Sulfonated nanoplates in proton conducting membranes for fuel cells†
Hikmatun
Ni'mah
a,
Wei-Fu
Chen
b,
Yu-Cheng
Shen
a and
Ping-Lin
Kuo
*a
aDepartment of Chemical Engineering, National Cheng Kung University, Tainan City, Taiwan. E-mail: plkuo@mail.ncku.edu.tw; Fax: +886 6 276 2331; Tel: +886 6 275 7575 ext. 62658
bChemistry Department, Brookhaven National Laboratory, Upton, NY, USA. E-mail: wfchen@bnl.gov; Fax: +1 631 344 5815; Tel: +1 631 344 4360
First published on 29th September 2011
Abstract
Surface-functionalized nanoplates are synthesized by anchoring sulfonic acid containing siloxanes on zirconium phosphate, and in turn blended with Nafion to fabricate proton conducting membranes. The effects of these sulfonated nanoplates on proton conduction, hydro-characteristics and fuel cell performance are reported.
The hydrogen-energy technology, combining the approach of proton exchange membrane fuel cells (PEMFCs) to enable cost-effective energy systems that can be used for transportation and electronic applications, has attracted intense attention in recent years. Perfluorosulfonated ionomers have been extensively used as the separator in PEMFCs.1–3 However, state-of-the-art cation exchange membranes such as Nafion are unstable at elevated temperatures. Perfluorosulfonated polymers become dry under conditions of high temperature (above 80 °C) or low humidity rather quickly due to the loss of water from the membrane.4,5 For this reason, widespread efforts have been dedicated to developing ionomers for operation at elevated temperatures based on inorganic–organic composites.6 Because of the perfluorinated nature of Nafion, a major research objective is to incorporate extrinsic species into Nafion in stable structures while maintaining high proton conductivity. In some cases, with the introduction of a nano-scale material, the miscibility of Nafion with extrinsic species is improved.
Watanabe et al.7 incorporated nanocrystallites Pt and oxides such as SiO2 and TiO2 in the Nafion matrix. They found that the Pt particles suppressed fuel crossover by catalyzing the oxidation of penetrating H2 with O2. The generated water subsequently was absorbed by the oxides which in turn humidified the membrane. Xu et al.8 studied a sol–gel approach of fabricating sulfonated polysilsesquioxane on Nafion that improves the compatibility between the inorganic phase and the hydrophilic domains. Ceramic oxides such as titania,9zirconia,10,11 heteropoly acids,12 montmorillonites13 and zirconium phosphates14–17 in the Nafion membrane have also been examined.
Among the reported inorganic fillers, α-zirconium phosphate (Zr(HPO4)2·H2O, α-ZrP) is known for its Brønsted acidity to donate protons,18,19 thermal stability to temperatures above 180 °C,20 and hygroscopic and hydrophilic character.21 Its compatibility with the chemical and physical limits of polymers enables easy synthesis in the fabrication procedure.22 As shown in Scheme 1, each layer of α-ZrP consists of planes of zirconium atoms connected to phosphate groups which alternate above and below the metal atom planes. The phosphate oxygen atom bears hydrogen atoms and points toward the adjacent layer or at the surface of the stacking of several layers that induce the surface acidity of the inorganic particle. Incorporating α-ZrP into the Nafion matrix could increase the hydration of the membrane and reduce methanol crossover.23–25 However, the composite approach does not always present distinctive advantages in fuel cell performance, mainly because the excessive addition of these less conductive fillers results in proton conductivity lower than that of pristine Nafion since the proton channel has been blocked. Alberti et al.26 have reported a 96% drop in conductivity at 100 °C for the composite membrane consisting of 5 nm ZrP nanoparticles in the Nafion matrix when the humidity was decreased from 95% RH to 30% RH. Kuan et al.27 have investigated the hybrid of exfoliated ZrP with Nafion; the proton conductivity was only 7% lower than neat Nafion-117. Siet al.28 have studied a Nafion-Teflon-ZrP composite membrane at high temperature and low humidity; the current density at a cell voltage of 0.6 V decreased from 1500 to 450 mA cm−2 when the relative humidity of the cell was reduced from 100 to 31% RH. Therefore, Nafion doped with fillers containing chemically anchored proton conducting groups would be highly desirable.
