Solid carbon nanorod whiskers: application to the electrochemical sensing of biologically relevant molecules
Philip M.
Hallam
a,
Bill L.
Riehl
b,
Bonnie D.
Riehl
b and
Craig E.
Banks
*a
aFaculty of Science and Engineering, School of Science and the Environment, Division of Chemistry and Environmental Science, Manchester Metropolitan University, Chester Street, Manchester M1 5GD, Lancs, UK. E-mail: c.banks@mmu.ac.uk; Tel: ++44(0)1612471196; Fax: +44(0)1612476831
bSCNTE, 417W. Dayton Drive, Fairborn, Ohio OH 45420, USA
First published on 18th July 2011
Abstract
We introduce solid carbon nanorod whiskers, a derivative of carbon nanotubes which are fabricated via a solid state methodology and are completely free from metallic impurities. We explore the electrochemical properties of these unique carbon nanostructures towards the electrochemical oxidation of NADH, dopamine and uric acid, and compare and contrast to other carbon nanomaterials/composites where appropriate. It is shown that thin layer behaviour dominates when the coverage of the nanomaterials is increased giving the false impression of electro-catalysis. The SCNR whiskers are analytically similar to other reported carbon nanotube structures and yet do not suffer from problems associated with metallic impurities, suggesting their beneficial use in many areas of electrochemistry.
Introduction
Carbon nanotubes (CNTs) are considered by many to be a novel class of nanomaterial, consequently having a profound impact on several diverse areas of science and technology, none more so than in electroanalysis and electrochemistry where their use has risen considerably.1–10 The properties and applications that make carbon nanotubes of vast interest have been well summarised in the literature with many reviews having an electrochemical emphasis.11 Structurally CNTs can be portrayed as “rolled up” sheets of graphene:12 a single rolled sheet in the case of a single walled (SWCNTs) or concentric tubes fitted one inside the other in the case of multi-walled carbon nanotubes (MWCNTs).13 It should also be noted that the way in which the graphene sheets are rolled influences the electronic properties of the CNTs. CNTs tender unique advantages including enhanced electronic properties, a large edge/basal plane ratio, and rapid electrode kinetics.4,14 Thus, CNT-based devices frequently display higher sensitivities, lower limits of detection, and faster electron transfer kinetics than traditional carbon electrodes.14 Electrochemists have efficiently exploited CNTs, taking advantage of these distinctive properties to accelerate the electron transfer reaction involving a wide range of biomolecules and environmentally significant compounds such as proteins, nucleic acids, NADH, neurotransmitters, cytochrome c, cysteine and hydrazine compounds.2Several unique morphological variations of the MWCNTs are possible;14 such as “hollow tube”, “herringbone” or “bamboo-like” MWCNTs.13 The divergence between these different MWCNT configurations is that herringbone and bamboo variants contain a proportionally higher number of edge plane like-sites/defects when compared to that of the hollow-tube MWCNTs; this is owed, in these two cases, to the plane of the graphite sheet, which is at an angle to the axis of the tube, necessitating a high percentage of the graphite sheets to terminate at the surface of the tube. It has been established that CNTs have two distinctive electrochemical reactive sites: basal plane sites which occur along the side wall of the CNTs and edge plane like-sites/defects at the open ends of the nanotube, certain variants may also include these edge plane like-sites defects along the tubes axis.13,15 The term basal and edge plane like-sites/defects arise from a structural comparison with that of highly ordered pyrolytic graphite.3 It has been established that it is these edge plane like-sites/defects that are responsible for a large proportion of the chemical and electrochemical activity of the CNTs.3–4,14
Carbon nanotubes can be produced from a range of methods such as via arc discharge,16laser ablation,17 and most commonly, chemical vapour deposition (CVD).18
Such fabrication processes unavoidably take advantage of a metal catalyst, resulting in the assembly of carbon nanotubes which contain metal impurities.19 The notion that a homogenous material is attained is often a false one, even a small amount of heterogeneity in the sample can yield potentially erroneous results.20 This has implications in producing sensors based on carbon nanotubes since the amount of metallic impurities can vary between samples, producing large divergences in the electrochemical response from sensor to sensor.21 Thus mechanistic information is not easily de-convoluted as these trapped metallic impurities can result in the misinterpretation of the electrochemical origins of the heterogeneous charge transfer.22 Conversely, technological advancements are dependent on the high purity of the carbon nanotube material. This difficulty can be overcome by using a novel solid-state production method, which can fabricate high-purity (99.99+%), metal-impurity-free MWCNTs, providing an obvious advantage over other commercially available carbon nanotubes.22
Recently fabricated derivatives of carbon nanotubes are solid carbon nanorod “whiskers” formed through a Carbo-Thermal Carbide Conversion process.23 A previous literature report has demonstrated a facile approach to quantifying the global coverage of the origin of electron transfer, viz edge plane like-sites/defects which were shown to be ∼13% for SCNR whiskers,24 which is significantly more than standard, commercially available nanotubes.24 Consequently in this paper we explore solid carbon nanorod (SCNR) whiskers for the first time and electrochemically “benchmark” these with the model electrochemical systems of NADH, dopamine and uric acid.
