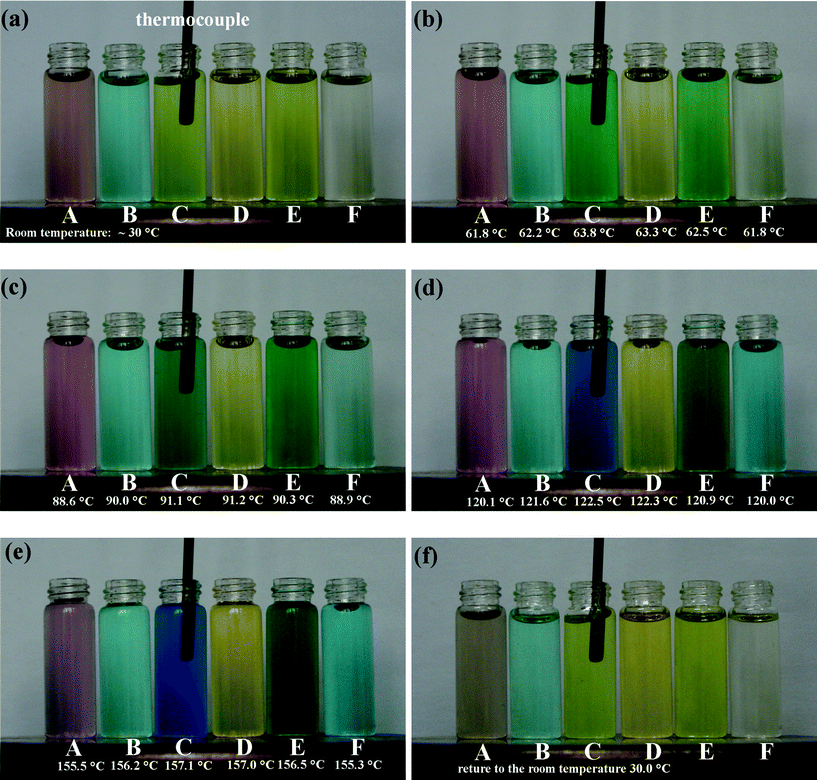 | ||
| Fig. 2 Photographs of CE-based solutions at various temperaures. Solutions labeled by A, B, C, D, E, and F are corresponding to 0.005 M CrCl2·6H2O (A), 0.00125 CoCl2·6H2O (B), 0.1 M NiCl2·6H2O (C), 0.005 M FeCl3 (D), the mixture solution of 0.00125 M FeCl3 and 0.025 NiCl2·6H2O (E), and 0.01 M NiCl2·6H2O (F) in CE-based DES, respectively. The solutions were heated by a magnetic stirrer. The temperature of each solution shown in the pictures was confirmed by a thermocouple. | ||
It should be noticed that the Ni(II)-CE solution exhibited a different thermochromic behavior from the previous reported [bmim]2NiCl4 dissolved CnOHmim+ based ILs.2 The [bmim]2NiCl4 dissolved CnOHmim+ based ILs can fulfil the colour change process from yellow or green (cold) to blue (hot) in a relatively narrow temperature range, 30–85 °C. However, the present Ni(II)-CE solution has a relatively wide temperature range to finish the colour change from yellow or green at room temperature to blue at about 120–150 °C. It implies that the tetrahedral–octahedral configuration conversion of the Ni(II)-CE solution might require more energy than the Ni(II)-CnOHmim+ based ILs. Being different from the absorption spectrum of the [bmim]2NiCl4 dissolved CnOHmim+ based ILs,2 the absorption spectrum of the Ni(II)-CE solution exhibits a pronounced peak in Band I with a comparable intensity to the peaks in Band II at the temperature range of 30–70 °C (see Fig. 1a). This feature of the absorption spectrum should be related to the variation of the tetrahedral and octahedral complexes in the solutions.
