One-pot alkaline vapor oxidation synthesis and electrocatalytic activity towards glucose oxidation of CuO nanobelt arrays†
Tetsuro
Soejima
*ab,
Hitomi
Yagyu
a,
Nobuo
Kimizuka
bc and
Seishiro
Ito
a
aDepartment of Applied Chemistry, School of Science and Engineering, Kinki University, 3-4-1 Kowakae, Higashi-osaka, Osaka, 577-8502, Japan. Tel: +81-6-6721-2332; Fax: +81-6-6721-2024; E-mail: soejima@apch.kindai.ac.jp
bCREST, Japan Science and Technology Agency, 744 Moto-oka, Nishi-ku, Fukuoka, 819-0395, Japan
cDepartment of Chemistry and Biochemistry, Graduate School of Engineering, Kyushu University, 744 Moto-oka, Nishi-ku, Fukuoka, 819-0395, Japan
First published on 10th August 2011
Abstract
CuO nanobelt arrays supported on copper substrates are synthesized by a simple and one-pot low-temperature vapor oxidation method. The CuO nanobelt arrays show high electrocatalytic activity towards glucose oxidation.
Cupric oxide (CuO) compounds have recently received significant attention due their application in important devices, including high-temperature semiconductors1 and magnetic storage media.2 CuO is a p-type semiconducting material (Eg = 1.2 eV) that exhibits field emission,3 photovoltaic,4 photoconductive,5 and gas-sensing properties.6 Due to its photochemical and (photo)conductive properties, CuO has been exploited for photocatalysts,7 solar cells,8 and lithium ion batteries.9 Additionally, CuO materials facilitate the catalysis of many reactions including cross-couplings,10 CO and NO oxidation,11 propylene oxidation to acrolein,12 and olefin epoxidation.13
Recently, arrayed one- or two-dimensional nanocrystals (nanowires, nanosheets, nanobelts), nanoarrays, have attracted considerable interest14 because of their potential for a variety of different applications, such as nanolasers,15 dye-sensitized solar cells,16 lithium ion batteries,17 nano-generators,18 unique adhesive nanomaterials,19 and optical sensors.20 The design and fabrication of nanoarrays is essential for the creation of smart, high-performance nanodevices. To date, CuO nanoarrays have been prepared by several methods. For instance, CuO nanoribbons were obtained by the heat treatment of Cu(OH)2 nanoribbons at 180 °C with a constant flow of N2.21 Xia et al. reported the high-temperature (500 °C) synthesis of CuO nanowires supported on the surfaces of various copper substrates including grids, foils, and wires.22 Thus, relatively high-temperatures and/or a controlled atmosphere are necessary to form CuO crystals in the gas-phase. CuO nanoarray structures have also been formed at low temperatures (<60 °C) in alkaline aqueous solutions. However, the liquid-phase synthetic methods require a long reaction time (>20 h) to form complete CuO nanoarrays.23–24
In this paper, we report a novel one-pot and low-temperature synthetic route to CuO nanobelt arrays on a Cu substrate. The simple growth approach via alkaline vapor oxidation (VO, Fig. S1†)25 under mild and facile conditions makes it possible to rapidly fabricate CuO nanobelts compared with those of liquid-phase synthetic methods. Additionally, CuO/ZnO composite nanoarrays can be obtained utilizing the VO process. The CuO nanobelt arrays/Cu electrode shows excellent oxidation of glucose in a basic solution and high sensitivity as a nonenzymatic glucose sensor.
To synthesize the nanobelt arrays, a copper plate was half immersed in an aqueous solution of 100 mM NH3–100 mM H2O2 at an angle against the wall of a glass vial (Fig. 1A) and kept at 80 °C for 7 h in a laboratory drying oven. The sample was then washed with distilled water and dried in air at room temperature. Fig. 1B shows the changes in the appearance of the Cu plate before and after the reaction. There was no significant change in the color of the portion that was immersed in the NH3–H2O2 aqueous solution. On the other hand, the surface of the Cu plate that was not immersed became entirely black. The field-emission scanning electron microscopy (SEM) image of the Cu plate before the reaction showed a smooth surface (Fig. S2†). Interestingly, nanobelt arrays were densely formed on the surface of the non-immersed portion (Fig. 1C), which was quite different from the rough surface of the immersed portion (Fig. S3B). The crystal structures of the samples were investigated by measuring the X-ray diffraction patterns (XRD). The XRD pattern of the immersed portion only showed the presence of Cu (Fig. S3C,† JCPDS No. 4-836). However, the XRD pattern of the non-immersed portion featured two diffraction patterns attributed to Cu and CuO (JCPDS No. 5-661) (Fig. 1D). Thus, the CuO nanobelt arrays were only formed on the non-immersed portion of the Cu plate by the VO process.