Experimental section
All chemicals used were of analytical grade and were used as received without any further purification and were obtained from Sigma-Aldrich. All solutions were prepared with deionised water of resistivity not less than 18.2 MΩ cm. Voltammetric measurements were carried out using a μ-Autolab III (ECO-Chemie, The Netherlands) potentiostat. All measurements were conducted using a three electrode configuration comprising a screen printed carbon working electrode (3 mm diameter), a carbon counter and a Ag/AgCl reference electrode. The manufacture of the screen printed electrodes (SPEs) has been reported previously and the SPEs were shown to exhibit a heterogeneous rate constant of ∼1.7 × 10−3 cm s−1 using the ferro/ferricyanide redox probe in 1 M KCl.25 Basal plane-like screen printed electrodes were obtained commercially (Product: KS 520).26 These sensors are on a flexible substrate consisting of a graphite working electrode (3.1 mm diameter), a carbon counter, and onboard silver/silver chloride reference electrode. Note that these electrodes are basal plane like in nature with electron transfer rates of the order ∼10−4 cm s−1 as determined with potassium ferrocyanide/1 M KCl.26 Connectors for the efficient connection of the screen printed electrochemical sensors were purchased from Kanichi Research Services Ltd.26 Metallic impurity free SCNR “whiskers”, formed through a Carbo-Thermal Carbide Conversion (CTCC) are commercially available.23 Solid carbon nanorod whiskers were immobilised onto the electrode by dropping aliquots of a solid carbon nanorod whisker solution (25 mg/2 ml ethanol/water 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) onto the electrode surface which was allowed to evaporate at room temperature.
50) onto the electrode surface which was allowed to evaporate at room temperature.
Results and discussion
A typical SEM image of the solid carbon nanorod (SCNR) whiskers is depicted in Fig. 1A which shows cylindrical type structures with lengths in the order of 1–5 microns with a diameter of 20 nm to 1 μm. Closer inspection, as depicted in Fig. 1B, reveals that these are made up of highly ‘kinked’ bundles of nanorods. Raman Spectroscopy is arguably the most powerful analytical tool available for the study of nanophase crystalline allotropes of carbon such as carbon nanotubes.27 There are 3 main features in the spectra of a CNT sample: the Radial Breathing Mode (RBM), the tangential mode (G band), and disorder induced mode (D band, G* band). Raman analysis of the SCNR whiskers, shown in Fig. 2A, (using a 785 nm laser excitation wavelength) exhibits sharp/well defined G peaks with a very large G* intensity and a smaller D![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) G ratio as compared with the commercial CNT material. The 785 nm excitation frequency allows observation of two key features in SCNR morphology as compared with MWCNTs, alluding to the consistency and utility of the material. The first feature of note is the RBM vibrations, present at 115 and 170 cm−1. Secondly, note that the D and G peaks are significantly narrower in width than commercial MWCNT materials,22 indicating a homogeneous material, in particular the G band, highlighting the consistency of the SCNR whiskers. This is supported by the Thermogravimetric Analysis (TGA) data (see below). Peak locations vary with material dimensions and defect content and type, resulting in a smearing of multiple peaks into a single, broader peak present in commercial material. Thus greater consistency is evident in the material produced via the fabrication process by the G peak separation (into G+ and