Low temperature densification process of solid-oxide fuel cell electrolyte controlled by anode support shrinkage
Toshio
Suzuki
*,
Toshiaki
Yamaguchi
,
Koichi
Hamamoto
,
Hirofumi
Sumi
and
Yoshinobu
Fujishiro
National Institute of Advanced Industrial Science and Technology (AIST), Nagoya, Japan. E-mail: toshio.suzuki@aist.go.jp
First published on 14th September 2011
Abstract
A low temperature densification process of an electrolyte for solid-oxide fuel cells (SOFCs) has been developed by utilizing controlled electrode microtubular support shrinkage. This technology enables reduction of the co-sintering temperature for the electrolyte and the electrode (anode) support to temperatures as low as 1250 °C, resulting in the realization of a new anode microstructure using conventional, commercially available materials, NiO and Sc stabilized ZrO2. The new microtubular SOFC has shown high fuel cell performances from 0.36, 0.52, and 0.56 W cm−2 and energy efficiencies of 31, 44 and 47% (lower heating value) at 600, 650, and 700 °C respectively. Impedance analysis has also shown that the main contributing factor of the cell performance varies depending upon the operating temperatures, which is of importance for further optimization of the cell structures.
1. Introduction
Solid oxide fuel cells (SOFCs) are attractive future power sources due to their high energy efficiency.1–5 Because the SOFCs are operated at relatively high temperatures, between 800–1000 °C, it is possible to utilize the heat to steam-reform hydrocarbon fuel internally, resulting in high energy conversion efficiency. On the other hand, such high operating temperatures of SOFCs becomes a drawback due to reducing the lifetime of SOFC components including sealant, and interconnects, as well as requiring start up times of several hours. Thus, lowering the operation temperature is expected to accelerate early commercialization of SOFC systems since it could widen the variety of material used for the SOFC components, and enable quick start-up ability, which in turn allows for their use in applications such as domestic power sources, transportable power sources and auxiliary power units for automobiles.Recent developments of intermediate temperature SOFCs has mainly focused on new materials for the electrolyte and electrodes,6–11 since SOFC performance strongly depends upon the electrochemical properties of the electrode materials as well as the ionic conductivity of the electrolyte materials. While the search for new electrolyte and electrode materials continues, the use of a conventional zirconia based electrolyte is still attractive due to its long term stability and high reliability. In fact, SOFCs utilizing such materials have been shown to be operable below 700 °C by reducing the thickness of the electrolyte.12,13 Furthermore, lowering the cell operating temperature below 650 °C, utilizing ceria based or zirconia based electrolytes, was also succeed by introducing a microtubular design, which is considered to be one of the ideal shapes for real application since it has been shown to have high electrode area per unit volume, and high thermal stability under rapid heating.14–16 The microtubular SOFCs showed a power density of over 1 W cm−2 using gadolinia doped ceria (GDC)17 or scandia stabilized zirconia (ScSZ)18 with nickel cermet anode at/below 600 °C with controlled anode microstructure.
In order to control the microstructure of electrodes, typically, graphite (carbon powder) was selected as a pore-former19–21 and different kinds of pore-formers such as flour,22polymer (PVA) fibers,23 paper-fibres24 and polymethylmethacrylate (PMMA)17,18,25 were also investigated and showed promising electrode performance. Such microstructures were also strongly affected by the sintering process, where a lower processing temperature is preferable for obtaining high performance electrodes such as the one with high porosity.18 The typical sintering temperatures, however, especially for electrode supported cells requiring the co-sintering process, is 1400–1500 °C due to the requirement of electrolyte densification. The key to reduce the co-sintering temperature electrode could be found in the report showing that the shrinkage rate of the anode support strongly influenced the densification of the electrolyte when they were co-sintered.26
In this study, the fabrication process of the microtubular SOFCs has been reconstructed, (1) looking for a new anode pore-former allowing the control of the anode shrinkage during the co-sintering process, (2) and lowering the processing (co-sintering of the anode and electrolyte) temperature of the cell. For these purposes, a cellulose type pore-former was selected and the effect of the pore-former on the densification process of the electrolyte and anode microstructure, as well as the fuel cell performance, including fuel utilization and energy efficiency of the single cell, investigated.