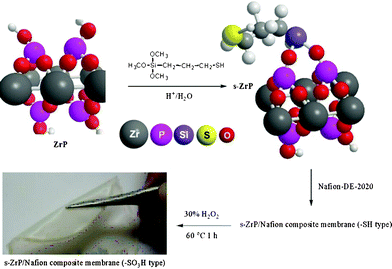 | ||
| Scheme 1 Illustration of the layered structure of MPTMS-functionalized zirconium phosphate and the preparation of s-ZrP/Nafion composite membranes. | ||
We have previously reported organic–inorganic hybrids by introducing triply cross-linked polysiloxane networks29 or hydrophilic polysiloxane-modified carbon nanotubes30 into the Nafion matrix. It was found that the existence of siloxane facilitates the compatibility between the intrinsic species and Nafion. In this communication, the structure of the inorganic filler has been designed at the molecular level in order to retain indispensible properties such as proton conductivity, water sorption, and dimensional stability. The composite membrane is fabricated by blending surface-functionalized α-zirconium phosphate nanoplates with Nafion. Herein, the architecture of α-ZrP is tailored in order to modify the proton transportation behavior by connecting sulfonated siloxane with the nanoplates. The sulfonated siloxanes on the ZrP surface act as proton donors, retain water inside nanoplates, and thus allow proton conduction to take place by surface transportation through the interlayer regions in the presence of water. Attention was focused on the ability of the filler to alter the hydro-characteristics of the membrane and to enhance the conductivity at high temperature and low humidity conditions.
The preparation of the surface-functionalized ZrP nanoplates/Nafion hybrid membrane is shown in Scheme 1. Layer-structured α-zirconium phosphate is synthesized according to a modified procedure given in the literature;31 we introduced Nafion into the stock solution of ZrOCl2 and H3PO4 to act as a stabilizer for ZrP nanoplates. 3-Mercaptopropyl-trimethoxysilane (MPTMS) was further reacted with α-ZrP colloidal dispersion based on sol–gel chemistry. MPTMS-modified ZrP nanoplates were then blended with 20% Nafion DE-2020 dispersion followed by casting and slowly removing the solvent to form a thin membrane. In this manner, a good dispersion of s-ZrP nanoplates in the Nafion matrix is achieved. The MPTMS-modified ZrP nanoplates contain sulfide groups on the surface, which are further converted to sulfonic acid groups through subsequent oxidation of the composite membranes in 30% H2O2. Different amounts of s-ZrP nanoplates, which have a MPTMS to Zr(HPO4)2·H2O ratio (Si/Zr) = 1, were mixed with Nafion (solid content (s-ZrP/s-ZrP + Nafion solid) = 5%, 10%, 20%, 30%) denoted as A, B, C, and D composite membranes, respectively (Table 1). Two control membranes, denoted as C2.0 and C0.5, were prepared with Si/Zr = 2 and 0.5, respectively.
The SEM and TEM images, as shown in Fig. 1a and Fig. 1b respectively, display the morphology of the Nafion-stabilized α-ZrP. α-ZrP is characterized by thin, flat and flaky plates with high radial-axial ratios; the thickness of the plate is ca. 15 nm (from the image looking along the radial axes in the inset in Fig. 1a). The TEM image shown in Fig. 1b indicates a good size-distribution of α-ZrP plates with an average diameter ca. 100 nm. It is noticed that the coherence between nanoplates and Nafion is observed; Nafion (grey color region) surrounds the nanoplates as shown in the highlighted inset image in Fig. 1b. Also shown in Fig. 1a is the XRD pattern of the Nafion-stabilized α-ZrP. The pattern gives a typical α phase zirconium phosphate (α-Zr(HPO4)2·H2O). The basal interlayer distance for the α-zirconium phosphate is 7.6 Å. Half of the –POH groups which are involved in inter- and intra-planar bonds are believed to form hydrogen bonds with phosphate oxygen atoms in an adjacent layer and the others are thought to be hydrogen bonded to the water molecules.32 Therefore, water molecules are trapped in the inorganic plate, even at high temperatures.33 In conduction terms, Alberti et al.34 have stated that the internal transport mainly occurs in the parallel direction to the layers and the intercalated molecules (alkali ions or water molecules) are mobile inside the α-ZrP layers which contribute to ionic conduction through diffusion within the interlayer.