G−). This separation is a result of the variation in the elastic vibrations of the crystal structure in the direction of the rod axis, and that of the vibration tangential to the tube circumference. G-Band splitting is typically used in order to determine the chirality of individual CNTs. A typical spectrum is shown in Fig. 2B (magnification of Fig. 2A) which demonstrates that the SCNR whiskers are highly consistent since isolation of individual structures is not necessary to obtain spectra in which G-Band splitting can be observed. The Raman data also show that SCNRs produced via CTCC are metallically conductive. Analysis of the purity (measured viaICP-MS) reveals a carbon mass % of 99.98 with an oxygen mass % of <0.05 and a silicon mass % of 0.01 indicating the complete absence of metallic impurities as is the case in other commercially available nanomaterials.28TGA has been used successfully in the past to study homogeneity and degree of functionalization of SWCNTs and MWCNTs.29 In this paper, TGA is used to demonstrate the high degree of homogeneity. This is shown in Fig. 3A by the near zero loss of mass until approximately 550 °C and the peak in the derivative plot (Fig. 3B) at 718 °C. Amorphous carbon content has the effect of shielding CNT electron transfer, effectively reducing the active electrode sites. This leads to reduced electrode efficiency.
G ratio as compared with the commercial CNT material. The 785 nm excitation frequency allows observation of two key features in SCNR morphology as compared with MWCNTs, alluding to the consistency and utility of the material. The first feature of note is the RBM vibrations, present at 115 and 170 cm−1. Secondly, note that the D and G peaks are significantly narrower in width than commercial MWCNT materials,22 indicating a homogeneous material, in particular the G band, highlighting the consistency of the SCNR whiskers. This is supported by the Thermogravimetric Analysis (TGA) data (see below). Peak locations vary with material dimensions and defect content and type, resulting in a smearing of multiple peaks into a single, broader peak present in commercial material. Thus greater consistency is evident in the material produced via the fabrication process by the G peak separation (into G+ and G−). This separation is a result of the variation in the elastic vibrations of the crystal structure in the direction of the rod axis, and that of the vibration tangential to the tube circumference. G-Band splitting is typically used in order to determine the chirality of individual CNTs. A typical spectrum is shown in Fig. 2B (magnification of Fig. 2A) which demonstrates that the SCNR whiskers are highly consistent since isolation of individual structures is not necessary to obtain spectra in which G-Band splitting can be observed. The Raman data also show that SCNRs produced via CTCC are metallically conductive. Analysis of the purity (measured viaICP-MS) reveals a carbon mass % of 99.98 with an oxygen mass % of <0.05 and a silicon mass % of 0.01 indicating the complete absence of metallic impurities as is the case in other commercially available nanomaterials.28TGA has been used successfully in the past to study homogeneity and degree of functionalization of SWCNTs and MWCNTs.29 In this paper, TGA is used to demonstrate the high degree of homogeneity. This is shown in Fig. 3A by the near zero loss of mass until approximately 550 °C and the peak in the derivative plot (Fig. 3B) at 718 °C. Amorphous carbon content has the effect of shielding CNT electron transfer, effectively reducing the active electrode sites. This leads to reduced electrode efficiency.