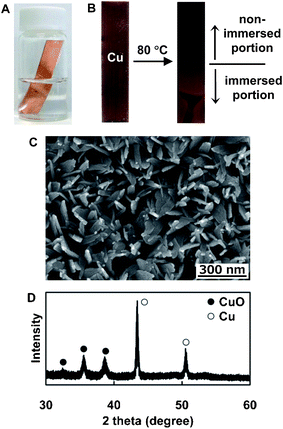 | ||
| Fig. 1 (A) A picture of the screw vial containing NH3–H2O2 aqueous solution and an immersed Cu substrate during the reaction. (B) Pictures of the Cu plate before (left) and after (right) the reaction. An SEM image (C) and XRD pattern (D) of CuO nanobelt arrays formed on the non-immersed portion of the Cu plate by the VO reaction. | ||
Fig. S4, S5 and S6 show color changes of copper substrates and SEM images and XRD patterns of the non-immersed portion obtained at different reaction periods, respectively.† The only non-immersed portion gradually changed to black, indicating formation of CuO nanoarrays. Tiny thorn-like nanostructures formed on the Cu substrate within 5 min and further heating led to the growth of quasi one-dimensional nanowire arrays (from 5 min to 1 h). These structures did not show any X-ray diffraction peaks besides those attributed to Cu, which indicates that they are amorphous. After 3 h, nanobelt arrays formed and their density increased with increasing reaction time (Fig. S5D–E†). The XRD diffraction pattern of the nanobelts corresponds to the CuO crystal (Fig. S6D–E).† The formation of CuO nanobelt arrays did not require immersion of Cu plate into the reaction media. Instead, the CuO nanobelt structures formed on the underside of the Cu plate as shown in Fig. S7.† This indicates that NH3 (and/or H2O2) vapor was involved in the formation of the CuO nanobelt arrays.
Fig. 2 shows a plausible formation mechanism for CuO nanobelt arrays on a Cu substrate. Water vapor containing NH3 was generated by heating an NH3 aqueous solution at 80 °C and then was absorbed onto the surface of the Cu plate. The Cu substrate was oxidized to Cu(II) by the O2 adsorbed from the gas phase and copper ammine complexes were formed by the reaction of the Cu(II) ion and ammonia. Under basic conditions (pH = 11.2), OH− replaces NH3 in the Cu[NH3]n2+ complex giving rise to square planar Cu(OH)42− units. It has been reported that Cu(OH)2 nanoribbons preferentially grow along the [100] direction by the assembly of >Cu(OH)2Cu< chains.21 Accordingly, the Cu(OH)2 nanobelt arrays formed by one-dimensional assembly of the Cu(OH)42− units. After further heat treatment at 80 °C, the Cu(OH)2 nanobelt arrays rapidly transformed to CuO. The XRD signals attributed to Cu(OH)2 crystal were not found for all samples which were obtained at varied reaction periods. It has been reported that Cu(OH)2 is a metastable phase which easily transforms into CuO more stable, either in the solid state by a thermal dehydration or at room temperature in aqueous basic solutions.26 Unfortunately in the present synthetic system, however, observation of metastable Cu(OH)2 is hindered by the rapid transformation process at 80 °C. The growth reactions continuously occurred during the heat treatment, and the perfect CuO nanoarrays were obtained. On the other hand, significant nanostructures were not formed on the immersed portion (Fig. S3†). The amount of copper in the NH3/H2O2 aqueous solutions after the VO reaction was determined by ICP-AES measurements, as shown in Fig. S8.† The cocentration of copper was increased with increasing reaction time. It is assumed that the immersed portion of the Cu substrate was oxidized by dissolved oxygen and soluble copper-amine complexes were formed in the aqueous solution. Thus, nanoarray structures could not be obtained on the immersed portion.
