Dehydration of fructose into 5-hydroxymethylfurfural in acidic ionic liquids
Furong
Tao
ab,
Huanling
Song
a and
Lingjun
Chou
*a
aState Key Laboratory for Oxo Synthesis and Selective Oxidation, Lanzhou Institute of Chemical Physics, Chinese Academy of Sciences, Lanzhou, 730000, P. R. China. E-mail: ljchou@licp.cas.cn; Fax: +86 931 4968129; Tel: +86 931 4968066
bGraduate School of Chinese Academy of Sciences, Beijing, 100049, P. R. China
First published on 24th August 2011
Abstract
A simple and effective process for the dehydration of fructose into 5-hydroxymethylfurfural (HMF) using ionic liquid 1-(4-sulfonic acid) butyl-3-methylimidazolium hydrogen sulfate (IL-1) as the catalyst was developed. High fructose conversion of 100% with HMF yield of 94.6% was obtained at 120 °C for 180 min reaction time in water-4-methyl-2-pentanone (MIBK) biphase system. Generally, the increase of water content had a negative effect on the reaction, the HMF selectivity decreased as the excessive elevation of temperature and prolonging of time, which suggested the decomposition of HMF. The ionic liquid IL-1 could be recycled and exhibited constant activity for six successful runs. This paper provided a new strategy for HMF production from fructose.
1. Introduction
In recent years, an increasing effort has been devoted to find ways to utilize hexoses as feedstocks for the production of valuable chemicals.1–6Hexoses are six-carbonic carbohydrates and are the most abundant monosaccharide existing in nature, because of their abundance and economy, the conversion of glucose and fructose to useful platform chemicals is very important.7–11 Nowadays, the catalytic transformation of glucose and fructose into furans is very interesting because it involves several steps, such as dehydration, hydrolysis, isomerization, aldol condensation, etc., which are of great interest.12–16Fructose is a carbohydrate obtained directly from biomass17–18 or by the isomerization of glucose.19 The conversion of fructose to 5-hydroxymethylfurfural (HMF) has been carried out in high-boiling organic solvents, such as dimethylsulfoxide (DMSO), dimethylformamide (DMF), acetone and sub-critical or high temperature water, 13,20–23 this approach necessitates difficult and energy-intensive isolation procedures. Also, in pure water, the dehydration of fructose is generally nonselective, which lead to many by-products besides HMF.24 To enhance the selectivity and facilitate the extraction of HMF, biphase system of water and organic solvent were used recently.25
In addition, room temperature ionic liquids (ILs), which are organic salts with low melting points, have attracted much attention in recent years. It was reported that many ionic liquids could dissolve carbohydrates effectively.26–28 With the increasing concern about the environmental protection and sustainable development, in our previous work,29 we investigated the conversion of xylose to furfural with an acidic ionic liquid. Here, we describe an efficient method for the dehydration of fructose into HMF with biphase system; the acidic ionic liquid, 1-(4-sulfonic acid) butyl-3-methylimidazlium hydrogen sulfate (IL-1), was used as the catalyst. With the given catalytic process, a HMF yield of 94.6% could be obtained, fructose conversion was up to 100%, and the IL-1 exhibited constant activity over six tests. The HMF production from fructose is shown in Scheme 1.