Zheng's group studied the solvatochromic changes of the Ni(II) complex by dissolving Ni(ClO4)2 in mixed [bmim]Cl and [C3OHmim]ClO4.2 They found that upon increasing the Cl/Ni molar ratio from 0 to >20, the gradual formation of different octahedral complex species, from [Ni(Sol)6]2+, [NiCl(Sol)5]+, [NiCl2(Sol)4], [NiCl3(Sol)3]− to [NiCl4(Sol)2]2− (Sol stands for solvent or the donor or ligand species in the solution), and finally to the tetrahedral NiCl42− species.2 These findings in the ILs differ from those in conventional solvents in which the high chloride and hence high energy octahedral configurations such as [NiCl3(Sol)3]− and [NiCl4(Sol)2]2− are usually absent.2 As for the CE-based solutions, there are abundant Cl− ions in the solution due to the component of ChCl, which means low Cl/Ni ratio could be obtained as long as high concentrated Ni(II) salt is dissolved. Therefore, it is hard to investigate the dependence of the concentration of Cl− on the Ni(II) complex in CE-based solutions. However, the absorption spectra of NiCl2·6H2O in CE were recorded at different concentrations and are presented in Fig. 3a. The different concentrations of NiCl2·6H2O (CNiCl2·6H2O) are corresponding to the different Cl/Ni ratios, as shown in Table 1. Fig. 3b indicates that the absorption intensity increased linearly with the concentration of NiCl2·6H2O and peaks belonging to Band I and II were all observed in this system. The peak position remained constant when the concentration was changed. However, it is found that the intensity ratio of the peaks centered at 705 nm and 417 nm, I705 nm/I417 nm was 2-fold increased with the increasing the Cl/Ni molar ratio from 12 to 1002, which might be attributed to the Cl− dependent formation of the octahedral complexes. Further experimental evidence should be done to explore the formulas of the octahedral complexes.
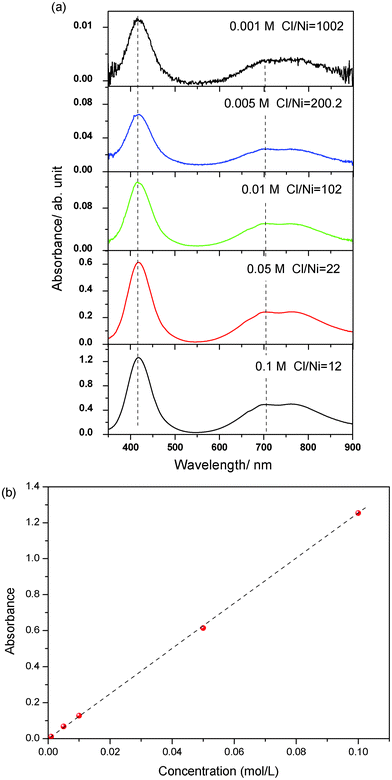 | ||
| Fig. 3 (a) Visible absorption spectra of NiCl2·6H2O in CE at different concentrations at room temperature (∼30 °C). (b) The intensity of the absorption peak at 705 nm as a function of the concentration of NiCl2·6H2O. | ||
| C NiCl2·6H2O/mol L−1 | Cl/Ni molar ratio | I 705 nm/I417 nm |
|---|---|---|
| 0.001 | 1002 | 2.89 |
| 0.005 | 200 | 2.50 |
| 0.01 | 102 | 2.49 |
| 0.05 | 22 | 2.50 |
| 0.1 | 12 | 1.45 |
One recent work done by Abbott et al. may shed light on the octahedral Ni(II) complexes in CE, in which the fast atom bombardment mass spectrometry (FABMS) was used to determine the speciations of the CE and CU containing NiCl2·6H2O.12 It was found that only ionic Ni containing species is NiCl3− and no cationic Ni species were observed showing that the naked or hydrated ions are not stable in solution.12 Interestingly, FABMS also shows the cluster ChCl2− but no (Cl·EG)− in the CE-based solution whereas in the CU-based solution, the ChCl2−cluster is small but the (Cl·urea)− is the largest signal.12 Therefore, the fundamental difference between the chloride speciation for CE- and CU-based solutions might be responsible for the distinguished thermochromic behavior of Ni complexes in the two DES-based solutions. In this case, it is possible that the octahedral Ni(II) complex in CE-based solution might have the formula of [NiCl3(EG)3]−. Therefore, the coordination reaction for the thermochromism of the Ni(II)-CE solution is supposed to be as below:
Anyway, further experimental evidence should be done to explore the complexes and thermo-solvatochromism of the novel solution system.
