Detection of single-stranded nucleic acids by hybridization of probe oligonucleotides on polystyrene nanospheres and subsequent release and recovery of fluorescence
Lei
Wang†
a,
Hailong
Li†
ab,
Yonglan
Luo
a,
Yingwei
Zhang
a,
Jingqi
Tian
ab and
Xuping
Sun
*a
aState Key Lab of Electroanalytical Chemistry, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, Changchun, 130022, Jilin, China. E-mail: sunxp@ciac.jl.cn; Fax: (+86) 431-85262065; Tel: (+86) 431-85262065
bGraduate School of the Chinese Academy of Sciences, Beijing, 100039, China
First published on 29th September 2011
Abstract
In this contribution, we demonstrate that polystyrene (PS) nanospheres can serve as an effective sensing platform for fluorescence-enhanced DNA detection. This kind of assay can be completed by the following two steps: (1) PS quenches the fluorescence of dye-labeled single-stranded DNA (ssDNA) probes very effectively when they are brought into close proximity as a result of the adsorption of ssDNA on PS. The adsorption is ascribed to the strong π–π stacking between unpaired DNA bases and PS. (2) Upon presence of target ssDNA, the specific hybridization of the probe with its target produces a double-stranded DNA (dsDNA). The duplex detaches from PS due to its rigid conformation, the absence of unpaired DNA bases, and electrostatic repulsion between negatively charged dsDNA backbone and PS, leading to recovery of dye fluorescence. This assay system exhibits high selectivity and sensitivity with a detection limit as low as 5 nM. It suggests that this sensing platform can differentiate between perfectly complementary and mismatched targets. The fluorescence enhancement in response to single-base mismatched target T2, two-base mismatched target T3, and three-base mismatched target T4 is about 71%, 63%, and 58% of that from complementary target T1, respectively. The suggested method can also discriminate complementary and single-base mismatched sequences embedded in rather larger strands with short oligonucleotide probes. The fluorescence enhancement in response to single-base mismatched sequence embedded in large strand is 83% of that from the one embedded with complementary sequence. In further experiments, it is demonstrated that the present system can be used for multiple DNA detection. Finally, efforts are made toward its application in human blood serum system to evaluate the ability to withstand the interference arising from real sample.
Introduction
Rapid, cost-effective, sensitive, and specific methods for the detection of DNA possesses important applications in the fields, such as gene expression profiling, clinical disease diagnostics, clinical treatment, and so on.1 The increasing availability of nanostructures has created widespread interest in their use in biotechnological systems for diagnostic applications,2 and the employment of various nanostructures for this purpose has been well documented.3 Recently, nanostructures have also proven of particular utility as “nanoquenchers” in fluorescense-enhanced nucleic acid detection.4–8 Because the same nanostructure is capable of quenching dyes with different emission wavelengths, the selection issue of a fluorophore-quencher pair is eliminated from the nanostructure-based system.4 Dubertret et al. have pioneered the use of dye fluorescence quenching ability of small gold nanoparticles (AuNPs) for DNA detection.5 In their study, a DNA moiety is decorated to a 1.4-nm AuNP surface and the DNA strand is curved to a hairpin structure by Watson–Crick hydrogen bonding. This hairpin conformation brings fluorescent dye into close proximity of the nanoparticle, leading to quenching of dye fluorescence. The subsequent specific hybridization of the moiety with target opens the hairpin and thus separates the fluorophore from the AuNP at a sufficient distance to allow fluorescence recovery. Maxwell et al. have also developed a similar AuNP-based nanobiosensor to detect nucleic acid.6 Although both of them are able to differentiate single-base mismatch in nucleic acid, they require tedious and laborious surface attachment chemistry for probe immobilization and suffer from slow response. To solve these