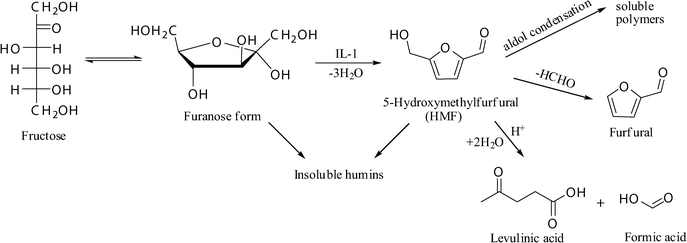 | ||
| Scheme 1 A typical reaction scheme for HMF production from fructose. | ||
2. Experimental
2.1 Materials
D-Fructose (99%) was commercially produced by Sinopharm Chemical Reagent Co., Ltd; 5-HMF (>99%) was from Aldrich; 1-methyl imidazole (CP, >99%), 1,4-butanesultone (>99%), were purchased from Alfa Aesar and used without further purification; 4-methyl-2-pentanone (AR, >90%), furfural (CP, >90%) and acetonitrile (HPLC) were purchased from Tianjin chemical reagent company (Tianjin, China). All the other reagents and solvents were reagent grade and were used as received.2.2 Preparation of the catalysts
The synthesis procedure of ionic liquid IL-1: 16.4 g (0.2 mol) 1-methyl imidazole, 27.28 g (0.2 mol) 1,4-butanesultone were mixed in a flask (250 mL) and stirred at 42–45 °C for 17 h to produce a white solid, then the white solid was ground, washed repeatedly with small portions of ether, filtered, vacuumized for 4 h at room temperature. An equimolar equivalent of the white solid was added to 98% H2SO4, and the mixture was stirred at 80 °C for 6 h, the obtained viscous liquid was washed with ether three times and dried under vacuum to form ionic liquid 1-(4-sulfonic acid) butyl-3-methylimidazolium hydrogen sulfate (IL-1). 1H NMR (400 MHz, D2O): δ 1.531–1.607 (m, 2H), 1.802–1.915 (m, 2H), 2.724 (t, 2H), 3.675 (s, 3H), 4.028 (t, 2H), 7.179 (s, 1H), 7.242 (s, 1H), 8.471 (s, 1H); 13C NMR (100 MHz, D2O): δ 20.907, 28.204, 35.851, 49.063, 50.258, 122.336, 123.719, 136.018. ESI-MS: m/z(+) 218.6, m/z(−) 96.3.The synthesis procedure of ionic liquid C4mimH2PO4 was similar to that of IL-1. Its NMR and ESI-MS spectra was shown as follows: 1H NMR (400 MHz, CDCl3): δ 0.89 (t, 3H), 1.26 (m, 2H), 1.77 (m, 2H), 3.47 (s, 3H), 4.21 (t, 2H), 7.81 (d, 1H), 7.89 (d, 1H), 9.54 (s, 1H); ESI-MS: m/z(+) 139.4, m/z (−) 97.2.
The synthesis procedure of phospho-12-tungsten (H3PW12O40):30 25 g (0.076 mol) Na2WO4·2H2O and 35 mL H2O were dissolved in a 250 mL beaker, then 2.5 mL H3PO4 (85%) and 20 mL concentrated HCl was added, cooled for 4 h and filtered. The obtained deposition was dissolved in 30 mL H2O. The solution was then transferred to a 250 mL separating funnel, 17.5 mL ether and 10 mL concentrated HCl were added and shaken for 5 min. After standing for 10 to 15 min, three layers formed. The lowest layer was collected, evaporated on the water bath with occasional stirring until crystals began to form on the surface. It was allowed to cool slowly and white octahedral crystals of the composition H3PW12O40·25H2O were obtained. The structure of H3PW12O40 was confirmed by FT-IR analysis, the four characteristic absorption peaks of heteropolyacid appeared: υ (P–O) 1080.46 cm−1, υ (W–O) 983.72 cm−1, υ (W–O1–W) 888.86 cm−1, υ (W–O2–W) 803.99 cm−1.