2. Experimental
The micro tubular SOFCs we report here consist of nickel oxide–scandia stabilized zirconia (NiO/ScSZ) for the anode, ScSZ for the electrolyte, and La0.6Sr0.4Co0.2Fe0.8O3–gadolinia doped ceria (LSCF/GDC) for the cathode, with an inter-layer of GDC between the cathode and the electrolyte. Note that all materials used for the SOFC fabrication are commercially available powders.Fabrication
Anode tubes were made from a NiO powder (Sumitomo Metal Mining Co., Ltd.), an ScSZ powder (Daiichiki-genso Co., Ltd.), a microcrystalline cellulose powder (average particle size: 20 μm, density: 1.6 g cm−3, Asahi Kasei Chemicals Co., Ltd.) as a pore-former and a binder (Yuken Kogyo Co., Ltd.). The amount of the cellulose pore-former added in the mixture was determined to be the same volume of the PMMA pore-former (average particle size: 5 μm, density: 1.2 g cm−3) in the previous study.18 These powders were mixed for 1 h by a mixer 5DMV-r (Dalton Co., Ltd.), and after adding the correct amount of water, were stirred for 30 min in a vacuumed chamber. The mixture that was prepared from these powders was left to age for 15 h. The tubes were extruded, using the aforementioned extrudate, from a metal mould (2.4 mm diameter with 2.0 mm diameter pin) by using a piston cylinder type extruder (Ishikawa-Toki Tekko-sho Co., Ltd.). A slurry for dip-coating the electrolyte was prepared by mixing the ScSZ powder, solvents (toluene and ethanol), binder (poly vinyl butyral), dispersant (polymer of an amine system) and plasticizer (dioctyl phthalate) for 24 h. The anode tubes were dipped in the slurry and coated at the pulling rate of 1.0 mm s−1. The coated films were dried in air, and co-sintered at 1250–1400 °C for 1 h in air. An inter-layer slurry of a GDC powder (Shinetsu Kagaku, Co., Ltd.) was dip-coated on the electrolyte layer of the tube and sintered at 1100 °C. A cathode slurry of a LSCF powder (DOWA Electronics Materials, Co., Ltd.) and the GDC powder (LSCF/GDC) were dip-coated on the inter-layer. The SOFCs were completed by sintering at 1050 °C.Characterization
The microstructure of the electrodes and electrolyte of the tubular cell was observed by using a mercury porosimeter and a scanning electron microscope (SEM) (JEOL, JSM6330F). It is not possible however to determine the density of the electrolyte, since the electrolyte and the anode support are not separable. Thus, the density of the electrolyte is checked by the open circuit voltage measurement. The electrochemical performance of the cell co-sintered at 1250 °C was investigated by using a potentiostat (Solartron 1296) and impedance analyzer at the furnace temperature of 500–700 °C, which were monitored by a thermocouple placed at the sample. Detailed information of experimental set up was discussed elsewhere.27 The size of the cell of 1250 °C co-sintered at temperature was 1.65 mm in diameter and 30 mm in length with cathode length of 10 mm, and an effective electrode area of 0.52 cm2. Note that testing such small electrode area is still of importance, since the cell can be considered as the exact unit cell of the micro SOFC module. Ag wire was used for collecting current from the anode and cathode sides, which were both fixed using Ag paste. The current collection from the anode side was made from an edge of the anode tube, and the collection from the cathode side was made from the whole cathode area. Diluted hydrogen (20% H2 in Ar) was flowed inside of the tubular cell at a flow rate of 17–47 mL min−1(H2 flow rate: 6.5–18 mL min−1 per electrode area of 1 cm2). Dilute hydrogen can be considered as steam-reformed hydrocarbon fuel gas. Air was supplied at the cathode side at a flow rate of 100 mL min−1.3. Results and discussion