problems, Liet al. have designed a novel fluorescent assay for DNA hybridization,7 which is based on that single-stranded DNA (ssDNA) adsorbs on negatively charged AuNP while double-stranded DNA (dsDNA) does not. As a result, dye-labeled probes have their fluorescence efficiently quenched when they are mixed with AuNPs unless they hybridize with components of the analyte.7 Up to now, considerable efforts have been made to develop fluorescent methods for DNA detection based on AuNPs.5–8 Recently, both single-walled carbon nanotubes9 and graphene oxide10 have found their significant application for fluorescence-enhanced DNA detection. Till now, many other structures have been used in this assay, including silver nanoparticles,11multi-walled carbon nanotubes and single-walled carbon nanohorns,12carbon nanoparticles,13carbon nanospheres,14nano-C60,15 mesoporous carbon microparticles,16 poly(p-phenylenediamine) nanobelts,17polyaniline nanofibres,18 poly(o-phenylenediamine) colloids,19 poly(m-phenylenediamine) nanorods,20 poly(2,3-diaminonaphthalene) microspheres,21 coordination polymer colloids, nanobelts and microdendrites,22–25Ag@poly(m-phenylenediamine) core-shell nanoparticles,26tetracyanoquinodimethane nanoparticles,27 supramolecular microparticles,28 and porphyrin nanoparticles.29Polystyrene (PS) colloids are important in many areas of technology, such as paints, coatings, ceramics processing, and the construction of photonic bandgap crystals.30 More recently, Socholet al. have constructed a microfluidic system for parallel detection of multiple DNA via molecular beacon probes immobilized on polystyrene microbeads. However, the proposed procedure is somewhat complicated, involving dual-labeled molecular beacon and its immobilization on PS microbeads.31 In this paper, we demonstrate a simpler fluorescence-enhanced DNA detection using PS nanospheres as an effective sensing platform. DNA detection is accomplished by the following two steps. First, PS adsorbs and quenches a dye-labeled single-stranded DNA (ssDNA) probe. In the second step, the specific hybridization of the probe with its target produces a double-stranded DNA (dsDNA) which detaches from PS, leading to recovery of dye fluorescence. We further demonstrate that this sensing platform can differentiate between perfectly complementary and mismatched targets. It can also discriminate complementary and single-base mismatched sequences embedded in rather larger strands with short oligonucleotide probes. In further experiments, it is demonstrated that the present system can be used for multiple DNA detection. Finally, efforts are made toward its application in human blood serum system to evaluate its ability to withstand the interference arising from real sample.
Experimental
All chemically synthesized oligonucleotides were purchased from TaKaRa Biotechnology (Dalian). DNA concentration was estimated by measuring the absorbance at 260 nm.32 All the other chemicals and the aqueous dispersion of COOH-functionalized PS nanoparticles (2.5% w/v) with a mean diameter of about 100 nm were purchased from Aladin Ltd. (Shanghai, China) and used as received without further purification. The water used throughout all experiments was purified through a Millipore system.Transmission electron microscopy (TEM) measurements were made on a HITACHI H-8100 EM (Hitachi, Tokyo, Japan) with an accelerating voltage of 200 kV. Fluorescent emission spectra were recorded on a RF-5301PC spectrofluorometer (Shimadzu, Japan) using a 300-μL quartz cuvette. Zeta potential measurements were performed on a Nano-ZS Zetasizer ZEN3600 (Malvern Instruments Ltd., U.K.).
The desired DNA concentration was achieved by diluting the concentrated DNA stock solutions with 20 mM Tris-HCl buffer containing 100 mM NaCl, 5 mM KCl, and 5 mM MgCl2 (pH: 7.4). The volume of each sample for fluorescence measurement is 300 μL. All the experiments were carried out at room temperature (about 25 °C) if not specified.
The precise PS concentration was achieved following this protocol: Drawing the corresponding amount of 2.5% (w/v) PS, then the solvent was removed by centrifugation and the nanospheres were suspended in 300-μL Tris-HCl buffer.
