Blue phosphorescent iridium(III) complexes containing carbazole-functionalized phenylpyridine for organic light-emitting diodes: energy transfer from carbazolyl moieties to iridium(III) cores†
Hoe-Joo
Seo
a,
Myungkwan
Song
b,
Sung-Ho
Jin
b,
Jung Hei
Choi
a,
Seong-Jae
Yun
a and
Young-Inn
Kim
*b
aDepartment of Chemistry, Pusan National University, Jangjeon-dong Kumjeong-gu, Busan, 609-735, Korea
bDepartment of Chemistry Education and Interdisciplinary Program of Advanced Information and Display Materials, Pusan National University, Jangjeon-dong Kumjeong-gu, Busan, 609-735, Korea. E-mail: yikim@pusan.ac.kr; Fax: +82 51 581 2348
First published on 8th September 2011
Abstract
By introducing a hexylcarbazolyl substituent into a phenylpyridine-based ligand, a Förster energy transfer from the carbazole moieties to the Ir(III) cores was observed in the heteroleptic cyclometalated Ir(III) complexes, resulting in an enhancement of luminescent efficiency. The phosphorescent OLED gave CIE coordinates of (0.21, 0.55) with a maximum external quantum efficiency of 7.16%.
Cyclometalated phosphorescent Ir(III) complexes have been of great interest in the study of modern organic light-emitting diodes (OLEDs)1 since colour and emission efficiency were first tuned by structural modification of chromophore ligands. This was caused by a strong spin–orbit coupling in the presence of heavy metals, leading to a mixing of singlet and triplet manifolds so that both singlet and triplet excitons could be harvested.2 Recently, we prepared highly efficient deep-blue heterocyclometalated Ir(III) complexes by modulating the electron density of a phenylpyridine-based ligand and suggested them as potential materials for phosphorescent OLEDs.3 However, the performance of the device was influenced by a balance of the emitting guest Ir(III) complexes in a blend with a host material, such as small-molecule 4,4′-bis(9-carbazolyl)-2,2′-biphenyl (CBP) or polymer poly(N-vinylcarbazole) (PVK), of high triplet energy and good hole-transporting ability. Many Ir(III) complexes containing the modified phenylpyridine, through introduction of the carbazole, have been reported to study the effects of the improved hole transition properties and to decrease the triple–triple annihilation under nondoped conditions when used as an emitter.4 For example, Zhang and co-workers have reported linkage isomeric Ir(III) complexes, [Ir(2-PhPyCz)2(acac)] and [Ir(3-PhPyCz)2(acac)] (2- and 3-PhPyCz = 2- and 3-[4′-(2′′-phenylpyridinyl)]-N-[2-ethylhexyl] carbazole), which produced a maximum current efficiency of 20.1 and 22.4 cd A−1, corresponding to an external quantum efficiency of 8.94 and 9.90%, respectively.5
Herein, the authors designed a long-chain hexylcarbazolyl moiety that was then introduced at the 3-position of the phenyl ring in 2-(2′,4′-difluorophenyl)-4-methylpyridine (dfpmpy). Novel blue Ir(III) complexes containing two 2-(2′,4′-difluoro-3′-hexylcarbazolylphenyl)-4-methylpyridine (HL) as cyclometalated ligands and one ancillary ligand (L′⁁X): Ir(L)2(L′⁁X), L′⁁X = acetylacetone (1), picolinic acid N-oxide (2) and biimidazole (3) was also synthesized. The authors expected the carbazole-containing Ir(III) complexes to possess dual functions, which showed stable blue emissions and improved charge-transporting properties, Förster transfers, and morphological stability.
The synthetic method (Scheme S1) and details are given in the supplementary information (ESI).† Full characterization included NMR spectroscopy and elemental analysis (C, H, N), with HL confirmed by X-ray single crystal structure determination.‡
Single crystals of HL were grown from a methanol/chloroform solution by slow evaporation at room temperature. The ORTEP diagrams are shown in Fig. 1. The bond lengths of C11–C13 and C18–N2 were 1.505(2) and 1.453(2) Å, respectively. C11–C13 was shorter than the single bond length of C–C (1.52–1.53 Å), while C18–N2 was similar to the single bond length of C–N (∼1.43 Å). The dihedral angle between the mean plane C2/C3/C4/N1/C5/C6 and C7/C8/C9/C10/C11/C12 was 6.15°, which was nearly coplanar and caused by an interaction between the C6 hydrogen atom and the F2 fluorine atom, at a distance of 2.161 Å and contributing less to the steric hindrance.6 The HL had a plane-to-plane separation of 5.057 Å, showing a weak interaction between the C1 hydrogen atom and the neighbouring F2 fluorine atom of 2.820 Å.