 | ||
| Fig. 2 Plausible formation mechanism of CuO nanobelt arrays by the VO process. | ||
The formation of CuO nanobelt arrays requires the presence of NH3 vapor. Fig. S9, S10 and S11 show changes in appearance of copper substrates and the SEM images and XRD patterns, respectively, of a Cu plate immersed in an NH3 aqueous solution at 80 °C.† CuO nanobelt arrays gradually formed on the Cu plate. The addition of H2O2 enhances the formation of the CuO nanobelt arrays (Fig. S5A–B vs. Fig. S10A–B†). It is assumed that the H2O2 molecules are involved in the oxidation process of Cu by supplying O2via thermal decomposition (H2O2(aq) → H2O + O2(g)) and by the following direct redox reaction:27
| Cu = Cu2+ + 2e− +0.340 V vs. SHE | (1) |
| H2O2 + 2H+ + 2e− = 2H2O +1.763 V vs. SHE | (2) |
The rapid formation of Cu2+ in the initial reaction stage increased the rate of CuO nanobelt formation. The pH of the NH3 aqueous solution also affects the formation of CuO nanobelt structures. Fig. S12 shows SEM images of the Cu plate after the VO process in an NH3 aqueous solution with different pH values.† At pH 2, the nanobelt structures barely formed; however, the formation density of plate-like nanostructures increased with increasing pH. This indicates that OH− ions play a critical role in the growth of CuO nanobelts. It is likely that the precursor Cu(OH)2 nanostructures did not form under the low pH conditions.
CuO/metal oxides hetero-composite nanomaterials have also attracted much attention due to their fascinating semiconducting and catalytic properties.28 A brass plate (Cu–Zn alloy) was partly immersed in a 100 mM NH3 aqueous solution and the sample was heated at 80 °C for 24 h. The color of the non-immersed portion of the brass plate changed to black indicating the formation of CuO/ZnO nanobelt/nanorod composites (Fig. S13†). It is expected that various CuO/metal oxide nanoarrays can be obtained from Cu-based alloys using the VO synthetic method.
The electrocatalytic activity of the CuO nanobelt arrays/Cu electrode towards the oxidation of glucose in an alkaline solution was investigated. The cyclic voltammograms (CVs) of the CuO nanobelt arrays/Cu electrode in different concentrations of glucose were measured, as shown in Fig. S14.† In the alkaline solution, a broad reduction with a peak potential of about 0.3 V vs. Ag/AgCl was observed. This wave might be attributed to a Cu(II)/Cu(III) redox reaction.29 Upon addition of glucose, the electrode exhibited significant oxidation of the glucose starting at ca. 0.25 V vs. Ag/AgCl with a shoulder peak at 0.6 V vs. Ag/AgCl; the response was concentration-dependent. Thus, the CuO nanobelt arrays/Cu electrode is electrocatalytically active towards glucose oxidation. This may be attributed to the proposed involvement of Cu(II) and Cu(III) surface species in the oxidation of glucose.30Fig. 3 shows a typical amperometric response of the CuO nanobelt/Cu electrode to the successive addition of glucose at an applied potential of 0.6 V vs. Ag/AgCl. The CuO/Cu electrode responded to the changes in glucose concentration and shows fast response, i.e., the response reaches 95% of the steady-state value within 2 s. The corresponding calibration curve for the glucose sensor is shown in Fig. 3B. The sensor shows a sensitivity of 582.0 μA mM−1 cm−2 and a detection limit of less than 1 μM. The performance of the CuO nanobelt sensor was compared with other nonenzymatic glucose sensors, as shown in Table S1.† The sensitivity and detection limit of the CuO nanobelt/Cu electrode are higher and lower, respectively, than those of the other glucose sensors. This is partly due to the direct deposition of CuO nanocrystals onto the Cu plate with high electrical conductivity via the VO process. The good electrochemical ability and simple, one-pot, rapid, low-temperature fabrication would make the as-prepared CuO nanobelt arrays/Cu electrode an excellent sensing platform for various chemicals such as H2S,31 CO,32 and volatile organic compounds.33
 | ||
| Fig. 3 (A) CA response of CuO nanobelt arrays/Cu plate electrode at 0.6 V upon the addition of glucose solution to a 0.1 M NaOH aqueous solution. (B) Corresponding calibration curve (current density versus glucose concentration). | ||
Conclusions
In conclusion, CuO nanobelts and CuO/ZnO composite nanoarrays were successfully obtained by a one-pot, low-temperature, rapid alkaline vapor oxidation method. The resultant CuO nanobelt/Cu nonenzymatic glucose sensor presents attractive analytical features such as a fast response time, high sensitivity, and a low detection limit. We envisage that various CuO/metal oxide composites and other metal oxide nanoarrays with various properties can be obtained by adapting the VO method to other Cu-based alloys and metal plates.This work was partly supported by a Grant-in-Aid for Young Scientists (B, No. 22710102) from the Ministry of Education, Culture, Sports, Science and Technology, Japan and JST CREST.