2.3 Typical procedure for the dehydration of fructose
The as-received fructose was dried for 24 h at 90 °C prior to fructose dehydration. Experiments were carried out in a Teflon-lined stainless steel autoclave equipped with a heating jacket. After the catalysts (typically 0.3 g or 0.5 g) and fructose (1.0 g) were added to the autoclave pre-charged with 4-methyl-2-pentanone (MIBK) (typically 9 mL), the reaction was started under spontaneous pressure (typically 2.5 atm) by heating the mixture to the reaction temperature. After the reaction, the reactor was removed and quickly quenched in a cool water bath. Subsequently, filtration, extraction and separation steps were conducted and the organic and aqueous phases were collected to characterize the products. For the recycling of IL-1, HMF was extracted out from the water phase 5 times with 8 mL of ethyl acetate, after extraction, the aqueous phase was heated at 60 °C for 24 h in a vacuum oven to remove water and residual ethyl acetate. The IL-1 was then used directly for the next run by adding fructose and MIBK. All results were replicated at least three times.2.4 Analyses
After each dehydration run, the consumption of fructose was confirmed by the phenol-sulfuric acid method.31 A mixture containing 0.1 mL of the reaction sample (water-soluble portion), 0.9 mL deionized water, 1 mL 5% phenol (freshly distilled) and 5 mL 98% concentrated sulfuric acid was prepared. The analysis was performed on a HP 8453 UV-vis spectrophotometer at about 490 nm with a slit width of 0.06 mm. The concentration of fructose in the reacted solution was calculated based on the standard curve obtained with fructose.Analysis of HMF was performed by HPLC on a HP 1090 series equipped with a photodiode array UV detector and a zorbax eclipse plus C18 reversed-phase column (150 mm × 4.6 nm, 0.5 μm). During this process, the column temperature remained constant at 30 °C, while the mobile phase applied was water–acetonitrile (15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 85, v/v) at the flow rate of 0.5 mL min−1, with UV detection at 280 nm.
85, v/v) at the flow rate of 0.5 mL min−1, with UV detection at 280 nm.
In addition, the ionic liquids were characterized by NMR spectra which were recorded on an INOVA-400 spectrometer; ESI-MS analyses were performed by using a Waters micromass ZQ alliance spectrometer; the FT-IR spectra of H3PW12O40 were collected on a FT-IR spectrometer (Nicolet Nexus 870) with a resolution of 4 cm−1 and 64 scans in the region of 4000–400 cm−1.
3. Results and discussion
3.1 Fructose dehydration with various catalysts
To select efficient acidic catalysts for the dehydration of fructose, various catalysts were tested in the same conditions. As shown in Fig. 1, for a series of acids as catalysts, the present system was effective for the dehydration of fructose. The catalysts included mineral acids (HCl, H2SO4), organic acid (CH3COOH), Lewis acids (AlCl3, SnCl2), heteropolyacid (H3PW12O40) and acidic ionic liquids (C4mimH2PO4, IL-1). It could be seen that acetic acid exhibited little catalytic activity under the experimental conditions, whereas HCl and H2SO4 showed weak activities. On the other hand, AlCl3, SnCl2 and H3PW12O40 had high activities for the dehydration of fructose to HMF. Compared with ionic liquid C4mimH2PO4 and other catalysts, IL-1exhibited the highest activity, which gave 98.2% of fructose conversion and gave a HMF yield of 92.1%. These results indicated that the acidity of the solution was an important factor in fructose dehydration, there was an obvious relationship between activity and catalyst acidity in the reaction, higher catalyst acidity led to higher reaction activity; the results also suggested that the catalysis of mineral and organic acids were generally nonselective, leading to many by-products besides HMF. | ||
| Fig. 1 Dehydration of fructose in the presence of catalysts. Conditions: 1.0 g fructose, 0.5 g catalyst, 1 mL H2O, 9 mL MIBK, T = 120 °C, t = 120 min, P = 2.5 atm. | ||
Simultaneously, we compared other representative ionic liquids, such as 1-butyl-3-methylimidazolium chloride (C4minCl) and 1-butyl-3-methylimidazolium bromine (C4minBr); the results were not as expected. Of course, not all acidic ionic liquids showed high activity, weakly acidic ILs were also non-ideal. Among the ionic liquids we used, IL-1 showed the optimum activity. Since dehydration results were most favorable for IL-1, this acidic ionic liquid was chosen to study in detail.