 | ||
| Fig. 1 Typical SEM images of the SCNR whiskers. | ||
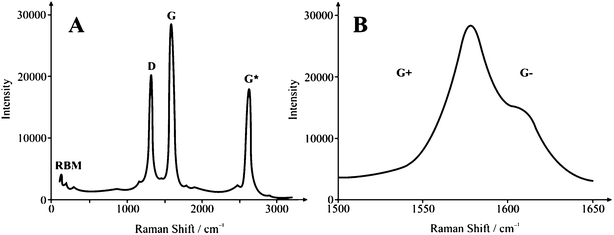 | ||
| Fig. 2 Typical Raman spectra of SCNR whiskers. | ||
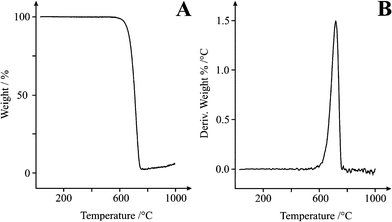 | ||
| Fig. 3 Thermal Gravimetric Analysis of SCNR whiskers between 0 and 1000 °C at a rate of 10 °C min−1 in air. A: Weight % vs. Temp, B: Derivative weight % vs. Temp. | ||
The SCNR whiskers are a unique subset of single-walled carbon nanotubes (SWCNTs) and clearly as a result of the unique process have no residual transition metal catalyst is present in the final product making them “ultra-pure”. In a recent paper a facile methodology to allow the density of defects of carbon nanomaterials to be readily determined was presented24 which demonstrated that this material had a high % of defects (viz edge plane like-sites defects) over that of commercially available MWCNTs and consequently we explore this unique material towards the sensing of biologically relevant molecules. IR spectra were obtained for the SCNR whiskers with the aim to distinguish the functional surface groups present. Bands at 1625, 1759, 2850, 2920 and 3416 cm−1 were observed within the IR spectrum. Assignment of the band at 1625 cm−1 was accredited to the stretching of conjugated C–C bonds. The C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching of aldehyde and ketone functional groups was present at 1759 cm−1 while the vibrations from the C–H bonds within alkyl groups contribute to the band shown at 2850 and 2920 cm−1. Finally the intense band at 3416 cm−1 was assigned to the presence of O–H from either alcohol or phenol active groups.
O stretching of aldehyde and ketone functional groups was present at 1759 cm−1 while the vibrations from the C–H bonds within alkyl groups contribute to the band shown at 2850 and 2920 cm−1. Finally the intense band at 3416 cm−1 was assigned to the presence of O–H from either alcohol or phenol active groups.
We now consider the voltammetric performance of screen printed electrodes (SPE) modified with 75 μg SCNR whiskers which are explored towards the electrochemical sensing of 1 mM NADH in a pH 7 phosphate buffer solution. Fig. 4 depicts a large voltammetric oxidation wave exhibiting a peak at ∼+0.148 V (at 0.1 Vs−1) which is observed to shift to higher potentials upon increasing the scan rate. Note that the response of the bare basal-plane-like-SPE is found to exhibit a voltammetric peak at ∼+0.685 V (Fig. 4A) which is as expected for an electrode with a low global coverage of edge-plane-like-sites/defects which is in agreement with previous studies of NADH on basal plane pyrolytic graphite.30 It is clear that the voltammetric peak in Fig. 4B lacks a diffusional tail suggesting thin-layer behaviour.22
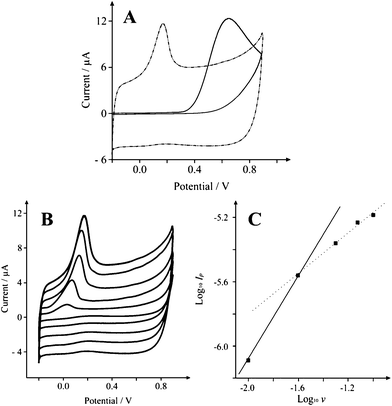 | ||
| Fig. 4 Typical cyclic voltammetric responses resulting from the electrochemical oxidation of 200 μM NADH in pH 7 at A: SCNR whiskers (75 μg) modified SPE (dot-dash) and a basal plane-like electrode (solid line) recorded at a scan rate of 0.1 Vs−1, B: SCNR whiskers (75 μg) modified SPE recorded over the scan rate range of 0.01 to 0.1 Vs−1. Part C depicts analysis of A in the form of Log10Ipvs. Log10ν. | ||
Analysis of the data presented in Fig. 4B in the form of Log Ipvs. Log ν should yield a gradient of 0.5 (δLog Ip/δLog ν = 0.5) for a diffusional controlled process while δLog Ip/δLog ν = 1 for thin layer behaviour. As shown in Fig. 4C, a low scan rate results in a gradient (δLog Ip/δLog ν) of 1 which is clearly substantially more than the expected gradient of 0.5 as governed by the Randles-Ševćik equation (as seen previously for multi-walled carbon nanotubes),31 while high scan rates produce a gradient (δLog Ip/δLog ν) of 0.5. This is compelling evidence to suggest that the mechanistic process is in-fact moving from a thin layer regime