Oligonucleotide sequences are listed as below (mismatch underlined):
(1) PHIV (FAM dye-labeled ssDNA):
5’-FAM-AGT CAG TGT GGA AAA TCT CTA GC-3’
(2) T1 (complementary target to PHIV):
5’-GCT AGA GAT TTT CCA CAC TGA CT-3’
(3) T2 (single-base mismatched target to PHIV):
5’-GCT AGA GAT T![[G with combining low line]](https://www.rsc.org/images/entities/char_0047_0332.gif) T CCA CAC TGA CT-3’
T CCA CAC TGA CT-3’
(4) T3 (two-base mismatched target to PHIV):
5’-GCT AGA GAT T![[G with combining low line]](https://www.rsc.org/images/entities/char_0047_0332.gif) T
T ![[A with combining low line]](https://www.rsc.org/images/entities/char_0041_0332.gif) CA CAC TGA CT-3’
CA CAC TGA CT-3’
(5) T4 (three-base mismatched target to PHIV):
5’-GCT ![[A with combining low line]](https://www.rsc.org/images/entities/char_0041_0332.gif) TA GAT T
TA GAT T![[G with combining low line]](https://www.rsc.org/images/entities/char_0047_0332.gif) T
T ![[A with combining low line]](https://www.rsc.org/images/entities/char_0041_0332.gif) CA CAC TGA CT-3’
CA CAC TGA CT-3’
(6) T5 (non-complementary target to PHIV):
5’-TTT TTT TTT TTT TTT TTT TTT TT-3’
(7) TL1 (The middle part of a long strand as a target complementary to PHIV):
5’-TTT TTT TTT TTT TTT TTT TTT TGC TAG AGA TTT TCC ACA CTG ACT TTT TTT TTT TTT TTT TTT TTT T-3’
(8) TL2 (The middle part of a long strand as a single-base mismatched target to PHIV):
5’-TTT TTT TTT TTT TTT TTT TTT TGC TAG AGA TT![[G with combining low line]](https://www.rsc.org/images/entities/char_0047_0332.gif) TCC ACA CTG ACT TTT TTT TTT TTT TTT TTT TTT T-3’
TCC ACA CTG ACT TTT TTT TTT TTT TTT TTT TTT T-3’
(9) PK167 (Cy5 dye-labeled ssDNA):
5’-Cy5-TCT GCA CAC CTC TTG ACA CTC CG-3’
(10) T6 (complementary target to PK167):
5’-CGG AGT GTC AAG AGG TGT GCA GA-3’
Results and discussion
Fig. 1 shows representative TEM images of the PS nanospheres used in this study. The low magnification image indicates that the nanospheres range in diameter from 80 to 120 nm, as shown in Fig. 1a. The high magnification image further reveals that these nanospheres have a smooth surface, as shown in Fig. 1b. | ||
| Fig. 1 (a) Low and (b) high magnification TEM images of PS nanospheres with a mean diameter of about 100 nm. | ||
To demonstrate that PS nanospheres can be used as an effective fluorescent sensing platform for DNA detection, an oligonucleotide sequence associated with human immunodeficiency virus (HIV) was chosen as a model system according to related literature,10 labeled at the 5’ end with the dye carboxyfluorescein (FAM). In the absence of PS, these species (PHIV) exhibit strong fluorescence emission in Tris-HCl buffer due to the presence of the fluorescein-based dye (Fig. 2, curve a). In contrast, the presence of 0.83% PS (w/v) leads to about 59% quenching of the fluorescence emission (curve c), demonstrating that the nanosphere can adsorb ssDNA and quench the dye effectively. It should be noted that the addition of the complementary sequence T1 in the absence of PS has a small influence on the fluorescence of the free PHIV (curve b), whereas the PHIV-PS complex exhibits significant fluorescence enhancement upon its incubation with T1 over a period of 15 min, as is evidenced by a 84% fluorescence recovery (curve d). It should be noted that this hybridization between complementary target and probe is competitive with ssDNA probe binding to PS, which means that the complementary strands can’t hybridize with 100% efficiency. Curve e shows that PS itself exhibits no fluorescence emission and thus makes no contribution to the fluorescence intensity of each PS-involved sample measured. Fig. 2 inset illustrates the fluorescence intensity changes (F/F0–1) of PHIV-PS complex upon addition of different concentrations of T1, where F0 and F are FAM fluorescence intensities at 517 nm in the absence and the presence of T1, respectively. In the DNA concentration range of 5–2000 nM, a dramatic increase of FAM fluorescence intensity is observed, suggesting that the PS/DNA assembly approach is effective in probing biomolecular interactions. The detection limit is estimated to be 5 nM (three times the standard deviation of blank sample).