3.2 Influence of IL-1 dosage on fructose dehydration
Fig. 2 shows the effect of IL-1 dosage on fructose conversion and HMF yield. The amount of IL-1 used was 0.1 g, 0.3 g, 0.5 g and 0.7 g, respectively. In the absence of IL-1, at a reaction temperature of 120 °C, no HMF yield was observed for 120 min reaction time, the conversion of fructose was only 5.7% (not shown). When the dosage of IL-1 was 0.1 g, the fructose conversion reached 96.2% and HMF yield was up to 89.8%; when the amount of IL-1 increased from 0.1 g to 0.3 g, the yield of HMF increased obviously, from 89.8% to 93.7%, fructose conversion reached 100%. However, when the IL-1 dosage increased from 0.3 g to 0.5 g, and from 0.5 g to 0.7 g, there was a little decrease in fructose conversion and HMF yield and a sticky and black solid residue formed after the reaction. We considered that in the reaction environment IL-1 dosage was above 0.5 g, side effects occurred, such as aldol condensation and rehydration of HMF, which led to the decrease of HMF selectivity and fructose conversion. As the HMF yield did not increase with further IL-1 dosage over 0.3 g, which implied that there were sufficient catalytic sites available for the substrate fructose (1.0 g) in our system at the experimental conditions.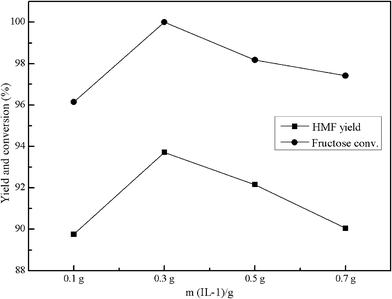 | ||
| Fig. 2 Effect of IL-1 dosage on fructose dehydration and HMF yield. Conditions: 1.0 g fructose, 1 mL H2O, 9 mL MIBK, T = 120 °C, t = 120 min, P = 2.5 atm. | ||
3.3 Effect of initial fructose concentration
Kuster et al.32 demonstrated that in the dehydration of fructose, the product HMF can combine with fructose and cross-polymerize to form humins, especially in aqueous systems and aqueous mixture systems, so the initial fructose concentration has a large effect on the selectivity of HMF. In this paper, the effect of initial fructose amount on fructose conversion and HMF selectivity is illustrated in Fig. 3. When the fructose weight increased from 0.3 g to 1.0 g, the HMF selectivity changed from 88.4% to 93.7%. As the fructose weight increased from 1.0 g to 1.5 g, the yield of HMF decreased by 2.5%, fructose conversion also decreased to 95.3%. Additional increase in the fructose weight (2.0 g) led to lower of HMF selectivity and fructose conversion, maybe caused by the formation of humins in the aqueous-MIBK biphase system or that reactive compounds, such as fructose and HMF, would collide with each other or cross polymerize. So in our reaction system, we chose 1.0 g as the optimum initial fructose amount. | ||
| Fig. 3 Effect of initial fructose concentration on fructose conversion and HMF yield. Conditions: 0.3 g catalyst, 1 mL H2O, 9 mL MIBK, T = 120 °C, t = 120 min, P = 2.5 atm. | ||
3.4 Effect of reaction temperature and time
Table 1 shows the effect of reaction time and temperature on fructose dehydration. As indicated in Table 1, the reaction time and temperature played an important role both in fructose conversion and HMF yield. With the elevation of temperature and prolonging of time, the conversion of fructose and HMF yield were improved correspondingly. The fructose conversion was up to 100% for a HMF yield of 94.6% at 120 °C for 180 min reaction time. When the reaction temperature was 100 °C, the fructose conversion was 92.9% for a HMF yield of 87.0% for 150 min. The HMF yield increased from 87.0% to 92.1% when the temperature changed from 100 °C to 150 °C.| T (°C) | t (min) | Yield (%) | Conv. (%) | |||
|---|---|---|---|---|---|---|
| HMF | Furfural | LA | HCOOH | |||
| 80 | 60 | 76.19 | 0.22 | 0.61 | 0.24 | 83.36 |
| 120 | 80.10 | 0.46 | 1.17 | 0.46 | 87.17 | |
| 180 | 83.43 | 0.75 | 1.48 | 0.59 | 89.98 | |
| 100 | 45 | 75.72 | 0.33 | 0.75 | 0.30 | 83.53 |