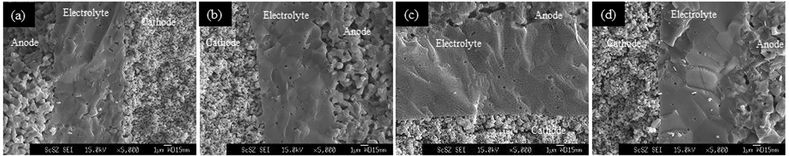
Fig. 1A–1D show cross-sectional fracture SEM image of the anode microstructure prepared at 1250, 1300, 1350, and 1400 °C co-sintering temperature, respectively. As can be seen, the grain growth of the NiO and ScSZ particles was observed, as the co-sintering temperature increased. If one compared this to the anode microstructure prepared using PMMA as pore-former18 as shown in Fig. 1E (co-sintering temperature = 1350 °C), the pore volume of the anodes with the cellulose pore-former apparently reduced even at a low co-sintering temperature of 1250 °C. Unlike that observed for the microstructure prepared using the PMMA pore-former, the microstructure prepared using the cellulose pore-former did not include apparent residual pores even though the particle size of the cellulose pore-former was about 20 μm. This can be explained by the high solubility of the cellulose pore-former in the water; it could become fine particles during the kneading process of the clay mixture. | ||
| Fig. 1 The cross-sectional fracture SEM image of the anode microstructure prepared using the cellulose pore-former at (a) 1250, (b) 1300, (c) 1350, and (d)1400 °C co-sintering temperature, and (e) the anode microstructure prepared using the PMMA pore-former (co-sintering temperature = 1350 °C). | ||
Fig. 2A shows the shrinkage rate and the final tube diameter of the anode tubes using the cellulose pore-former (without electrolyte layer) prepared at various sintering temperatures, along with those values of the anode tube using the PMMA pore-former, sintered at 1350 °C. The shrinkage rate of the anode tube using the cellulose pore-former is quite large, about 20% even at a sintering temperature of 1250 °C. As a result, the final tube diameter and the pore volume of the anode tube using the cellulose pore-former became about 1.65 mm and 0.1 cm3 g−1, respectively, much smaller than those of the anode tube using the PMMA pore-former.18 Accumulative pore volume of the anode tube was in the range of 0.02–0.1 cm3 g−1, while the samples prepared using the PMMA pore-former was in the range of 0.07–0.13 cm3 g−1. The peak size of pore was about 0.5 μm for all sintering temperatures and similar to the ones prepared using the PMMA pore-former. Table 1 summarized the porosity of the samples with various sintering temperatures. Even though the porosity of the samples using the cellulose pore-former is much smaller than those from the PMMA pore-former, there is an advantage of use of the cellulose pore-former that it is possible to realize a high shrinkage rate of the anode tube at lower sintering temperatures. Fig. 3A–3D show cross-sectional fracture SEM image of the electrolyte prepared at 1250, 1300, 1350, and 1400 °C co-sintering temperature, respectively. As can be seen, fully densified structures of the electrolyte were observed even at the low sintering temperature of 1250 °C, which was the result of the high shrinkage rate of the anode tube support. A high shrinkage rate of the anode tube prepared with the cellulose pore-former can be explained by the fact that the cellulose pore-former itself could absorb water and expand during the kneading process, which required more water than that prepared using the PMMA pore-former. As a result, the anode tube could shrink further during the sintering process. In order to confirm the effectiveness of the use of the cellulose pore-former, the electrochemical measurement was conducted for the sample prepared at 1250 °C, which had a reasonably high porosity of about 29% before reduction.