We also prepared the other three CE-based solutions by dissolving iron chloride (FeCl3), cobalt chloride (CoCl2·6H2O), and chromium chloride (CrCl3·6H2O) in CE respectively to see whether the similar thermochromism exist in these chlorides as shown above. Visual observations and spectral measurements indicate that these chlorides do not exhibit obvious thermochromic property in the temperature range of 30–70 °C, which is shown in Fig. 2 and 4, respectively. However, it should be noted that when heated in an oven in air to about 150 °C, the colour of 0.005 M FeCl3 dissolved CE solution was changed from yellow to dark orange confirmed by visual observation (see the solution D in Fig. 2), which implies that the FeCl3 dissolved CE solution may need more heat energy to fulfil the thermochromism compared with the Ni(II)-CE solution. Moreover, the colour-change of FeCl3 dissolved CE solution is reversible upon the repeated heating-cooling cycles as well as the NiCl2·6H2O dissolved CE solution. Strangely, the colour of 0.005 M CrCl3·6H2O dissolved CE solution changed from light pink at room temperature to deep pink at about 150 °C. However, when the CrCl3·6H2O dissolved CE solution was cooled to room temperature, its original colour was not recovered and changed to light grey, as shown in Fig. 2. No visible thermochromism is found in the CoCl2·6H2O dissolved CE solution in this case (see the solution B in Fig. 2).
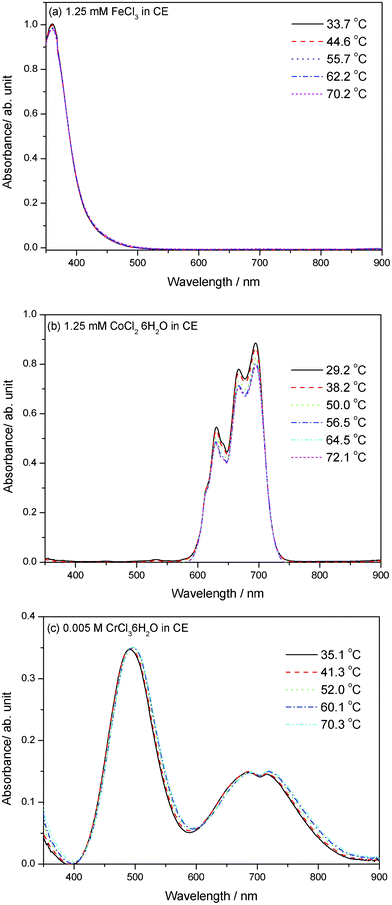 | ||
| Fig. 4 Visible absorption spectral changes of (a) 0.00125 M FeCl3, (b) 0.00125 M CoCl2·6H2O, and (c) 0.005 M CrCl3·6H2O dissolved CE solutions upon increasing the temperature from room temperature to about 70 °C. | ||
In the temperature range of 30–70 °C, the vis-spectra of 0.00125 M FeCl3 in CE at different temperatures are nearly repeated as shown in Fig. 4a. The same phenomenon is also appeared for 0.00125 M CoCl2·6H2O in CE (Fig. 4b) and 0.005 M CrCl3·6H2O (Fig. 4c). The 0.00125 M FeCl3 in CE has a pale yellow colour with an obvious adsorption peak band centered at about 360 nm and no significant adsorption is found at a wide wavelength range from 500 nm to 900 nm. The 0.00125 M CoCl2·6H2O in CE has a pale blue colour as shown in Fig. 2, exhibiting a broad adsorption peak band from about 570 nm to 750 nm (see Fig. 4b). Upon increasing the temperature from 30 to 70 °C, the intensity of peaks in the band of 570–750 nm decreased a little accordingly. Therefore, an idea is proposed that many more colours can be produced by mixing two or more kinds of chlorides with the Ni(II)-CE solution. As a representative case of study, we prepared a mixture with 1.25 mM FeCl3 and 0.025 M NiCl2·6H2O dissolved in CE, which has a colour of pale yellow at room temperature. When increasing the temperature of the mixture solution from 30 °C to 73 °C, a colour change from pale yellow to yellow green occurs confirmed by visual observation (see the inset of Fig. 5 and the solution E in Fig. 2) and spectral measurement as shown in Fig. 5. In Fig. 5, the peak centered at 386 nm and the peak band of 550–800 nm should be assigned to Fe(III) and Ni(II) complexes, respectively. Similar to the system of Ni(II)-CE, the thermochromic behavior of the mixture is reversible. Therefore, it is an effective method to produce more colorful solutions with thermochromic behavior by mixing Ni(II) with other metal ions in CE.