on longer timescales, to semi-infinite diffusion on shorter timescales, thus presenting a ‘Catch 22’ when it comes to interpretation of peak potentials for electron transfer rates since for a quasi-reversible system such as ours, one would ideally work at slow scan rates, however faster scan rates are required to avoid falling into the thin layer regime. Therefore the response is clearly a contribution from both semi-infinite and thin layer diffusion. In the literature it is common to explore the effect on the peak height as a function of added nano-material. To further explore this and to determine the optimum coverage of nanomaterial which exhibits the most beneficial electrochemical response, as commonly undertaken in electroanalysis, we consequently explored the effect of increasing amounts of SCNR whiskers over the range 25–100 μg. A notable shift in the peak potential is clearly displayed, from +0.685 V as observed at the bare underlying electrode, to +0.148 V (vs. Ag/AgCl) when 75 μg of the SCNR whiskers is added (Fig. 4A). Note that at each mass addition of SCNR whiskers, the voltammetric peaks compared to the electrochemical oxidation was performed over a range of scan rates and analysed as a function of Log Ipvs. Log ν. A plot depicting the response of this gradient as a function of the mass addition of SCNR whiskers is shown in Fig. 5, clearly showing a thin-layer effect once a mass of 50 μg or greater is utilized. Table 1 depicts the peak potential (Ep) for the observed voltammetric profiles of various carbon based electrode materials towards NADH. It is clear that the response of the SCNR whiskers exhibits a facile electrochemical response but on further investigations it is clear that this is actually due to a thin layer response as identified above where the diffusional contribution has changed and thus cannot be directly compared to the response of EPPG, GC, and BPPG, as is commonly (misleadingly) undertaken in the literature.31 Note that the peak potential shifts with a function of coverage as the thin layer behaviour dominates which is often mistaken for ‘electro-catalysis’; clearly in comparing CNT modified electrodes, the coverage is critical. Additionally, a comparison across the literature is also misleading as we have shown there is a clear demonstration that Ep varies with both scan rate (quasi-reversible electrode kinetics) and coverage, and thus a comparison without an explicit knowledge of which scan rate/coverage was employed is meaningless; this approach is rife within the scientific literature. Returning to the electrochemical response in Fig. 4, the electrochemical mechanism for NADH on carbon surfaces has been reported to be:30
| NADH − e− → NADH˙+ | (1) |
| NADH˙+ → NAD˙ + H+ | (2) |
| NAD˙ − e− → NAD+ | (3) |
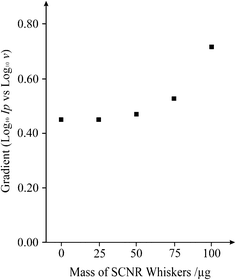 | ||
| Fig. 5 A plot depicting the response of the gradient (Log Ipvs. Log ν) as a function of the mass addition of SCNR whiskers onto a basal plane-like screen printed electrode over the range 0–100 μg. | ||
We next explore the electroanalytical performance of the SCNR whiskers towards the electrochemical sensing of NADH. Additions of NADH were made into a pH 7 buffer solution and as depicted in Fig. 6, two linear regions are observed over the range of 20 to 65 μM (Ip/A = 6 × 10−8 A μM−1 − 9 × 10−7 A; R2 = 0.998; N = 4) and 0.2 to 2.5 mM (Ip/A = 1 × 10−5 A μM−1 + 5 × 10−6 A; R2 = 0.995; N = 5). Both ranges are analytically relevant and based on the former, a limit of detection (based on 3-sigma) is found to correspond to 10 μM (n = 3) which is in excellent agreement with previous studies on edge plane pyrolitic graphite.28Table 2 shows a short overview of literature reports for NADH sensing using a range of electrode composites, it is clear that the SCNR whiskers are comparable to other carbon nanomaterials.