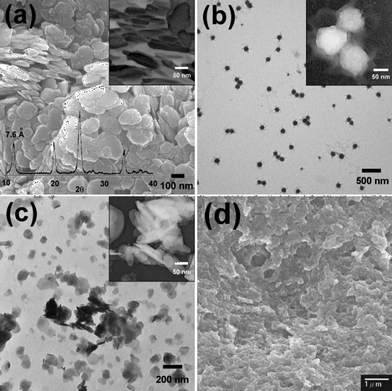 | ||
| Fig. 1 SEM (a) and TEM (b) micrographs of as-prepared α-ZrP. The XRD pattern of as-prepared α-ZrP in shown overlaid on the SEM (a). (c) TEM image of MPTMS-functionalized α-ZrP nanoplates. (d) SEM image of s-ZrP/Nafion composite membrane C. The inset pictures in (a)–(c) are negative photographs with high magnification. | ||
The smaller size of the Nafion-stabilized nanoplates makes them easy to be utilized in a homogenous dispersion. The presence of acidic hydroxyphosphate groups (P–OH) on a plane would increase the hydrolysis reaction of SiOCH3, forming SiOH which further condenses with P–OH. Thus, MPTMS is attached to the surface of ZrP nanoplates, forming anchored propylsulfide chains. Energy-dispersive X-ray spectroscopy (EDX) has confirmed the presence of sulfur and silicon elements for the MPTMS-functionalized ZrP (see Fig. S1 in ESI†). 31P MAS NMR with 1H decoupling shows the signal of P–O–Si bridging at δ = −12.5 ppm together with the main peak at δ = −20 ppm which is labeled as the phosphorous group of zirconium phosphate (Fig. S2a).35XPS analysis in the Si 2p region of the s-ZrP nanoplates (Fig. S2b), which shows a peak at 104 eV attributed to Si–O bonding, also confirms the presence of siloxane on s-ZrP nanoplates. The transformation of –SH to –SO3H of MPTMS-functionalized ZrP was confirmed by the S 2p lines in XPS spectra (Fig. S3). The TEM images of MPTMS-functionalized ZrP nanoplates are shown in Fig. 1c. The structure of ZrP is not changed by the attachment of MPTMS. Casting from isopropanol solution of the ionomers gives a tough and flexible membrane with a thickness ca. 90 μm. The picture in Scheme 1 displayed a bent film without cracking. We performed SEM on the fracture surfaces of the s-ZrP/Nafion composite membrane. The image is shown in Fig. 1d, which displays the morphology of the composites loaded with 20 wt% s-ZrP, i.e.membrane C. The fracture surface shows good homogeneity and dispersion. No stack of ZrP plates was found, demonstrating that good interfacial compatibility exists between the Nafion matrix and functionalized nanoplates.
The measured ion exchange capacity (IEC) and water content values of composite membranes and commercial Nafion-117 are listed in Table 1. The IEC value becomes higher as the s-ZrP solid content increases from 5% to 30%, however, water content decreases from 21.7% to 13.4%, respectively. Normally, the amount of water absorption in proton conducting polymers depends strongly on the concentration of sulfonic acid groups. The considerable reduction in water content of the membranes with high solid contents, e.g. 20 and 30 wt%, can be ascribed to the presence of uniformly distributed s-ZrP nanoplates, which occupy the space of adsorbed water, throughout the membrane.