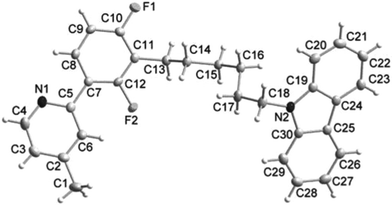 | ||
| Fig. 1 ORTEP diagram of L. Selected bond lengths (Å) and bond angles (°); C10–C11 1.381(2), C11–C13 1.505(2), C18–N2 1.453(2), C19–N2 1.388(2), C5–C7–C8 119.54(15), C10–C11–C12 114.92(15), C10–C11–C13 122.99(16), C18–N2–C19 126.84(14), N2–C19–C20 129.16(16). | ||
Fig. 2 shows the absorption spectra of the main ligand (HL) and its heteroleptic cyclometalated Ir(III) complexes (1, 2, 3) in dichloromethane, along with the photoluminescence (PL) spectrum of pure HL. The results are in Table S1. The intense absorption bands in the ultraviolet region below 320 nm were attributed to spin-allowed 1π–π* ligand-centred (LC) transitions of the HL. The broad bands at the longer wavelengths of 330–370 nm range were conventionally assigned to the interligand energy transfer transition and the metal-to-ligand charge transfer (1MLCT) transition in the singlet manifold. The lower energy absorptions that extend to the visible region (ca. 500 nm) can be assigned to the spin-forbidden 3MLCT transition and a spin–orbital coupling enhanced 3π–π* transition.7 The absorption intensity of the Ir(III) complexes increased by a factor of 3–4 compared to that of free HL.
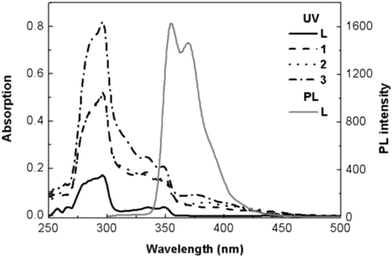 | ||
| Fig. 2 Absorption spectra of Ir(III) complexes and PL spectra of HL in solution (1.0 × 10−5 M in CH2Cl2). | ||
The photoluminescence (PL) spectra of the Ir(III) complexes (1, 2, 3) in dichloromethane are presented in Fig. S1. Fig. S1(a) and S1(b) are the PL spectra excited at 380 nm and 300 nm, respectively. The results are summarized in Table S1. The emission peaks near 470 nm, examined in both Fig. S1(a) and S1(b), were assigned to arise from the Ir(III) cores. The emission peak near 360 nm in Fig. S1(b) was believed to be from the carbazole unit in the Ir(III) complexes, since a similar emission peak was observed in the corresponding HL ligand. Furthermore, the luminescent intensities from the Ir(III) cores in Fig. S1(b) were stronger by 3–7 times than those in Fig. S1(a), under identical conditions. This observation supported the conclusion that an efficient energy transfer (ET) from the carbazole moieties to the Ir(III) cores was achieved, resulting in an increase in the luminous intensity without a change in the emission colour. This energy transfer led to photoluminescent quantum efficiencies of 18–26% in the carbazole-functionalized Ir(III) complexes.
To confirm whether the intermolecular energy transfer existed, this lab investigated the emissions in films at room temperature as shown in Fig. 3. The emission peak at 355 nm from the carbazole unit was nearly quenched in films, implying that an efficient intermolecular energy transfer from the carbazole unit to the Ir(III) core was achieved, resulting from the decreased distances between the molecules in films. The emission wavelengths in films were nearly identical to those in solution at room temperature. The lower energy shoulders (ca. 550 nm) in Fig. 3 are typical of vibrational energy levels of mixed MLCT/π–π* transitions.8 The calculated quantum yields in films are listed in Table S1. The quantum yields in films are larger than those of solutions, leading to an improvement of the device performances.
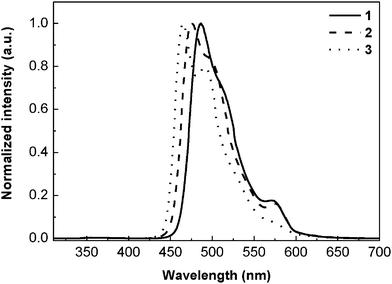 | ||
| Fig. 3 PL spectra of Ir(III) complexes in 5 wt% doped PMMA films. | ||
Cyclic voltammetry (CV) experiments were carried out to study the electrochemical behaviour of the Ir(III) complexes (Fig. S2). The experimental results are in Table S2. The cyclic voltammograms showed two chemically quasi-reversible oxidation waves in the range of 0.88 to 1.39 V versusAg/AgNO3 in CH3CN. The oxidation potentials at ca. 0.90 V could be assigned to metal-centred Ir(III)/Ir(IV) couples in the studied Ir(III) complexes.9 The neutral Ir(III) complexes with anionic ancillary ligands were oxidized more readily (0.96 and 0.93 V for 1 and 2, respectively) than the cationic Ir(III) complex 3 (0.88 V), due to the higher electron density on 1 and 2 than 3, indicating that 1 and 2 exhibited more negative HOMO energy levels in comparison to 3 (−5.68, −5.65 and −5.60 eV for 1, 2 and 3, respectively). The carbazole-based, ligand-centred oxidation potentials were clearly in the range of 1.32 to 1.39 V since the similar oxidation potential was observed in the free main ligand (HL) at 1.36 V. This indicated that the carbazole unit had no substantial effect upon the electrochemical properties of the iridium-cored complexes. No reduction waves were observed under the experimental conditions.