References
- (a) J. G. Bendnorz and K. A. Muller, Z. Phys. B: Condens. Matter, 1986, 64, 189 CrossRef; (b) M. K. Wu, J. R. Ashburn, C. J. Torng, P. H. Hor, R. L. Meng, L. Gao, Z. J. Huang, Y. Q. Wang and C. W. Chu, Phys. Rev. Lett., 1987, 58, 908 CrossRef CAS.
- J. Ziolo, F. Borsa, M. Corti, A. Rigamonti and F. Parmigiani, J. Appl. Phys., 1990, 67, 5864 CrossRef CAS.
- C. T. Hsieh, J. M. Chen, H. H. Lin and H. C. Shih, Appl. Phys. Lett., 2003, 83, 3383 CrossRef CAS.
- L. Reijnen, B. Meester, A. Goossens and J. Schoonman, Chem. Vap. Deposition, 2003, 9, 15 CrossRef CAS.
- M. K. Wu, J. R. Ashburn, C. J. Torng, P. H. Hor, R. L. Meng, L. Gao, Z. J. Huang, Y. Q. Wang and C. W. Chu, Phys. Rev. Lett., 1987, 58, 908 CrossRef CAS.
- J. Chen, K. Wang, L. Hartman and W. Zhou, J. Phys. Chem. C, 2008, 112, 16017 CAS.
- A. P. L. Batista, H. W. P. Carvalho, G. H. P. Luz, P. F. Q. Martins, M. Gonçalves and L. C. A. Oliveira, Environ. Chem. Lett., 2010, 8, 63 CrossRef CAS.
- S. Anandan, X. Wen and S. Yang, Mater. Chem. Phys., 2005, 93, 35 CrossRef CAS.
- P. Podhájeckýa, Z. Zábranskýa, P. Nováka, Z. Dobiášováb, R. Černýb and V. Valvodab, Electrochim. Acta, 1990, 35, 245 CrossRef.
- (a) S. Jammi, S. Sakthivel, L. Rout, T. Mukherjee, S. Mandal, R. Mitra, P. Saha and T. Punniyamurthy, J. Org. Chem., 2009, 74, 1971 CrossRef CAS; (b) M. L. Kantam, J. Yadav, S. Laha, B. Sreedhar and S. Jha, Adv. Synth. Catal., 2007, 349, 1938 CrossRef CAS.
- (a) Y. Liu, Q. Fu and M. F. Stephanopoulos, Catal. Today, 2004, 93–95, 241 CrossRef CAS; (b) Y. Feng and X. Zheng, Nano Lett., 2010, 10, 4762 CrossRef CAS.
- J. B. Reitz and E. I. Solomon, J. Am. Chem. Soc., 1998, 120, 11467 CrossRef CAS.
- L. Xu, S. Sithambaram, Y. Zhang, C.-H. Chen, L. Jin, R. Joesten and S. L. Suib, Chem. Mater., 2009, 21, 1253 CrossRef CAS.
- (a) Y. Xia, P. Yang, Y. Sun, Y. Wu, B. Mayers, B. Gates, Y. Yin, F. Kim and H. Yan, Adv. Mater., 2003, 15, 353 CrossRef CAS; (b) X. Cao, H. Zeng, M. Wang, X. Xu, M. Fang, S. Ji and L. Zhang, J. Phys. Chem. C, 2008, 112, 5267–5270 CrossRef CAS; (c) R. Gao, L. Yin, C. Wang, Y. Qi, N. Lun, L. Zhang, Y.-X. Liu, L. Kang and X. Wang, J. Phys. Chem. C, 2009, 113, 15160 CrossRef CAS; (d) Y. Wang, H. Xia, L. Lu and J. Lin, ACS Nano, 2010, 4, 1425 CrossRef CAS; (e) W. Liu, Z.-M. Cui, Q. Liu, D.-W. Yan, J.-Y. Wu, H.-J. Yan, Y.-L. Guo, C.-R. Wang, W.-G. Song, Y.-Q. Liu and L.-J. Wan, J. Am. Chem. Soc., 2007, 129, 12922 CrossRef CAS.