High IEC enables a proton-conducting membrane to possess good proton conductivity. However, poor mechanical strength is, unfortunately, the proverbial “Achilles' heel” for most proton exchange membranes with an ultra-high IEC value. In the present study, a membrane, denoted as C2.0, is prepared by doubling the Si/Zr ratio. The doubled Si/Zr ratio increases IEC by about 7.2%, and an increase by 40.7% in water content is observed. Another control sample with Si/Zr = 0.5 (membrane C0.5) was synthesized as well, which has an IEC similar to membrane B (1.02 meq g−1) but possesses lower water content (13.2 vs. 18.0 wt%).
Shown in Fig. 2a are the 3D mesh plots displaying the relationships between proton conductivities, s-ZrP solid contents and Si/Zr ratios of the composite membranes measured at 30 and 95 °C with 95% RH. The conductivity increases when the s-ZrP solid content increases, and reaches an optimum when the solid content = 20 wt%. Considering the relationship between conductivities, water contents and IEC, it is found that more s-ZrP filler in membranes results in lower water content, higher IEC value, but higher proton conductivity, except for membrane D. As we mentioned above, the percentage of s-ZrP filler up to 30 wt% is such a high loading that nanoplates occupy the space for water absorption in the ionic channels in Nafion. Thus, the increase in conductivity with increasing s-ZrP content can be ascribed to the increased IEC values. Generally, it is believed that in the fully hydrated state, sulfonated polymers may dissociate immobile sulfonate groups and mobile protons. The free protons can move along with cationic mixtures such as H3O+ in a localized ionic network within fully water-swollen sulfonated polymer membranes. The correlation between water and ionomers dominates the transportation of protons across the membrane. It has been shown that the water which associates with species such as ionic and polar groups forming a true solution does not freeze at 0 °C.36,37 The DSC thermograms of the fully hydrated composite membranes present a broad endothermic signal at approximately 0 °C to −40 °C (Fig. S4a in ESI†). The membranes with higher s-ZrP contents have a lower melting point of frozen water and a higher bound water degree (Table S2). It has been reported that confinement in nano-sized domains dominates the thermal transitions of water molecules. We have previously shown that the strong interaction between water molecules and polar groups in polysiloxane/Nafion hybrids brought about high bound water degrees and low melting temperatures.26 In the present study, we ascribed the enhanced proton conductivity to the formation of a bound-water layer on the s-ZrP's surface. These abundant proton carriers in a confined domain facilitate the binding of water, as demonstrated by the shift of water melting behavior and high bound water degree, so as to form a continuous water passage for proton transportation along the planes of s-ZrP.
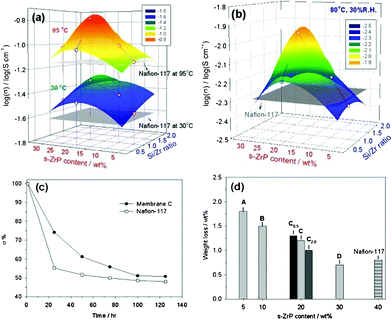 | ||
| Fig. 2 Proton conductivity of s-ZrP/Nafion composite membranes and Nafion-117 measured (a) under highly humidified conditions (30 and 95 °C; 95% RH) and (b) in a low humidity environment (80 °C; 30% RH). (c) Retention of proton conductivity as a function of time at 80 °C and 30% RH. (d) Oxidative stability compared by the loss of the membrane weight obtained after testing in Fenton's reagent at 80 °C for 1 h. | ||
The DSC thermogram of the fully hydrated membrane C0.5 shows two major melting peaks (Fig. S4b). However, the thermogram of the membrane C2.0 shows similar features to membrane C and has a much smaller melting enthalpy than the latter. The increased Si/Zr ratio brings about a high water content (19.4 wt%) but a low freezing water fraction (0.3 wt%), which gives a bound water degree up to 98.5%. On the other hand, the membrane C0.5 has a reduced bound water degree of 87.1%. These results suggest that the sulfonic acid groups on s-ZrP dominate the interaction between water and the composite membranes. We considered that the hydrogen bonding between –SO3H groups on the s-ZrP lamella and the –POH or –SO3H groups on the adjacent lamella forms a localized hygroscopic lamellar structure. Water absorbed in a confined space could be beneficial to reduce methanol crossover (see page S9–10 in ESI† for the results about methanol-crossover behavior). Moreover, a decrease in proton conductivity was observed for membrane D. This might result from the excess of s-ZrP filler in Nafion. The massive inorganic fillers in Nafion may result in agglomeration between the plates which blocks the water channel and prolongs the transporting pathway for protons.