 | ||
| Fig. 5 Visible absorption spectral changes of the mixture solution of 0.00125 M FeCl3 and 0.025 NiCl2·6H2O dissolved in CE. The upper-left inlet shows the photographs of the corresponding solutions at room temperature and at about 73 °C and the upper-right inset is the enlarged view of the spectrum at the wavelength of 550–800 nm. | ||
So far, the Ni(II)-CE solution with the thermochromic behavior at a lower temperature range was facilely produced. It would be much more promising to produce the solar-thermochromism polymer composites by using this novel solution. The thermochromic PVDF composite film was obtained by simply mixing the Ni(II)-CE solution with PVDF powders. The composite film is named as the Ni(II)-CE@PVDF film hereafter. The pure PVDF film is transparent and with a very smooth surface observed by SEM (see Fig. 6a). However, the Ni(II)-CE@PVDF film becomes translucent with a white colour. Under the SEM observations, the Ni(II)-CE@PVDF film exhibits a microporous structure with a ligament width of about 1–3 μm and pore size in the range of 10–20 μm, as shown in Fig. 6b. The magnified SEM image as the inset of Fig. 6b indicates that the Ni(II)-CE nanodroplets were uniformly dispersed on the ligaments and channels of the porous structure, which is also confirmed by the EDS mapping analysis. Fig. 6c–f presents the element mappings of C, F, Ni, and Cl, respectively. As expected, the Ni and Cl signals are very strong all over the film surface, especially in the channel regions. The C and F signals are overlapped and mainly from the ligament regions. This result confirms again that the Ni(II)-CE droplets incorporated PVDF composite film was successfully fabricated. Wei et al. used XRD analysis to reveal the presence of a pseudocrystalline nanodroplets of the CnOHmim+ based IL-Ni(II) complexes in the PVDF composite films.3Fig. 6g gives the XRD patterns of the Ni(II)-CE@PVDF film. For comparison, the XRD patterns of pure PVDF film are also shown as Fig. 6g. Two broad peaks of diffractions are all observed in the two films, peaking at around 2θ = 18.1° and 20.0°, respectively, which can be assigned to the (020) and (021) planes of the α phase of PVDF.17 However, the very noticeable difference between these XRD patterns is the broad peak centered at 2.9° appears in the patterns of the Ni(II)-CE@PVDF film, but is absent in those of the pure PVDF film. Therefore, this peak should be assigned to the CE-based Ni(II) complexes with a pseudocrystalline structure. The thermochromic behavior of the Ni(II)-CE@PVDF film is inspected visually, and recorded using a digital camera and a visible spectrometer. Fig. 7a gives the temperature dependent visible spectra of the Ni(II)-CE@PVDF film. The absorption features of the Ni(II)-CE@PVDF film are in agreement with those of the CE-based NiCl2·6H2O solution system, as shown in Fig. 1a. Fig. 7b shows the two typical colour of the Ni(II)-CE@PVDF film at room temperature and at ∼70 °C. The Ni(II)-CE@PVDF film changed its colour from white at low temperature to blue when it was heated to ∼70 °C. This color-temperature relation was fully reversible for the composite film. It is also found that the reversible thermochromic properties of the Ni(II)-CE solution and the Ni(II)-CE@PVDF film are much stable in the open air.