The thermal stabilities of the Ir(III) complexes were evaluated using thermogravimetric analysis (TGA) under a nitrogen atmosphere. The 5% weight loss temperatures indicated that the studied Ir(III) complexes exhibited excellent thermal stabilities over 370 °C (Table S1), which is required for an electroluminescent device.
Typical OLED devices of 1, 2 and 3 were fabricated by spin-coating from a chlorobenzene solution, to give a final configuration of ITO/PEDOT:PSS (40 nm)/PVK:OXD-7:UGH3 + Ir(III) complex (70 nm)/OXD-7 (20 nm)/Ba (3 nm)/Al (100 nm), where 8 wt% of the Ir(III) complexes were used as a dopant, the PVK:OXD-7:UGH3 (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was used as a host, and OXD-7 was used as both the hole blocking and electron transport layer. The host:Ir(III) complex films showed a good uniform dispersion of the dopants. The energy diagram of the electrophosphorescent devices is shown in Fig. S3.
1) was used as a host, and OXD-7 was used as both the hole blocking and electron transport layer. The host:Ir(III) complex films showed a good uniform dispersion of the dopants. The energy diagram of the electrophosphorescent devices is shown in Fig. S3.
The current density–voltage–luminance (J–V–L) curves and luminance and power efficiencies of the OLEDs prepared from the studied Ir(III) complexes are shown in Fig. S4 and S5, respectively. The results are summarized in Table 1. The device of 1 demonstrates the best performance with a maximum external quantum efficiency (EQE) of 7.16%, a maximum luminance efficiency (LE) of 20.75 cd A−1 at 10 mA cm−2, a maximum luminance (L) of 2286 cd m−2, and an 8 V turn-on voltage. The highest EQE of 1 can be explained by the fact that the LUMO triplet energy level (ET,LUMO) of 1, calculated from the PL spectrum at 77 K (2.42 and 2.59 eV), as shown in Fig. S3, is lower than those of the hosts, resulting in better charge transport within device 1, whereas the ET,LUMO of 2 and 3 are higher than those of PVK and OXD-7. Fig. 4 shows the electroluminescence (EL) spectra using the prepared devices. The EL spectra were red shifted by nearly 3–9 nm, compared with the PL spectra in films, and the second stronger emission peaks were observed in the region of 490–510 nm. The emission peaks from the carbazole moieties were not found in the EL spectra, demonstrating that an energy transfer from the carbazole unit to the iridium-based luminophore was allowed under the experimental electrical excitations. The Commission International de L'Éclairage (CIE) (x, y) coordinates were calculated to be (0.21, 0.55), (0.23, 0.41) and (0.21, 0.48) for complexes 1, 2 and 3, respectively.
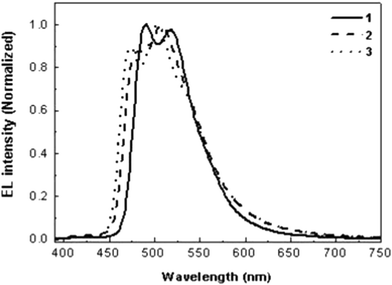 | ||
| Fig. 4 Electroluminescence spectra of Ir(III) complexes at a current density of 10 mA cm−2. | ||
In summary, we have reported three blue phosphorescent carbazolyl-substituted phenylpyridine-based Ir(III) complexes and observed an energy transfer from the carbazolyl moieties to the Ir(III) cores. This approach offers advantages in terms of obtaining a wide triplet band gap and enhancing luminescent efficiencies without a red shift.
This work was supported by National Research Foundation of Korea Grant funded by the Korean Government (No. 2010-0017080).