| 90 | 81.67 | 0.55 | 0.79 | 0.31 | 88.80 | |
| 150 | 87.01 | 0.83 | 0.42 | 0.17 | 92.92 | |
| 120 | 90 | 89.73 | 0.26 | 0.18 | 0.007 | 98.43 |
| 120 | 93.72 | 0.58 | 0.76 | 0.30 | 100 | |
| 180 | 94.59 | 0.92 | 0.55 | 0.22 | 100 | |
| 150 | 30 | 88.70 | 0.25 | 0.06 | 0.02 | 96.52 |
| 90 | 90.57 | 1.06 | 0.35 | 0.14 | 100 | |
| 150 | 92.09 | 1.18 | 0.73 | 0.30 | 100 | |
However, the HMF selectivity decreased as the reaction proceeded, and the color of the solution changed from achromatism to deep brown, which was an evidence for the decomposition of the formed HMF. Generally, in acid catalyzed dehydration of fructose, there are three pathways for HMF decomposition. The first pathway is the rehydration of HMF into levulinic acid (LA) and formic acid; the second pathway is the aldol condensation between HMF molecules into soluble polymers; the third way is the cross-polymerization between HMF and fructose to form insoluble humins (Scheme 1). In our reaction system, very little furfural was generated; we used HMF as the substrate to prove that the furfural was from HMF in the same conditions. The color of the reacted solutions was brown; remaining even after HMF was extracted from the mixture. The brown by-products were thought to be soluble polymers and humins, which could not be quantified according to the present methods. In this work, the decrease in HMF selectivity with reaction time and temperature was supposed to be due to the formation of these by-products, which consumed the initial fructose and the formed HMF, and hence reduced the selectivity of HMF.
3.5 Influence of water content on fructose dehydration
In the previous work,8water was proved to have a large negative effect on the dehydration of fructose; our study also confirmed this point. As shown in Fig. 4, when the reaction was performed in MIBK without water, the fructose conversion was just 47.6% and HMF yield was only 20.4%; when the water content was below 1.0 mL, with the increase of water content, the fructose conversion and HMF yield were improved. However, as water content increased to above 1.0 mL, both fructose conversion and HMF selectivity decreased. This is to say, when the V (H2O)/V (MIBK) was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9, we get the optimum results, the conversion of fructose was 100% and HMF yield reached 93.7%. Therefore, water content in our system had a large influence on fructose dehydration, and also, water–MIBK biphase system was a very effective method for the reaction. A previous study24 indicated that in pure water, the dehydration of fructose is generally nonselective; in our system, MIBK was added to extract continuously the HMF from the aqueous phase, minimizing degradation reactions arising from extended HMF residence in the reactive aqueous phase and to achieve more efficient recovery of HMF in the subsequent isolation step. Larger amounts of water would result in the loss of the catalytic activity, hence led to the decrease of HMF selectivity.
9, we get the optimum results, the conversion of fructose was 100% and HMF yield reached 93.7%. Therefore, water content in our system had a large influence on fructose dehydration, and also, water–MIBK biphase system was a very effective method for the reaction. A previous study24 indicated that in pure water, the dehydration of fructose is generally nonselective; in our system, MIBK was added to extract continuously the HMF from the aqueous phase, minimizing degradation reactions arising from extended HMF residence in the reactive aqueous phase and to achieve more efficient recovery of HMF in the subsequent isolation step. Larger amounts of water would result in the loss of the catalytic activity, hence led to the decrease of HMF selectivity.