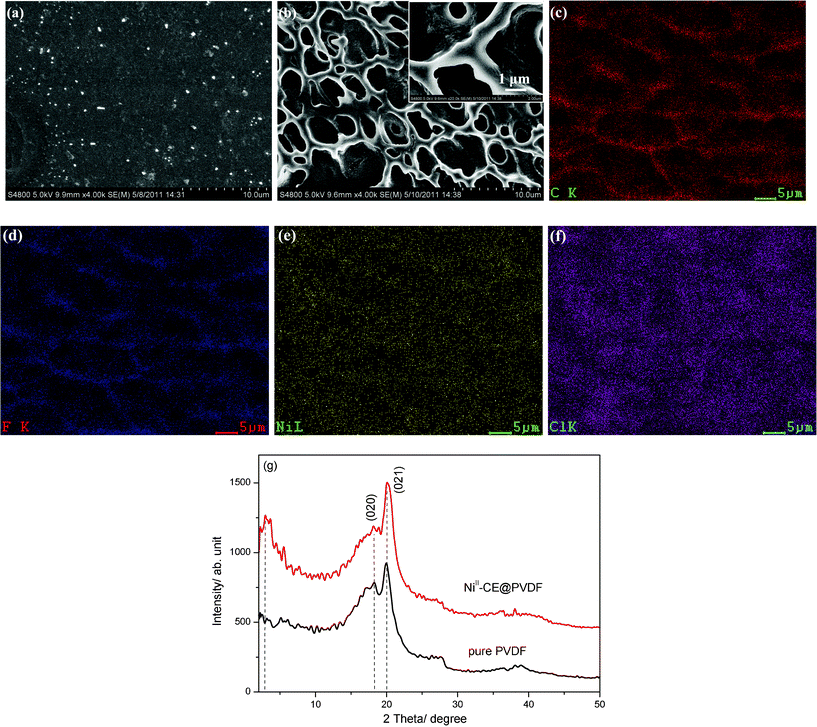 | ||
| Fig. 6 SEM images (a–b) and XRD patterns (g) of the pure PVDF film and the Ni(II)-CE@PVDF film, respectively. Inset of (b) is the corresponding magnified SEM picture. DES analysis of the Ni(II)-CE@PVDF film is shown in (c)–(f). Photos of (c), (d), (e), and (f) are the element mappings of C, F, Ni, and Cl, respectively. The XRD peaks at around 2θ = 18.1° and 20.0° are assigned to the (020) and (021) planes of the α phase of PVDF film. | ||
 | ||
| Fig. 7 (a) Temperature dependent visible spectra of the Ni(II)-CE@PVDF film. (b) Photographs of the Ni(II)-CE@PVDF film at room temperature and at ~70 °C. | ||
4. Conclusion
In summary, novel non-aqueous thermochromic systems have been facilely prepared in this work by dissolving transition metal chlorides in CE based DESs, typically such as NiCl2·6H2O, FeCl3, CrCl3·6H2O, and CoCl2·6H2O. The NiCl2·6H2O dissolved CE solution exhibited a stable and significant thermochromic behavior over a wide working temperature ranging from the room temperature to about 150 °C. The colour of the 0.1 M NiCl2·6H2O dissolved CE solution changed in the order from pale green (room temperature), spring green (∼70 °C), to blue (∼120 °C). A possible coordination reaction for the thermochromism of the Ni(II)-CE solution was proposed based on the published FABMS results. However, CrCl3·6H2O, FeCl3, and CoCl2·6H2O dissolved CE solutions did not show an obvious thermochromic property at the temperature range of 30–70 °C. But the CrCl3·6H2O and FeCl3 dissolved CE solutions deepened their colours when heated in air to a relatively high temperature above 100 °C revealed by visual observations. The thermochromic behavior of FeCl3–CE solution is reversible while the CrCl3·6H2O–CE is not. A strategy was proposed to produce more thermochromic solutions by mixing Ni(II) with other metal ions in CE. The thermochromic PVDF composite film was fabricated by incorporating CE-based Ni(II) solution into the microporous PVDF film. The preliminary results communicated here are expected to stimulate interest of the development of high performance thermochromic materials for the facile fabrications from DESs. Anyway, the complexes and thermo-solvatochromism of the novel thermochromic solutions are open questions and deserved to be investigated in details.Acknowledgements