- M. H. Huang, S. Mao, H. Feick, H. Yan, Y. Wu, H. Kind, E. Weber, R. Russo and P. Yang, Science, 2001, 292, 1897 CrossRef CAS.
- M. Law, L. E. Greene, J. C. Johnson, R. Saykally and P. Yang, Nat. Mater., 2005, 4, 455 CrossRef CAS.
- Y. Li, B. Tan and Y. Wu, Nano Lett., 2008, 8, 265 CrossRef CAS.
- Y. Qin, X. Wang and Z. L. Wang, Nature, 2008, 451, 809 CrossRef CAS.
- L. Qu, L. Dai, M. Stone, Z. Xia and Z. L. Wang, Science, 2008, 322, 238 CrossRef CAS.
- T. Zhao, J. Wu and J. Huang, J. Am. Chem. Soc., 2009, 131, 3158 CrossRef.
- X. Wen, W. Zhang and S. Yang, Langmuir, 2003, 19, 5898 CrossRef CAS.
- X. Jiang, T. Herricks and Y. Xia, Nano Lett., 2002, 2, 1333 CrossRef CAS.
- X. Wen, Y. Xie, C. L. Choi, K. C. Wan, X.-Y. Li and S. Yang, Langmuir, 2005, 21, 4729 CrossRef CAS.
- J. Liu, X. Huang, Y. Li, K. M. Sulieman, X. He and F. Sun, J. Mater. Chem., 2006, 16, 4427 RSC.
- X. Yang, J. Zhuang, X. Li, D. Chen, G. Ouyang, Z. Mao, Y. Han, Z. He, C. Liang, M. Wu and J. C. Yu, ACS Nano, 2009, 3, 1212 CrossRef CAS.
- Y. Cudennec and A. Lecerf, Solid State Sci., 2003, 5, 1471–1474 CrossRef CAS.
- Handbook of Chemistry: Pure Chemistry, Maruzen, Tokyo, 5th edn, 2004 Search PubMed.
- (a) D. H. Yoon, J. H. Yu and G. M. Choi, Sens. Actuators, B, 1998, 46, 15 CrossRef; (b) S.-T. Jun and G. M. Choi, J. Am. Ceram. Soc., 1998, 81, 695 CrossRef CAS; (c) Y. Zhu, C.-H. Sow, T. Yu, Q. Zhao, P. Li, Z. Shen, D. Yu and J. T.-L. Thong, Adv. Funct. Mater., 2006, 16, 2415 CrossRef CAS; (d) N. Wu, M. Zhao, J.-G. Zheng, C. Jiang, B. Myers, S. Li, M. Chyu and S. X. Mao, Nanotechnology, 2005, 16, 2878 CrossRef CAS; (e) H. A. Zaidi and K. K. Pant, Ind. Eng. Chem. Res., 2008, 47, 2970 CrossRef CAS.
- (a) L. D. Burke, G. M. Bruton and J. A. Collins, Electrochim. Acta, 1998, 44, 1467 CrossRef CAS; (b) Z Zhang, X. Su, H. Yuan, Q. Sun, D. Xiao and M. M. F. Choi, Analyst, 2008, 133, 126 RSC; (c) Q. Xu, Y. Zhao, J. Z. Xu and J.-J. Zhu, Sens. Actuators, B, 2006, 114, 379 CrossRef.
- X. Kang, Z. Mai, X. Zou, P. Cai and J. Mo, Anal. Biochem., 2007, 363, 143 CrossRef CAS.
- J. Chen, K. Wang, L. Hartman and W. Zhou, J. Phys. Chem. C, 2008, 112, 16017 CAS.
- J. H. Yu and G. M. Choi, Sens. Actuators, B, 2001, 75, 56 CrossRef.
- D. Barreca, E. Comini, A. Gasparotto, C. Maccato, C. Sada, G. Sberveglieri and E. Tondello, Sens. Actuators, B, 2009, 141, 270 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Explanation of the VO process. SEM images and XRD patterns of Cu or brass plates after the VO reaction. CV data for the CuO nanobelt arrays/Cu electrode. See DOI: 10.1039/c1ra00109d |
| This journal is © The Royal Society of Chemistry 2011 |