The proton conductivity at low humidity is measured after treating the s-ZrP/Nafion composite membranes at 80 °C and 30% RH for 1 h, as shown in Fig. 2b. The conductivities of all the membranes are reduced by 1 to 1.2 orders of magnitude, due to a serious loss of water. The 3D mesh plot shows an optimum of 0.011 S cm−1 at s-ZrP content = 20 wt% and Si/Zr ratio = 1.0 (membrane C), which is distinctly higher than that of Nafion-117 (5.1 × 10−3 S cm−1). The long-term conductivity of PEMs is also crucial to the performance stability of the fuel cells. The conductivity-retaining ability of the composite membranes is determined by measuring the conductivity at 80 °C and 30% RH up to 125 h. Fig. 2c shows the changes in proton conductivity of membrane C and Nafion-117. It is seen that the loss in conductivity occurs via similar processes and in two steps. In the first step (from 0 to 20 h), the conductivity of Nafion-117, for example, decreases rather rapidly to 55%, mainly due to the evaporation of free water; in the second step (20 to 125 h), the conductivity reduces slowly, most likely due to the loss of bound water. For membrane C, a 25% drop in conductivity was observed within 20 h. Note that the conductivity reduced gradually, and that equilibrium is reached after about 100 h. The gradual decrease in conductivity of membrane C gives evidence that s-ZrP confers water-retaining ability on the hybrid. It should be pointed out here that the stability test conditions in low humidity environment are much more severe than normal fuel cell operating conditions.
The oxidative stability of the composite membranes is evaluated in Fenton's reagent at 80 °C as an accelerated testing and is shown in Fig. 2d. Nafion-117 shows a high oxidative stability. All the composite membranes possess good oxidative stability. Their weight losses are less than 2% after the Fenton's treatment. As expected, the membranes with the higher s-ZrP content had the better oxidative stability. Membrane D even shows a comparable stability to Nafion-117.
To demonstrate the applicability of the s-ZrP/Nafion composites as proton exchange membranes in PEMFCs, a single cell was assembled with membrane C and a Pt/C cathode and anode. Fig. 3 shows the cell polarization performance measured at 80 and 100 °C in H2/O2. The open circuit voltage (OCV) of the cell composed of membrane C was 1.0 V, very close to that of the single cell assembled with Nafion-117, 0.99 V. The maximum power density is 323 mW cm−2 at 80 °C and 541 mW cm−2 when the temperature increases to 100 °C. The maximum power density of membrane C at 100 °C is found to be 19.2% higher than that of Nafion-117 (454 mW cm−2).