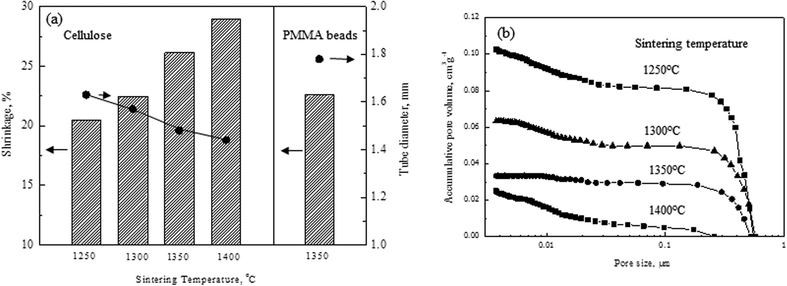 | ||
| Fig. 2 (a) The shrinkage rate and the final tube diameter of the anode tubes prepared using the cellulose pore-former at various sintering temperatures, along with those of the anode tube prepared using the PMMA pore-former sintered at 1350 °C and (b) accumulative pore volume as a function of pore size for various sintering temperatures. | ||
 | ||
| Fig. 3 The cross-sectional fracture SEM image of the electrolyte on the anode support prepared using the cellulose pore-former at co-sintering temperatures of (a) 1250, (b) 1300, (c) 1350, and (d) 1400 °C. | ||
| Sintering temperature (°C) | Porosity (%) (Cellulose) | Porosity (%) (PMMA) |
|---|---|---|
| 1250 | 29 | 54 |
| 1300 | 19 | 47 |
| 1350 | 11 | — |
| 1400 | 9 | 37 |
Fig. 4 shows the performance of the microtubular SOFC prepared using the cellulose pore-former at a sintering temperature of 1250 °C at various fuel flow rates at furnace temperatures of (a) 600 °C, (b) 650 °C, and (c) 700 °C, respectively. The cell temperature of each furnace was 591, 642 and 695 °C, respectively, and were not affected by the fuel flow rates. As can be seen, the open circuit voltages (OCV) of the cell were over 1.1 V, which was considered to be the theoretical value and confirmed that the electrolyte was fully dense even at a co-sintering temperature of 1250 °C. The maximum power density of the cell increased as the furnace temperature and the fuel gas flow increased, from 0.36 to 0.41 W cm−2, from 0.52 to 0.77 W cm−2 and from 0.56 to 0.97 W cm−2 at 600, 650, 700 °C, respectively. Note that the performance of the cell at a lower fuel flow rate was simply limited by the concentration polarization due to low fuel concentration. At the fuel flow rate of 17 mL min−1, which converted to 6.5 mL min−1 of H2 per 1 cm2 of electrode area, it was equivalent to 0.94 A cm−2. On the other hand, it was observed that the performance of the cell at the low current density region did not change significantly as the fuel gas flow rate was changed. Thus, impedance analysis was employed near the OCV at various furnace temperatures using a gas flow rate of 47 mL min−1.
 | ||
| Fig. 4 The performance of the microtubular SOFC prepared using the cellulose pore-former at a sintering temperature of 1250 °C at various fuel flow rates using furnace temperatures of (a) 600 °C, (b) 650 °C, and (c) 700 °C. | ||
Fig. 5 shows impedance spectra of the microtubular SOFC prepared using the cellulose pore-former at a sintering temperature of 1250 °C obtained at various furnace temperatures, 600 °C, 650 °C, and 700 °C. As can be seen, two semi-circles were observed which generally correspond to the electrochemical processes (high frequency semi-circle (left)) and gas transport process (low frequency semi-circle (right)) in both the anode and cathode. The high frequency semi-circle was strongly influenced by the furnace temperature compared to the change in the low frequency semi-circle. Thus it can be said that the main contribution of the cell performance is the electrochemical processes such as charge transfer reactions in the anode and/or the cathode. Note that the overpotential for the gas transport process—the low frequency semi-circle—showed rather a small influence with the furnace temperature, and thus the understanding of the electrochemical property of the anode and cathode will be the key for further improvement of the fuel cell performance. These results also indicated that the cell structure must be optimized with consideration of the operating temperature.