 | ||
| Fig. 2 Fluorescence emission spectra of PHIV (100 nM) at different conditions: (a) PHIV; (b) PHIV + 600 nM T1; (c) PHIV + PS; (d) PHIV + PS + 600 nM T1. Curve e is the emission spectra of the PS sample. Inset: fluorescence intensity changes (F/F0–1) of PHIV−PS complex (F0 and F are the fluorescence intensities without and with the presence of T1, respectively) plotted against the logarithm of the concentration of T1. The concentration of PS used in each sample is 0.83% (w/v). All measurements were done in Tris-HCl buffer in the presence of 5 mM Mg2+ (pH: 7.4). Excitation was at 480 nm, and the emission was monitored at 517 nm . | ||
The zeta potential of the PS nanospheres in Tris-HCl buffer (pH: 7.4) was measured to be about −47.5 mV. Electrostatic repulsion between PS and the negatively charged backbone of ssDNA exists in this system, but is greatly weakened in the presence of a large amount of salt in buffer.26 The stronger π–π stacking between unpaired DNA bases and the styrene polymer dominates the mutual interactions between ssDNA and PS.33 In contrast, dsDNA does not bind to the surface because of its rigid conformation, the absence of unpaired DNA bases, and electrostatic repulsion between the negatively charged dsDNA backbone and PS. The adsorption of dye-labeled ssDNA on PS is accompanied by fluorescence quenching. This quenching may be ascribed to unimolecular collision between excited fluorophore and aromatic group in PS when they are brought into close proximity.34 In the presence of target, the specific hybridization generates a dsDNA, which detaches from PS and leads to the dye’s fluorescence recovery. Since PS effectively quenches the fluorescence of adsorbed dye-labeled ssDNA, the ssDNA-selective adsorption provides a mechanism for optical sensing of target oligonucleotide (Scheme 1).
 | ||
| Scheme 1 A schematic (not to scale) illustrating the PS-based fluorescence-enhanced DNA detection. | ||
To get further insight into the kinetics of the fluorescence quenching and recovery, we collected the time-dependent fluorescence spectra of PHIV and PS, as well as of the PHIV-PS complex with T1, as shown in Fig. 3. Plot a shows the fluorescence quenching of PHIV by PS as a function of incubation time. In the absence of the target, the curve exhibits a rapid reduction in the first 1 min and reaches equilibrium over a period of 10 min, indicating that the adsorption process of ssDNA on PS is very fast. Plot b shows the fluorescence recovery of PHIV-PS by T1 as a function of incubation time. In the presence of the target T1, the curve shows a fast increase in the first 1 min, and the best fluorescence response is obtained over a period of 15 min in terms of the tendency as there is almost no obvious change in fluorescence intensity from 2 to 15 min. All the above observations indicate that PS is superior to SWCNT9 and GO10 in detection speed and the present method is time-saving.