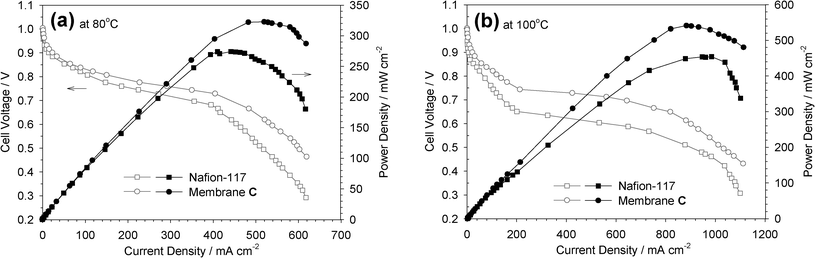 | ||
| Fig. 3 Comparison of polarization curves for the single PEMFC with membrane C and Nafion-117 as the electrolyte membranes at cell temperatures (a) 80 °C and (b) 100 °C (anode: humidified H2 flow rate: 100 ml min−1; cathode: humidified O2 flow rate: 100 ml min−1; atmospheric pressure). | ||
The electrode kinetic parameters, including the open circuit potential (Eo), Tafel slope (b) and the ohmic resistance (R), were derived from Fig. 3 for membrane C and Nafion-117 (see Table S4 in the ESI†). Membrane C possesses a lower ohmic resistance than Nafion-117 (R = 0.178 ohm cm2vs. 0.287 ohm cm2 at 80 °C; R = 0.040 ohm cm2vs. 0.079 ohm cm2 at 100 °C). The film thicknesses of membrane C and Nafion-117 are 90 μm and 150 μm, respectively. The lower resistance for the cell composed of membrane C is most likely due to a joint outcome combining thinner film, lower equivalent weight and better proton conductivity over Nafion-117. At a cell temperature of 100 °C, better membrane/electrode interfacial assembly was demonstrated for the cell consisting of the s-ZrP/Nafion hybrid than for Nafion-117 (see the discussion of Tafel slopes in the ESI†).
The durability test was carried out at a given cell voltage of 0.5 V at a cell temperature of 100 °C, as shown in Fig. 4. The relative humidities of the cell are 73 and 7% RH for the “humidifier-on “ and “humidifier-off” periods. After the first “humidifier off” point (∼90 h), the power density reduced gradually from 520 mW cm−2 to 460 mW cm−2 (about 15% drop). The data fluctuated between 450 and 480 mW cm−2, which apparently resulted from the loss of water in the membrane. We then turned the humidifiers on at ∼220 h, the power density went up to 515 mW cm−2. At the 2nd “humidifier-off” period, the power density decreased to ∼400 mW cm−2 and fluctuated between 380 and 410 mW cm−2. As soon as the humidifiers were turned on, the power density of the cell returned to 500 mW cm−2. The cell after testing for 600 h produced 501 mW cm−2 power density, which is a 6.5% drop from the initial power output. It is noteworthy that, under dry conditions (100 °C and humidifier-off), a drop of 22.3% in power density is observed with the s-ZrP-Nafion composite membrane. It has been reported by Sahu et al.38 that the performance of a PEMFC with Nafion membrane decreased by 65% when the relative humidity changed from 100 to 18% RH. Sahu incorporated highly hygroscopic mesoporous zirconium phosphate in the Nafion matrix; a drop of about 51% in peak power density was observed. Yang et al.39 have reported a self-humidifying membrane which consists of a Pt/Nafion layer-by-layer structure. The PEMFC comprising the self-humidifying membrane showed a 33% drop in power density with dry H2 and O2. Son et al.40 have utilized a Pt/zeolite-Nafion composite membrane in PEMFCs. Under dry H2 and O2, the cell consisting of the Pt/zeolite-Nafion composite membrane gave 75% of the performance obtained with humidified fuels. In summary, the s-ZrP nanoplate/Nafion hybrid in the present work afforded significantly higher performance with dry fuels than the previous ZrP/Nafion or self-humidifying membranes.