 | ||
| Fig. 4 Effect of water content on fructose dehydration. Conditions: 1.0 g fructose, 0.3 g catalyst, 9 mL MIBK, T = 120 °C, t = 120 min, P = 2.5 atm. | ||
3.6 Recycling of catalyst IL-1
To study the reusability of the catalyst IL-1, the main product HMF was separated from reaction mixture using extraction. After the first reaction run for fructose, the catalyst IL-1 only existed in the aqueous phase, so the organic phase was separated. Then 1 g of distilled water was added into the reaction liquid to decrease the viscosity of ionic liquid and facilitate the extraction of HMF. Subsequently, the HMF was separated from aqueous phase by extracting 8 times with 5 mL of ethyl acetate.7 The amount of extracted product was examined by HPLC, which demonstrated that ethyl acetate extraction could recover about 90–95% of HMF. After extraction, the solution was heated at 60 °C in a vacuum drier until the water and residual ethyl acetate were removed completely. The dried IL-1 catalyst was used directly in the next run by adding fresh fructose and MIBK under the same reaction conditions. In order to examine the reusability of IL-1, the recycling tests were conducted six times. As shown in Fig. 5, the catalytic activity of the IL-1 for the dehydration of fructose did not decrease obviously after six runs, which demonstrated that the catalyst IL-1 was stable in this system. Anyway, the non-completely extraction of little products and the residue of unreacted fructose in the previous run may have a slight influence on the results of the recycling test. So, the separation and purification of HMF will remain the subject of future studies.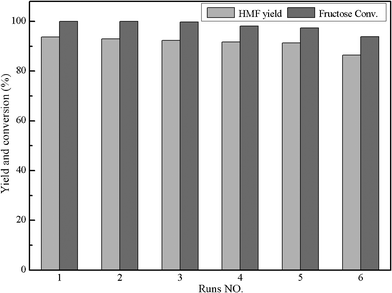 | ||
| Fig. 5 Recycling of IL-1 catalyst. Conditions: 1.0 g fructose, 0.5 g catalyst, 1 mL H2O, 9 mL MIBK, T = 120 °C, t = 120 min, P = 2.5 atm. | ||
Conclusion
In summary, we demonstrated that acidic ionic liquid 1-(4-sulfonic acid) butyl-3-methylimidazolium hydrogen sulfate (IL-1) was an excellent catalyst for fructose dehydration into 5-hydroxymethylfurfural (HMF). The acidity of the solution had a large effect on fructose conversion and HMF selectivity, usually, higher acidity led to higher catalytic activity. The dosage of IL-1 and the initial amount of fructose also played an important role in the dehydration reaction. Generally, water had a negative effect on the reaction, the HMF selectivity decreased as the excessive elevation of temperature and prolonging of time, which suggested side reactions were taking place, such as rehydration, condensation, and reversion of HMF. The reaction conditions were optimized to obtain the satisfactory results. Fructose conversion reached 100% and the yield of HMF was up to 94.6% when the reaction was performed at 120 °C for 180 min time. The catalyst IL-1 was recycled which exhibited favorable catalytic activity over six repeated runs. To conclude, the simple, efficient, low toxicity and reusable catalytic system has great potential.Acknowledgements
We gratefully acknowledge the financial support of the State Key Laboratory for Oxo Synthesis and Selective Oxidation of China.References
- Z. Zhang and Z. Zhao, Bioresour. Technol., 2011, 102(4), 3970–3972 CrossRef CAS.
- G. Yong, Y. Zhang and J. Y. Ying, Angew. Chem., Int. Ed., 2008, 47, 9345–9348 CrossRef CAS.
- K. B. Sidhpuria, A. L. Daniel-da-Silva, T. Trindade and J. A. P. Coutinho, Green Chem., 2011, 13, 340–349 RSC.
- Y. Zhang, V. Degirmenci, C. Li and E. J. M. Hensen, ChemSusChem, 2011, 4(1), 59–64 CrossRef CAS.
- Z. Zhang, Q. Wang, H. Xie, W. Liu and Z. Zhao, ChemSusChem, 2011, 4(1), 131–138 CrossRef CAS.