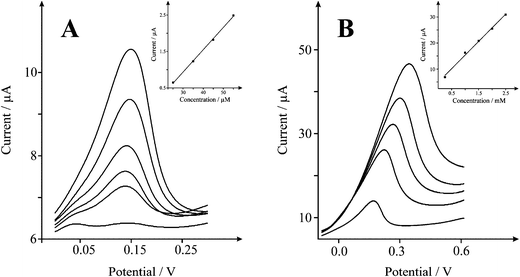 | ||
| Fig. 6 Cyclic voltammetric responses from the electrochemical oxidation of NADH A) 25–65 μM B) 0.2–2.5 mM at a solid carbon nanorod (SCNR) whiskers (75 μg) modified SPE in 0.1 M PBS at pH 7 vs.Ag/AgCl at 0.1 Vs−1. | ||
| Sensing scheme/applied E | Linear range/μM L−1 | Limit of detection/μM | Ref. |
|---|---|---|---|
| a LOD determined through amperometric analysis. | |||
| HOPG and CNT modified GC electrode | — | 5 | 30 |
| E (vs.SCE) = 0.4 V | |||
| SPE (pretreated), flow injection analysis | 10–500 | 0.15 | 44 |
| E (vs.Ag/AgCl) = 0.4 V | |||
| Boron doped CNT on GC electrodea | Up to 1.400 | 0.05 | 45 |
| E (vs.SCE) = 0.3 V | |||
| CNT/chitosal film on GC electrode | 5–1000 | 3 | 46 |
| E (vs.SCE) = 0.4 V | |||
| Carbon nanofiber film on GC electrodea | 0.2–686 | 0.11 | 47 |
| E (vs.SCE) = 0.06 V | |||
| Meldola's blue functionalised CNTsa | Up to 4.500 | 0.05 | 48 |
| E (vs.SCE) = −0.1 V | |||
| Toludine blue/CNTs electropolymeriseda | Up to 4.500 | 0.5 | 49 |
| E (vs.SCE) = 0 V | |||
| Varimine blue adsorbed or covalently bound to CNTsa | Up to 2000 | 1.2 | 50 |
| E (vs.Ag/AgCl) = 0.05 V | |||
| Meldola's blue in SiO2/TiO2/graphite composite electrode | 18–7290 | 8 | 51 |
| E (vs.SCE) = −0.12 V |
Next, attention was turned to exploring the response of the SCNR whiskers towards the electrochemical oxidation of dopamine (DP), an essential neurotransmitter that plays a crucial role within the central nervous, renal, hormonal, and cardiovascular systems.34–35Neurotransmitters are chemical messengers, able to transmit signals from one neuron to the next. The determination of DP has therefore become of significant importance and the focus of much research. Fundamental investigation of its physiological function and improved diagnosis has resulted in an improved understanding of central nervous diseases, ensuing from atypical DP metabolism such as epilepsy, senile dementia, Parkinson and HIV infection.36–37 Many studies of dopamine optimize its electrochemical detection in the presence of other commonly occurring neurochemical interferents such as uric acid (UA), which is the primary end product of purine metabolism38 and often present in biological samples. Unusual levels of UA is a symptom displayed by several diseases such as hyperuricaemia, gout, and lesch-Nyhan disease,39 thus making its determination an important topic in clinic medicine.38 As depicted in Fig. 7, a voltammetric response is observed at −0.018 V (vs. Ag/AgCl) which increases with additions of dopamine. The observed peak potential compares well with +0.42 V at commercially available SPE,40 +0.3 V at ionic liquid modified SPEs40 and +0.175 V reported for magnetic particle (MP)/SWCNT modified SPEs.41
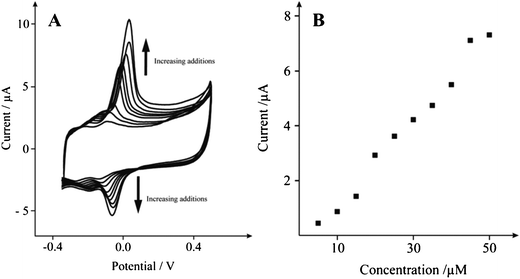 | ||
| Fig. 7 A: Typical cyclic voltammetric responses from the electrochemical oxidation of dopamine 10–50 μM at a solid carbon nanorod (SCNR) whiskers (75 μg) modified SPE in 0.1 M PBS at pH 7 vs.Ag/AgCl at 0.005 Vs−1. Part B depicts analysis of A in the form of peak height vs. concentration. | ||