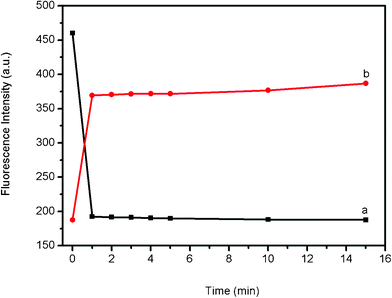 | ||
| Fig. 3 (a) Fluorescence quenching of PHIV (100 nM) by PS and (b) fluorescence recovery of PHIV-PS by T1 (600 nM) in Tris-HCl buffer as a function of incubation time. The concentration of PS used in this sample is 0.83% (w/v). All measurements were done in Tris-HCl buffer in the presence of 5 mM Mg2+ (pH: 7.4). Excitation was at 480 nm, and the emission was monitored at 517 nm. | ||
The sensing platform described herein can differentiate complementary and mismatched sequences. Fig. 4 shows the fluorescence responses of PHIV-PS complex toward perfect complementary target T1, single-base mismatched target T2, two-base mismatched target T3, and three-base mismatched target T4. The F/F0 value (F0 and F are the fluorescence intensities without and with the presence of target, respectively) obtained upon addition of 600 nM T2, T3, and T4 is about 71%, 63%, and 58% of the value obtained upon addition of 600 nM T1 into PHIV-PS complex, respectively. Compared to the complementary target, the mismatched target should have lower hybridization ability toward the adsorbed dye-labeled ssDNA probe, leading to decreased hybridization and thus decreased fluorescence recovery efficiency. However, only very small fluorescence enhancement was observed for the PHIV-PS complex upon addition of 600 nM non-complementary target T5, indicating that the observed fluorescence enhancement in our present system is indeed due to the base pairing between probe and its target other than competitive displacement. The inset presents the corresponding fluorescence intensity histograms with error bar. All the above observations suggest that the results exhibit good reproducibility and the PS-based system is likely to be capable of practically useful mismatch detection upon further development.
 | ||
| Fig. 4 Fluorescence emission spectra of PHIV (100 nM) at different conditions: (a) PHIV-PS complex; (b) PHIV-PS complex + 600 nM T1; (c) PHIV-PS complex + 600 nM T2; (d) PHIV-PS complex + 600 nM T3; (e) PHIV-PS complex + 600 nM T4; (f) PHIV-PS complex + 600 nM T5. Inset: the corresponding fluorescence intensity histograms with error bar. The concentration of PS used in each sample is 0.83% (w/v). All measurements were done in Tris-HCl buffer in the presence of 5 mM Mg2+ (pH: 7.4). Excitation was at 480 nm, and the emission was monitored at 517 nm. | ||
For genomic analysis, it is desirable to detect specific target sequence which is only a small fraction of much longer DNA strand. To do this, two 67-mer DNA strands were tested: the middle part of TL1 is complementary target sequence to PHIV and the middle part of TL2 is single-base-mismatched target sequence to PHIV. Fig. 5 shows the results of the experiments for distinguishing complementary and single-base matched sequences embedded in longer strands. The addition of 600 nM TL1 to PHIV-PS complex leads to about 67% fluorescence recovery, which is much lower than 84% observed when 600 nM T1 was used as the target. Such observation can be explained as follows: PHIV-TL1 is a complex with a duplex DNA in the middle and two single strands on both ends and thus there are unpaired DNA bases for binding to PS. The F/F0 value obtained upon addition of 600 nM TL2 is about 83% of the value obtained upon addition of 600 nM TL1 into PHIV-PS complex. All the above observations indicate that the present assay system is able to discriminate complementary and mismatched target sequences embedded in longer strands with the use of short probe. Thus, it is promising for practical detection of single-base mismatch in target sequences.
 | ||
| Fig. 5 Fluorescence emission spectra of PHIV (100 nM) in the presence of PS at different conditions: (a) PHIV-PS complex; (b) PHIV-PS complex + 600 nM TL1; (c) PHIV-PS complex + 600 nM TL2. Inset: fluorescence intensity histograms with error bar. Excitation was at 480 nm, and the emission was monitored at 517 nm. The concentration of PS used in each sample is 0.83% (w/v). All measurements were done in Tris-HCl buffer in the presence of 5 mM Mg2+ (pH: 7.4). | ||
In further experiments, we evaluated the application potential of this sensing system for real sample analysis by challenging it with human blood serum samples. Fig. 6 shows the fluorescence responses of PHIV-PS complex toward T1 and T2 in the presence of 50% (volume ratio) human blood serum, respectively. The F/F0 value obtained upon addition of T2 is about 89% of the value obtained upon addition of T1 into PHIV-PS complex. Fig. 6 inset is the corresponding fluorescence intensity histograms with error bar. These observations demonstrate that the present system holds great promise for real sample analysis upon further development.