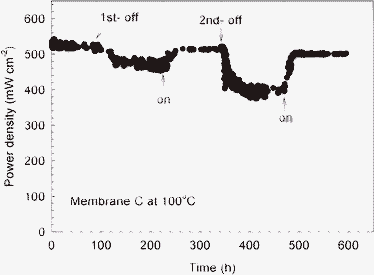 | ||
| Fig. 4 The durability test with cyclic dry/wet test of the MEA made with membrane C (cell temperature: 100 °C; anode: H2 flow rate: 100 ml min−1; cathode: O2 flow rate: 100 ml min−1; atmospheric pressure). Both the humidifiers of the anode and cathode were turned on and off in turn, as clearly marked with blue and red arrows. | ||
Conclusions
The hybrid of sulfonated siloxane-modified ZrP nanoplates with Nafion shows a significant effect on the behavior of proton transportation and on the enhancement of power output of PEMFC. The anchored sulfonic acids on the surface of ZrP plates are believed to promote proton conduction through producing a hydrous layer on the plate surface which connects the proton passage between the ionic clusters in the Nafion matrix and the ionic channels between ZrP lamellae. More significantly, the composite membranes display proton conductivity values over 2 times higher than Nafion-117 under low humidity conditions and excellent oxidative stability, and are capable of reducing methanol crossover. The single PEMFC comprising the s-ZrP/Nafion hybrid afforded 20% higher performance with humidified fuels at 100 °C than Nafion-117. A drop of 22.3% in power density was observed with dry fuels, and only a 6.5% drop in power density was detected after 600 h operation. These favorable properties make it a promising material for technological application in polymer electrolyte fuel cells.References
- O. Diat and G. Gebel, Nat. Mater., 2008, 7, 13–14 CrossRef CAS.
- M. A. Hickner, H. Ghassemi, Y. S. Kim, B. R. Einsla and J. E. McGrath, Chem. Rev., 2004, 104, 4587–4612 CrossRef CAS.
- L. Carrette, K. A. Friedrich and U. Stimming, Fuel Cells, 2000, 1, 5–39 CrossRef.
- S. J. Paddison, Annu. Rev. Mater. Res., 2003, 33, 289–319 CrossRef CAS.
- J. Wang, H. Zhang, X. Yang, S. Jiang, W. Lu, Z. Jiang and S. Z. Qiao, Adv. Funct. Mater., 2011, 21, 971–978 CrossRef CAS.
- J. A. Asensio, E. M. Sánchez and P. Gómez-Romero, Chem. Soc. Rev., 2010, 39, 3210–3239 RSC.
- M. Watanabe, H. Uchida, Y. Seki, M. Emori and P. Stonehart, J. Electrochem. Soc., 1996, 143, 3847–3852 CrossRef CAS.
- K. Xu, C. Chanthad, M. R. Gadinski, M. A. Hickner and Q. Wang, ACS Appl. Mater. Interfaces, 2009, 1, 2573–2579 CAS.
- V. Baglio, A. S.Aricò, A. Di Blasi, V. Antonucci, P. L. Antonucci, S. Licoccia, E. Traversa and F. Serraino Fiory, Electrochim. Acta, 2005, 50, 1241–1246 CrossRef CAS.
- A. D'Epifanio, M. A. Navarra, F. C. Weise, B. Mecheri, J. Farrington, S. Licoccia and S. Greenbaum, Chem. Mater., 2010, 22, 813–821 CrossRef CAS.
- M. A. Navarra, F. Croce and B. Scrosati, J. Mater. Chem., 2007, 17, 3210–3215 RSC.
- R. K. Nagarale, W. Shin and P. K. Singh, Polym. Chem., 2010, 1, 388–408 RSC.
- C. H. Rhee, H. K. Kim, H. Chang and J. S. Lee, Chem. Mater., 2005, 17, 1691–1697 CrossRef CAS.
- A. K. Sahu, S. Pitchumani, P. Sridhar and A. K. Shukla, Fuel Cells, 2009, 9, 139–147 CrossRef CAS.
- I. Nicotera, T. Zang, A. Bocarsly and S. Greenbaum, J. Electrochem. Soc., 2007, 154, B466–B473 CrossRef CAS.
- Y. Si, H. R. Kunz and J. M. Fenton, J. Electrochem. Soc., 2004, 151, A623 CrossRef CAS.
- F. Bauer and M. Willert-Porada, Fuel Cells, 2006, 3, 261–269 CrossRef.
- J. M. Troup and A. Clearfield, Inorg. Chem., 1977, 16, 3311–3314 CrossRef CAS.