Inspection of the voltammertric profile reveals prominent oxidation and reduction waves at ∼0.00 and ∼−0.074 V (at 0.005 Vs−1) respectively. Fig. 7B shows the analytical response of dopamine at the SCNR whisker modified SPE in a pH 7 buffer solution over the range of 10 to 50 μM (IP/A = 6.5 × 10−9 A μM−1 − 6.0 × 10−7 A; R2 = 0.985; N = 10). A limit of detection (based on 3-sigma) is found to correspond to 3.8 μM (n = 3), which compares favourably with the reported detection of 1 μM at SWCNTs viacyclic voltammetry.42Fig. 8 shows square wave analysis of additions of dopamine over the range of 10 to 50 μM (Ip/A = 7.3 × 10−8 A μM−1 − 6.2 × 10−7 A; R2 = 0.953; N = 4) at the SCNR whisker modified electrode, corresponding to a limit of detection (based on 3-sigma) of 7 μM (n = 3). Next the simultaneous sensing of dopamine and uric acid was explored which is required from an analytical sensing view point. As shown in Fig. 8, a new voltammertic peak close to the voltammetric peak arising from the electrochemical oxidation of dopamine is observed at +0.120 V with the inclusion of uric acid over the range of 20 to 120 μM (Ip/A = 1.99 × 10−8 A μM−1 − 3.54 × 10−7 A; R2 = 0.982; N = 4), where a 16 μM limit of detection (based on 3-sigma) was calculated, showing that the SCNR whisker electrode exhibits superior sensing performance over traditional noble metal (Au/Pt) nanoparticle modified carbon electrodes, with a detection limit of 24 μM and 21 μM reported for dopamine and UA respectively.43
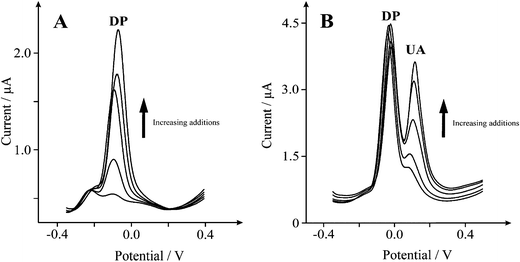 | ||
| Fig. 8 A: Typical square wave responses from the electrochemical oxidation of 10–50 μM dopamine (DP) in a 0.1 M PBS at pH 7 (frequency: 25 Hz). Part B depicts the addition of uric acid (UA) 20–120 μM. | ||
Conclusions
We have reported on the electrochemical performance of SCNR whiskers (a derivative of carbon nanotubes) which exhibit metallic conductivity and are completely free from metallic impurities unlike other available CNTs which imposes limitations due to inter and intra data viability. The electrochemical properties of these unique carbon nanostructures have been explored towards the electrochemical and analytical quantification of NADH, uric acid and dopamine, which has found to be useful over the low micro-molar to milli-molar range. These nanomaterials show great promise for sensing biologically relevant molecules and are clearly advantageous due to their high purity and high proportion of edge plane like-sites/defects and should be considered when designing electrochemical devices such as electro-analytical sensors.References
- J. J. Gooding, Electrochim. Acta, 2005, 50, 3049 CrossRef CAS.
- J. Wang, Electroanalysis, 2005, 17, 7 CrossRef CAS.
- C. E. Banks, R. R. Moore, T. J. Davies and R. G. Compton, Chem. Commun., 2004, 1804 RSC.
- C. E. Banks and R. G. Compton, Analyst, 2006, 131, 15 RSC.