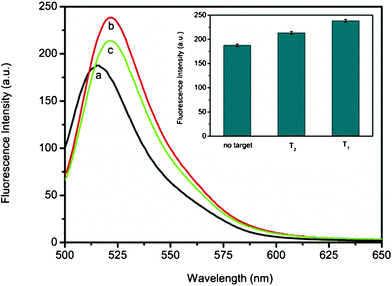 | ||
| Fig. 6 Fluorescence emission spectra of PHIV (100 nM) at different conditions: (a) PHIV-PS complex; (b) PHIV-PS complex + 600 nM T1; (c) PHIV-PS complex + 600 nM T2. Inset: the corresponding fluorescence intensity histograms with error bar. The concentration of PS used in each sample is 0.83% (w/v). All measurements were done in Tris-HCl buffer (pH: 7.4) containing 5 mM Mg2+ in the presence of 50% (volume ratio) human blood serum. Excitation was at 480 nm, and the emission was monitored at 525 nm. | ||
We also explored the feasibility of using the platform described herein to detect multiple DNA targets simultaneously. To this end, we chose FAM-labeled PHIV and another probe PK167 labeled with Cy5 (cyanine 5) as model systems. Because these two dyes are individually excited at 480 and 643 nm to emit at 518 and 660 nm, respectively, significant dye-to-dye energy transfer is avoided. In the presence of PS, the fluorescence of these two dyes in the probe mixture was quenched to a significant extent, suggesting that PS can be used to quench dyes of different emission frequencies. Fig. 7 shows the fluorescence intensity histograms of the probe mixture toward different target combinations in the presence of PS under excitation/emission wavelengths of 480/517 and 643/660 nm/nm. As expected, the addition of T1 only lead to fluorescence enhancement at 517 nm when excited at 480 nm while the addition of T6 only results in fluorescence enhancement at 660 nm emission peaks with the excitation at 643 nm. In contrast, the combination of T1+T6 results in fluorescence enhancement of both emission peaks. These observations suggest that this PS-based sensing platform can be used for multiple DNA detection.
![Fluorescence intensity histograms of the probe mixture toward different target combinations in the presence of PS under excitation/emission wavelengths of 480/518 and 643/660 nm/nm. ([PHIV]=[PK167]=100 nM; [T1]=[T6]=600 nM). The concentration of PS used in each sample is 0.83% (w/v). All measurements were done in Tris-HCl buffer in the presence of 5 mM Mg2+ (pH: 7.4).](/image/article/2011/RA/c1ra00241d/c1ra00241d-f7.gif) | ||
| Fig. 7 Fluorescence intensity histograms of the probe mixture toward different target combinations in the presence of PS under excitation/emission wavelengths of 480/518 and 643/660 nm/nm. ([PHIV]=[PK167]=100 nM; [T1]=[T6]=600 nM). The concentration of PS used in each sample is 0.83% (w/v). All measurements were done in Tris-HCl buffer in the presence of 5 mM Mg2+ (pH: 7.4). | ||
A survey of the influence of the concentration of PS on the efficiency of fluorescence quenching and recovery is shown in Fig. 8, revealing that larger amounts of PS provide increased quenching efficiency but decreased recovery efficiency. This is consistent with the concept that excess PS can adsorb target complementary sequences rather than allow them to hybridize with the adsorbed probe strands. An optimal balance between the amount of PS and fluorescence quenching-recovery was achieved when 0.83% (w/v) PS was used. So an optimal PS concentration 0.83% (w/v) was used throughout all experiments if not specified.