References
- (a) C. W. Tang and S. A. Vanslyke, Appl. Phys. Lett., 1987, 51, 913 CrossRef CAS; (b) M. A. Baldo, S. Lamansky, P. E. Burrows, M. E. Thompson and S. R. Forrest, Appl. Phys. Lett., 1999, 75, 4 CrossRef CAS; (c) Y. Chi and P.-T. Chou, Chem. Soc. Rev., 2007, 36, 1421 RSC; (d) Z. Liu, Z. Bian, F. Hao, D. Nie, F. Ding, Z. Chen and C. Huang, Org. Electron., 2009, 10, 247 CrossRef CAS; (e) S.-L. Lai, S.-L. Tao, M.-Y. Chan, M.-F. Lo, T.-W. Ng, S.-T. Lee, W.-M. Zhao and C.-S. Lee, J. Mater. Chem., 2011, 21, 4983 RSC; (f) E. Baranoff, S. Fanttacci, F. D. Angelis, X. Zhang, R. Scopelliti, M. Gatzel and M. K. Nazeeruddin, Inorg. Chem., 2011, 50, 451 CrossRef CAS; (g) S. Aoki, Y. Matsuo, S. Ogura, H. Ohwada, Y. Hisamatsu, S. Moromizato, M. Shiro and M. Kitamura, Inorg. Chem., 2011, 50, 806 CrossRef CAS.
- (a) K. Dedeian, J. Shi, N. Shepherd, E. Forsythe and D. C. Morton, Inorg. Chem., 2005, 44, 4445 CrossRef CAS; (b) L.-L. Wu, C.-H. Yang, I-W. Sun, S.-Y. Chu, P.-C. Kao and H.-H. Huang, Organometallics, 2007, 26, 2017 CrossRef CAS.
- H.-J. Seo, K.-M. Yoo, M. Song, S.-H. Jin, Y.-I. Kim and J.-J. Kim, Org. Electron., 2010, 11, 564 CrossRef CAS.
- (a) X. Zhang, Z. Chen, C. Yang, Z. Li, K. Zhang, H. Yao, J. Qin, J. Chen and Y. Cao, Chem. Phys. Lett., 2006, 422, 386 CrossRef CAS; (b) W.-Y. Wong, C.-L. Ho, Z.-Q. Gao, B.-X. Mi, C.-H. Chen, K.-W. Cheah and Z. Lin, Angew. Chem., Int. Ed., 2006, 45, 7800 CrossRef CAS; (c) S. Bettington, M. Tavasli, M. R. Bryce, A. Beeby, H. Al-Attar and A. P. Monkman, Chem.–Eur. J., 2007, 13, 1423 CrossRef CAS; (d) C.-L. Ho, W.-Y. Wong, Q. Wang, D. Ma, L. Wang and Z. Lin, Adv. Funct. Mater., 2008, 18, 928 CrossRef CAS; (e) C.-L. Ho, Q. Wang, C.-S. Lam, W.-Y. Wong, D. Ma, L. Wang, Z.-Q. gao, C.-H. Chen, K.-W. Cheah and Z. Lin, Chem.–Asian J., 2009, 4, 89 CrossRef CAS.
- K. Zhang, Z. Chen, C. Yang, X. Zhang, Y. Tao, L. Duan, L. Chen, L. Zhu, J. Qin and Y. Cao, J. Mater. Chem., 2007, 17, 3451 RSC.
- L.-L. Wu, C.-H. Yang, I.-W. Sun, S.-Y. Chu, P.-C. Kao and H.-H. Huang, Organometallics, 2007, 26, 2017 CrossRef CAS.
- (a) A. P. Wilde, K. A. King and R. J. Watts, J. Phys. Chem., 1991, 95, 629 CrossRef CAS; (b) M. C. Columbo, A. Hauser and H. U. Güdel, Top. Curr. Chem., 1994, 171, 143 Search PubMed.
- (a) D. W. Samuel, J. Am. Chem. Soc., 2009, 131, 16681 CrossRef; (b) C.-H. Yang, Y.-M. Cheng, Y. Chi, C.-J. Hsu, F.-C. Fang, K.-T. Wong, P.-T. Chou, C.-H. Chang, M.-H. Tsai and C.-C. Wu, Angew. Chem., Int. Ed., 2007, 46, 2418 CrossRef CAS.
- B.-L. Li, L. Wu, Y.-M. He and Q.-H. Fan, Dalton Trans., 2007, 2048 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available: details of experimental procedures, characterisation of HL, spectroscopic and EL device data for all compounds reported. CCDC reference number 771903. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c1ra00140j |
| ‡ Crystal data for HL: C30H28F2N2, Mr = 454.54, orthorhombic, space groupP212121, a = 5.0573(13), b = 14.471(4), c = 31.891(9) Å, V = 2333.9(11) Å3, Z = 4, T = 293(2) K, 4091 reflections (Rint = 0.0392), R1 (final) = 0.0335, wR2 = 0.0784, GoF = 1.082 for 3884 reflections (I > 2σ(I)) and 307 parameters. CCDC 771903. |
| This journal is © The Royal Society of Chemistry 2011 |