 | ||
| Fig. 5 Impedance spectra of the microtubular SOFC prepared using the cellulose pore-former at a sintering temperature of 1250 °C obtained at various furnace temperatures, 600 °C, 650 °C, and 700 °C in the fuel flow rate of 47 mL min−1. | ||
Fig. 6 summarized the relationship between maximum energy efficiency/maximum power density for various furnace temperatures and the fuel flow rate. The energy efficiency (η) of lower heating value (LHV) was calculated by following equation,
 | (1) |
 | (2) |
 | (3) |
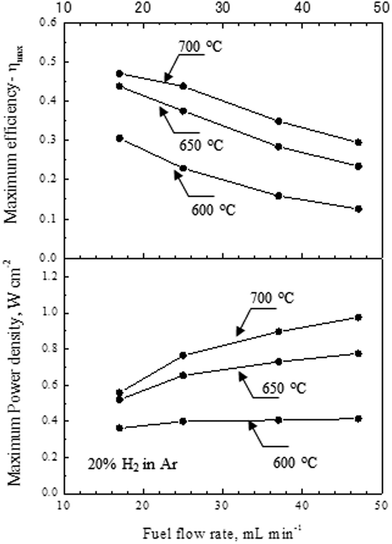 | ||
| Fig. 6 Summary of the maximum energy efficiency and the maximum power density of the microtubular SOFC prepared using the cellulose pore-former at a sintering temperature of 1250 °C as a function of fuel flow rate for various furnace temperatures. | ||
As can clearly be seen, the energy efficiency of the single cell reached over 30% at 600 °C, and 44% and 47% at 650 and 700 °C, respectively. Thus, the SOFC prepared at the lower co-sintering temperature of 1250 °C was able to realize high efficient operation in the intermediate temperature. To seek the possibility of lowering the operating temperature, the SOFCs were tested at furnace temperatures of 500 and 550 °C , corresponding to cell temperatures of 484 and 538 °C, respectively. Fig. 7 shows (a) I–V performance of the cell and (b) impedance spectra obtained at 500 and 550 °C, respectively. The fuel flow rate of 20% H2fuel gas was 47 mL min−1. As can be seen, the maximum power densities of the furnace temperatures were 0.03 and 0.13 W cm−2 for 500 and 550 °C, respectively. The huge drop in the performance at 500 °C can be explained by the large gas transport overpotential of the electrodes (anode and cathode) as shown in Fig. 7B. Such an increase in gas transport overpotential can be attributed to the change in the main contribution (one of the electrodes) of the overpotential, which could occur when the activation energy of the anode and cathode are different. Thus, further improvement of cell performance at lower temperature relied on the optimization of both the anode and cathode electrode microstructure.
 | ||
| Fig. 7 (a) I–V performance of the microtubular SOFC prepared using the cellulose pore-former at a sintering temperature of 1250 °C and (b) impedance spectra obtained at furnace temperatures of 500 and 550 °C. | ||
The results discussed in this paper were obtained from single cell measurements. Since the cell is the exact one used for module fabrication for the actual system, these results can be used for designing stack/module size as well as the system specification. After all, lowering the operating temperature is still a challenging issue from the energy efficiency point of view. It was shown that the limiting factor of the performance may vary depending upon the operating temperatures as shown in Fig. 5 and the direction of the optimization of the cell should be considered based on the operating temperature. After all, it can be said that improving the electrode microstructure is key for the development of SOFCs towards the commercialization.
4. Conclusions
A new microtubular fuel cell fabrication technology was developed using a cellulose pore-former in the hope of reducing the co-sintering temperature for an electrolyte and an anode support. It was shown that controlling the anode support shrinkage by using the cellulose pore-former was effective to realize fully dense electrolyte structure at a sintering temperature as low as 1250 °C. The microtubular SOFC used in this study, which was also the exact cell for stack/module, consisted of Ni/ScSZ for the anode, ScSZ for the electrolyte, GDC for the interlayer, and LSCF/GDC for the cathode, respectively, showed relatively high fuel cell performance from 0.36 to 0.41 W cm−2, from 0.52 to 0.77 W cm−2 and from 0.56 to 0.97 W cm−2 at 600, 650, 700 °C, respectively, depending upon the fuel supply. The energy efficiency of the microtubular SOFC was also studied in various experimental conditions. The energy efficiency increased as the temperature was increased, and reached over 30% (LHV), 44% and 47% at 600, 650 and 700 °C, respectively, in flowing 20% H2–Ar fuel inside the tube. Impedance analysis showed that the main contributing factor to the cell performance was the operating temperature. Further development of fabrication technique for optimizing the cell structure will improve the cell performance toward the commercialization of the SOFC system.References
- N. Q. Minh, J. Am. Ceram. Soc., 1993, 76, 563–588 CrossRef CAS.