![Fluorescence intensity histograms of PHIV + PS and PHIV + PS + T1 with the use of PS at the concentration of 0, 0.50%, 0.67%, 0.83%, and 1.00% (w/v), respectively. ([PHIV]=100 nM; [T1]=600 nM). All measurements were done in Tris-HCl buffer in the presence of 5 mM Mg2+ (pH: 7.4). Excitation was at 480 nm, and the emission was monitored at 517 nm.](/image/article/2011/RA/c1ra00241d/c1ra00241d-f8.gif) | ||
| Fig. 8 Fluorescence intensity histograms of PHIV + PS and PHIV + PS + T1 with the use of PS at the concentration of 0, 0.50%, 0.67%, 0.83%, and 1.00% (w/v), respectively. ([PHIV]=100 nM; [T1]=600 nM). All measurements were done in Tris-HCl buffer in the presence of 5 mM Mg2+ (pH: 7.4). Excitation was at 480 nm, and the emission was monitored at 517 nm. | ||
Conclusions
In conclusion, we demonstrate that inexpensive PS nanospheres can be used as a sensing platform for fluorescence-enhanced DNA detection. This sensing platform can differentiate perfectly complementary and mismatched targets, it can also discriminate complementary and single-base mismatched sequences embedded in rather larger strands with short oligonucleotide probes. Furthermore, this sensing platform is suitable for multiple DNA detection. Most importantly, it holds great potential for practical application in terms of its performance in human blood serum system. This is extended application of PS, and the method holds great potential as a platform for a variety of other target molecule analysis, such as proteins and heavy metal ions.Acknowledgements
This work was supported by National Basic Research Program of China (No. 2011CB935800).References
- D. Gresham, D. M. Ruderfer, S. C. Pratt, J. Schacherer, M. J. Dunham, D. Botstein and L. Kruglyak, Science, 2006, 311, 1932 CrossRef CAS.
- R. Brayner, Nano Today, 2008, 3, 48 CrossRef.
- N. L. Rosi and C. A. Mirkin, Chem. Rev., 2005, 105, 1547 CrossRef CAS.
- P. C. Ray, G. K. Darbha, A. Ray, J. Walker and W. Hardy, Plasmonics, 2007, 2, 173 CrossRef CAS.
- B. Dubertret, M. Calame and A. J. Libchaber, Nat. Biotechnol., 2001, 19, 365 CrossRef CAS.
- D. J. Maxwell, J. R. Taylor and S. Nie, J. Am. Chem. Soc., 2002, 124, 9606 CrossRef CAS.
- H. Li and L. J. Rothberg, Anal. Chem., 2004, 76, 5414 CrossRef CAS.
- (a) S. Song, Z. Liang, J. Zhang, L. Wang, G. Li and C. Fan, Angew. Chem., Int. Ed., 2009, 48, 8670 CrossRef CAS; (b) D. Li, S. Song and C. Fan, Acc. Chem. Res., 2010, 43, 631 CrossRef CAS.
- (a) R. Yang, Z. Tang, J. Yan, H. Kang, Y. Kim, Z. Zhu and W. Tan, Anal. Chem., 2008, 80, 7408 CrossRef CAS; (b) R. Yang, J. Jin, Y. Chen, N. Shao, H. Kang, Z. Xiao, Z. Tang, Y. Wu, Z. Zhu and W. Tan, J. Am. Chem. Soc., 2008, 130, 8351 CrossRef CAS.
- (a) C. Lu, H. Yang, C. Zhu, X. Chen and G. Chen, Angew. Chem., Int. Ed., 2009, 48, 4785 CrossRef CAS; (b) S. He, B. Song, D. Li, C. Zhu, W. Qi, W. Wen, L. Wang, S. Song, H. Fang and C. Fan, Adv. Funct. Mater., 2010, 20, 453 CrossRef CAS.
- (a) Y. Zhang, H. Li and X. Sun, Chinese J. Anal. Chem., 2011, 39, 998 CrossRef CAS; (b) C. Liu, X. Yang, H. Yuan, Z. Zhou and D. Xiao, Sensors, 2007, 7, 708 CrossRef CAS; (c) Y. Cao, X. Wu and M. Wang, Talanta, 2011, 84, 1188 CrossRef CAS; (d) J. Geng, J. Liang, Y. Wang, G. G. Gurzadyan and B. Liu, J. Phys. Chem. B, 2011, 115, 3281 CrossRef CAS; (e) H. I. Peng and B. L. Miller, Analyst, 2011, 136, 436 RSC; (f) H. I. Peng, T. D. Krauss and B. L. Miller, Anal. Chem., 2010, 82, 8664 CrossRef CAS; (g) Y. Wang, B. Liu, A. Mikhailovsky and G. C. Bazan, Adv. Mater., 2010, 22, 656 CrossRef CAS.