We thank Prof. Mao Peng and Mr. Honglei Guo from Department of Polymer Science and Engineering, Zhejing University for kind help with the spectral measurements. This work was supported by the National Natural Science Foundation of China (51001089), the Specialized Research Fund for the Doctoral Program of Higher Education of China (20100101120026), the Research Foundation of Education Bureau of Zhejiang Province (Y200906938), and the Fundamental Research Funds for the Central Universities (2009QNA4006).References
- W. Linert, Y. Fukuda and A. Camard, Coord. Chem. Rev., 2001, 218, 113 CrossRef CAS.
- X. Wei, L. Yu, D. Wang, X. Jin and G. Z. Chen, Green Chem., 2008, 10, 296 RSC.
- X. J. Wei, L. P. Yu, X. B. Jin, D. H. Wang and G. Z. Chen, Adv. Mater., 2009, 21, 776 CrossRef CAS.
- U. El-Ayaan, F. Murata and Y. Fukuda, Monatshe. Chem., 2001, 132, 1279 CrossRef CAS.
- T. R. Griffiths and R. K. Scarrow, Trans. Faraday Soc., 1969, 65, 1727 RSC.
- D. E. Scaife and K. P. Wood, Inorg. Chem., 1967, 6, 358 CrossRef CAS.
- T. Torimoto, T. Tsuda, K. Okazaki and S. Kuwabata, Adv. Mater., 2010, 22, 1196 CrossRef CAS.
- C. M. Tollan, R. Marcilla, J. A. Pomposo, J. Rodriguez, J. Aizpurua, J. Molina and D. Mecerreyes, ACS Appl. Mater. Interfaces, 2009, 1, 348 CAS.
- A. P. Abbott, D. Boothby, G. Capper, D. L. Davies and R. K. Rasheed, J. Am. Chem. Soc., 2004, 126, 9142 CrossRef CAS.
- A. P. Abbott, J. Griffith, S. Nandhra, C. O'Connor, S. Postlethwaite, K. S. Ryder and E. L. Smith, Surf. Coat. Technol., 2008, 202, 2033 CrossRef CAS.
- A. P. Abbott, K. El Ttaib, G. Frisch, K. J. McKenzie and K. S. Ryder, Phys. Chem. Chem. Phys., 2009, 11, 4269 RSC.
- A. P. Abbott, K. El Ttaib, K. S. Ryder and E. L. Smith, Trans. Inst. Met. Finish., 2008, 86, 234 CrossRef CAS.
- A. P. Abbott and K. J. McKenzie, Phys. Chem. Chem. Phys., 2006, 8, 4265 RSC.
- C. D. Gu, Y. H. You, Y. L. Yu, S. X. Qu and J. P. Tu, Surf. Coat. Technol., 2011, 205, 5536 Search PubMed.
- C. D. Gu, X. J. Xu and J. P. Tu, J. Phys. Chem. C, 2010, 114, 13614 CAS.
- A. P. Abbott, G. Capper, D. L. Davies, R. K. Rasheed and P. Shikotra, Inorg. Chem., 2005, 44, 6497 CrossRef CAS.
- R. Gregorio and E. M. Ueno, J. Mater. Sci., 1999, 34, 4489 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2011 |