- O. Yamamoto, Electrochim. Acta, 2000, 45, 2423 CrossRef CAS.
- S. C. Singhal, Solid State Ionics, 2002, 152–153, 405 CrossRef CAS.
- B. C. H. Steele and A. Heinzel, Nature, 2001, 414, 345 CrossRef CAS.
- H. Yokokawa, N. Sakai, T. Horita, K. Yamaji and M. E. Brito, MRS Bull., 2005, 30, 591 CrossRef CAS.
- J. Yan, H. Matsumoto, M. Enoki and T. Ishihara, Electrochem. Solid-State Lett., 2005, 8, A389 CrossRef CAS.
- Z. Shao and S. M. Haile, Nature, 2004, 431, 170 CrossRef CAS.
- S. Tao and J. T. S. Irvine, Nat. Mater., 2003, 2, 320 CrossRef CAS.
- K. Eguchi, T. Setoguchi, T. Inoue and H. Arai, Solid State Ionics, 1992, 52, 165 CrossRef CAS.
- T. Hibino, A. Hashimoto, K. Asano, M. Yano, M. Suzuki and M. Sano, Electrochem. Solid-State Lett., 2002, 5, A242 CrossRef CAS.
- E. D. Wachsman, Solid State Ionics, 2002, 152–153, 657 CrossRef CAS.
- H. Huang, M. Nakamura, P. C. Su, R. Fasching, Y. Saito and F. B. Prinz, J. Electrochem. Soc., 2007, 154, B20 CrossRef CAS.
- S. de Souza, S. J. Visco and L. C. DeJohnge, J. Electrochem. Soc., 1997, 144, L35 CrossRef CAS.
- N. M. Sammes, Y. Du and R. Bove, J. Power Sources, 2005, 145, 428 CrossRef CAS.
- K. Kendall and M. Palin, J. Power Sources, 1998, 71, 268 CrossRef CAS.
- K. Yashiro, N. Yamada, T. Kawada, J. Hong, A. Kaimai, Y. Nigara and J. Mizusaki, Electrochemistry, 2002, 70, 958 CAS.
- T. Suzuki, Y. Funahashi, T. Yamaguchi, Y. Fujishiro and M. Awano, Electrochem. Solid-State Lett., 2007, 10, A177 CrossRef CAS.
- T. Suzuki, Md. Z. Hasan, Y. Funahashi, T. Yamaguchi, Y. Fujishiro and M. Awano, Science, 2009, 325, 852 CrossRef CAS.
- L. Zhang, S.P. Jiang, W. Wang and Y. J. Zhang, J. Power Sources, 2007, 170, 55 CrossRef CAS.
- R. M. C. Clemmer and S. F. Corbin, Solid State Ionics, 2004, 166, 251 CrossRef CAS.
- F. Bozza, R. Polini and E. Traversa, Electrochem. Commun., 2009, 11, 1680 CrossRef CAS.
- K. F. Chen, Z. Lu, X. J. Chen, N. Ai, X. Q. Huang, B. Wei, J. Y. Hu and W. H. Su, J. Alloys Compd., 2008, 454, 447 CrossRef CAS.
- W. P. Pan, Z. Lu, K. F. Chen, X. Q. Huang, B. Wei, W. Y. Li, Z. H. Wang and W. H. Su, Electrochim. Acta, 2010, 55, 5538 CrossRef CAS.
- W. P. Pan, Z. Lu, K. F. Chen, X. Q. Huang, B. Wei, W. Y. Li, Z. H. Wang and W. H. Su, Fuel Cells, 2011, 11, 172 CrossRef CAS.
- J. C. Ruiz-Morales, J. Canales-Vazquez, J. Pena-Martinez, D. Marrero-Lopez and P. Nunez, Electrochim. Acta, 2006, 52, 278 CrossRef CAS.
- T. Yamaguchi, T. Suzuki, S. Shimizu, Y. Fujishiro and M. Awano, J. Membr. Sci., 2007, 300, 45 CrossRef CAS.
- T. Suzuki, T. Yamaguchi, Y. Fujishiro and M. Awano, J. Power Sources, 2006, 160, 73 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2011 |