- (a) S. Zhu, Z. Liu, W. Zhang, S. Han, L. Hu and G. Zhu, Chem. Commun., 2011, 47, 6099 RSC; (b) H. Li, J. Tian, L. Wang, Y. Zhang and X. Sun, J. Mater. Chem., 2011, 21, 824 RSC.
- H. Li, Y. Zhang, L. Wang, J. Tian and X. Sun, Chem. Commun., 2011, 47, 961 RSC.
- H. Li, Y. Zhang, T. Wu, S. Liu, L. Wang and X. Sun, J. Mater. Chem., 2011, 21, 4663 RSC.
- H. Li, Y. Zhang, Y. Luo and X. Sun, Small, 2011, 7, 1562 CrossRef CAS.
- S. Liu, H. Li, L. Wang, J. Tian and X. Sun, J. Mater. Chem., 2011, 21, 339 RSC.
- L. Wang, Y. Zhang, J. Tian, H. Li and X. Sun, Nucleic Acids Res., 2011, 39, e37 CrossRef CAS.
- S. Liu, L. Wang, Y. Luo, J. Tian, H. Li and X. Sun, Nanoscale, 2011, 3, 967 RSC.
- J. Tian, H. Li, Y. Luo, L. Wang, Y. Zhang and X. Sun, Langmuir, 2011, 27, 874 CrossRef CAS.
- Y. Zhang, H. Li, Y. Luo, X. Shi, J. Tian and X. Sun, PLoS One, 2011, 6, e20569 CAS.
- J. Tian, Y. Zhang, Y. Luo, H. Li, J. Zhai and X. Sun, Analyst, 2011, 136, 2221 RSC.
- H. Li and X. Sun, Chem. Commun., 2011, 47, 2625 RSC.
- H. Li, L. Wang, Y. Zhang, J. Tian and X. Sun, Macromol. Rapid Commun., 2011, 11, 899 CrossRef.
- Y. Luo, F. Liao, W. Lu, G. Chang and X. Sun, Nanotechnology, 2011, 22, 195502 CrossRef.
- H. Li, J. Zhai and X. Sun, RSC Advances, 2011 10.1039/c1ra00359c.
- Y. Zhang, L. Wang, J. Tian, H. Li, Y. Luo and X. Sun, Langmuir, 2011, 27, 2170 CrossRef CAS.
- H. Li, L. Wang, J. Zhai, Y. Luo, Y. Zhang, J. Tian and X. Sun, Anal. Methods, 2011, 3, 1051 RSC.
- H. Li, J. Zhai and X. Sun, PLoS One, 2011, 6, e18958 CAS.
- J. Zhai, H. Li and X. Sun, RSC Advances, 2011, 1, 36 RSC.
- (a) Polymer Colloids , ed. R. M. Fitch, Academic Press, San Diego, 1997 Search PubMed; (b) Y. Xia, B. Gates, Y. Yin and Y. Lu, Adv. Mater., 2000, 12, 693 CrossRef CAS.
- R. D. Sochol, B. P. Casavant, M. E. Dueck, L. P. Lee and L. Lin, J. Micromech. Microeng., 2011, 21, 054019 Search PubMed.
- O. Warberg and W. Christian, Biochem., 1942, 310, 384 Search PubMed.
- N. Varghese, U. Mogera, A. Govindaraj, A. Das, P. K. Maiti, A. K. Sood and C. N. R. Rao, ChemPhysChem, 2009, 10, 206 Search PubMed.
- R. Matsushima and S. Sakuraba, J. Am. Chem. Soc., 1971, 93, 7143 Search PubMed.
Footnote |
| † L. Wang and H. Li made equal contribution to this work. |
| This journal is © The Royal Society of Chemistry 2011 |