- W. H. J. Hogarth, J. C. Diniz da Costa, J. Drennan and G. Q. Lu, J. Mater. Chem., 2005, 15, 754–758 RSC.
- A. Clearfield and U. Costantino, Layered metal phosphates and their intercalation chemistry in two and three-dimensional inorganic networks, in Comprehensive Supramolecular Chemistry; G. Alberti and T. Bein, ed.; Pergamon, Elsevier Science Ltd Press: New York, 1996; Vol. 7, Chapter 4 Search PubMed.
- A. Clearfield, Annu. Rev. Mater. Sci., 1984, 14, 205–229 CrossRef CAS.
- M. Casciola, G. Alberti, A. Donnadio, M. Pica, F. Marmottini, A. Bottino and P. Piaggio, J. Mater. Chem., 2005, 15, 4262–4267 RSC.
- C. Laberty-Robert, K. Vallé, F. Pereira and C. Sanchez, Chem. Soc. Rev., 2011, 40, 961–1005 RSC.
- D. H. Jung, S. U. Peck, D. R. Shin and J. S. Kim, J. Power Sources, 2003, 118, 205–211 CrossRef CAS.
- F. Bauer and M. W. Porada, J. Power Sources, 2005, 145, 101–107 CrossRef CAS.
- G. Alberti, M. Casciola, D. Capitani, A. Donnadio, R. Narducci, M. Pica and M. Sganappa, Electrochim. Acta, 2007, 52, 8125–8132 CrossRef CAS.
- H.-C. Kuan, C.-S. Wu, C.-Y. Chen, Z.-Z. Yu, A. Dasari and Y.-W. Mai, Electrochem. Solid-State Lett., 2006, 9, A76–A79 CrossRef CAS.
- Y. Si, H. R. Kunz and J. M. Fenton, J. Electrochem. Soc., 2004, 151, A623–A631 CrossRef CAS.
- W.-F. Chen and P.-L. Kuo, Macromolecules, 2007, 40, 1987–1994 CrossRef CAS.
- W.-F. Chen, J.-S. Wu and P.-L. Kuo, Chem. Mater., 2008, 20, 5756–5767 CrossRef CAS.
- W. G. Grot and G. Rajendran, US Patent 5919583, 1999.
- A. Clearfield and J. A. Stynes, J. Inorg. Nucl. Chem., 1964, 26, 117–129 CrossRef CAS.
- R. C. T. Slade, J. A. Knowles, D. J. Jones and J. Rozie're, Solid State Ionics, 1997, 96, 9–19 CrossRef CAS.
- G. Alberti, M. Casciola, U. Costantino and M. Leonardi, Solid State Ionics, 1984, 14, 289–295 CrossRef CAS.
- F. Odobel, D. Massiot, B. S. Harrison and K. S. Schanze, Langmuir, 2003, 19, 30–39 CrossRef CAS.
- L. E. Karlsson, B. Wesslén and P. Jannasch, Electrochim. Acta, 2002, 47, 3269–3275 CrossRef CAS.
- Y. Yang and S. Holdcroft, Fuel Cells, 2005, 5, 171–186 CrossRef CAS.
- A. K. Sahu, S. Pitchumani, P. Sridhar and A. K. Shukla, Fuel Cells, 2009, 2, 139–147 CrossRef.
- B. Yang, Y. Z. Fu and A. Manthiram, J. Power Sources, 2005, 139, 170–175 CrossRef CAS.
- D. H. Son, R. K. Sharma, Y. G. Shul and H. Kim, J. Power Sources, 2007, 165, 733–738 CrossRef CAS.
Footnote |
| † Electronic Supplementary Information (ESI) available: Experimental section; EDS patterns; solid-state NMR spectra; XPS spectra; DSC thermograms; electrode kinetic parameters; methanol permeability; durability. See DOI: 10.1039/c1ra00203a |
| This journal is © The Royal Society of Chemistry 2011 |