- F. Benvenuti, C. Carlini, P. Patrono, A. M. R. Galletti, G. Sbrana, M. A. Massucci and P. Galli, Appl. Catal., A, 2000, 193, 147–153 CrossRef CAS.
- X. Qi, M. Watanabe, T. M. Aida and R. L. Smith Jr., Green Chem., 2009, 11, 1327–1331 RSC.
- X. Qi, M. Watanabe, T. M. Aida and R. L. Smith Jr., Green Chem., 2008, 10, 799–805 RSC.
- H. Zhao, J. E. Holladay, H. Brown and Z. C. Zhang, Science, 2007, 316, 1597–1599 CrossRef CAS.
- Q. Bao, K. Qiao, D. Tomida and C. Yokoyama, Catal. Commun., 2008, 9, 1383–1388 CrossRef CAS.
- C. Moreau, A. Finiels and L. Vanoye, J. Mol. Catal. A: Chem., 2006, 253, 165–169 CrossRef CAS.
- H. Yan, Y. Yang, D. Tong, X. Xiang and C. Hu, Catal. Commun., 2009, 10(11), 1558–1563 CrossRef CAS.
- M. Bicker, J. H irth and H. Vogel, Green Chem., 2003, 5, 280–284 RSC.
- K. Shimizu, R. Uozumi and A. Satsuma, Catal. Commun., 2009, 10(14), 1849–1853 CrossRef CAS.
- A. Takagaki, M. Ohara, S. Nishimura and K. Ebitani, Chem. Commun., 2009, 6276–6278 RSC.
- T. Ståhlberg, M. G. SØrensen and A. Riisager, Green Chem., 2010, 12, 321–325 RSC.
- M. A. Harmer, A. Fan, A. Liauw and R. K. Kumar, Chem. Commun., 2009, 6610–6612 RSC.
- J. Pang, A. Wang, M. Zheng and T. Zhang, Chem. Commun., 2010, 46, 6935–6937 RSC.
- J. Lecomte, A. Finiels and C. Moreau, Starch, 2002, 54(2), 75–79 CrossRef CAS.
- S. Hu, Z. Zhang, Y. Zhou, B. Han, H. Fan, W Li, J. Song and Y. Xie, Green Chem., 2008, 10, 1280–1283 RSC.
- Y. Nakamura and S. Morikawa, Bull. Chem. Soc. Jpn., 1980, 53, 3705–3706 CrossRef CAS.
- F. S. Asghari and H. Yoshida, Carbohydr. Res., 2006, 341, 2379–2387 CrossRef CAS.
- F. S. Asghari and H. Yoshida, Ind. Eng. Chem. Res., 2006, 45, 2163–2173 CrossRef CAS.
- K. M. Rapp, U.S. Patent 4, 740, 605 ( 1987) Search PubMed.
- Y. Román-Leshkov, J. N. Chheda and J. A. Dumesic, Science, 2006, 312, 1933–1937 CrossRef.
- R. P. Swatloski, S. K. Spear, J. D. Holbrey and R. D. Rogers, J. Am. Chem. Soc., 2002, 124, 4974–4975 CrossRef CAS.
- X. Tong, Y. Ma and Y. Li, Carbohydr. Res., 2010, 345, 1698–1701 CrossRef CAS.
- H. Zhao, J. E. Holladay, H. Brown and Z. C. Zhang, Science, 2007, 316, 1597–1599 CrossRef CAS.
- F. Tao, H. Song and L. Chou, Can. J. Chem., 2011, 89, 83–87 CrossRef CAS.
- H. Wu, J. Biol Chem., 1920, 43, 189–220 CAS.
- M. Dubois, K. A. Gilles, J. K. Hamilton, P. A. Rebers and F. Smith, Anal. Chem., 1956, 28, 350–356 CrossRef CAS.
- B. F. M. Kuster, Starch, 1990, 42, 314–321 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2011 |