- R. R. Moore, C. E. Banks and R. G. Compton, Anal. Chem., 2004, 76, 2677 CrossRef CAS.
- M. Pumera, S. Sánchez, I. Ichinose and J. Tang, Sens. Actuators, B, 2007, 123, 1195 CrossRef.
- A. Merkoςi, M. Pumera, X. Llopis, B. Perez, M. Del valle and S. Alegret, TrAC, Trends Anal. Chem., 2005, 24, 826 CrossRef.
- A. Fennimore, T. Yuzvinsky, T. D. Han, W.-Q. Han, M. S. Fuhrer, J. Cumings and A. Zettl, Nature, 2003, 424, 408 CrossRef CAS.
- J. A. Misewich, R. Martel, Ph. Avouris, J. C. Tsang, S. Heinze and J. Tersoff, Science, 2003, 300, 783 CrossRef CAS.
- G. G. Wildgoose, C. E. Banks, H. C. Leventis and R. G. Compton, Microchim. Acta, 2006, 152, 187 CrossRef CAS.
- X. Ji, R. O. Kadara, J. Krussma, Q. Chen and C. E. Banks, Electroanalysis, 2010, 22, 7 CrossRef CAS.
- D. A. C. Brownson and C. E. Banks, Analyst, 2010, 135, 2768–2778 RSC.
- B. Šljukić, C. E. Banks and R. G. Compton, Nano Lett., 2006, 6, 1556 CrossRef.
- C. E. Banks, T. J. Davies, G. G. Wildgoose and R. G. Compton, Chem. Commun., 2005, 829 RSC.
- C. E. Banks, A. Crossley, C. Salter, S. J. Wilkins and R. G. Compton, Angew. Chem., Int. Ed., 2006, 45, 2533 CrossRef CAS.
- R. B. Mathur, S. Seth, C. Lal, R. Rao, B. P. Singh, T. L. Dhami and A. M. Rao, Carbon, 2007, 45, 132 CrossRef CAS.
- A. P. Moravsky, E. M. Wexler and R. O. Loutfy, Carbon Nanotubes, 2005, 65 Search PubMed.
- V. Ivanov, J. B. Nagy, Ph. Lambin, A. Lucas, X. B. Zhang, X. F. Zhang, D. Bernaerts, G. Van Tendeloo, S. Amelinekx and J. Van Landuyt, Chem. Phys. Lett., 1994, 223, 329 CrossRef CAS.
- T.-J. Park, S. Banerjee, T. Hermraj-Bennya and S. S. Wong, J. Mater. Chem., 2006, 16, 141 RSC.
- M. E. Itkis, D. E. Perea, R. Jung, S. Niyogi and R. C. Haddon, J. Am. Chem. Soc., 2005, 127, 3439 CrossRef CAS.
- C. P. Jones, K. Jurkschat, A. Crossley and C. E. Banks, J. Iran. Chem. Soc., 2008, 5, 279 CAS.
- C. P. Jones, K. Jurkschat, A. Crossley, R. G. Compton, B. L. Riehl and C. E. Banks, Langmuir, 2007, 23, 9501 CrossRef CAS.
- http://www.scnte.com; accessed Jan 2011.
- P. M. Hallam and C. E. Banks, Phys. Chem. Chem. Phys., 2011, 13, 1210 RSC.
- R. O. Kadara, N. Jenkinson and C. E. Banks, Sens. Actuators, B, 2009, 138, 556 CrossRef.
- http://kanichi-research.com; accessed Jan 2011.
- A. Jorio, M. A. Pimenta, A. G. Souza Filho, R. Saito, G. Dresselhaus and M. S. Dresselhaus, New J. Phys., 2003, 5, 139 CrossRef.
- M. Pumera and Y. Miyahara, Nanoscale, 2009, 1, 260 RSC.
- D. Bom, R. Andrews, D. Jacques, J. Anthony, B. Chen, M. S. Meier and J. P. Selegue, Nano Lett., 2002, 2, 615 CrossRef CAS.
- C. E. Banks and R. G. Compton, Analyst, 2005, 130, 1232 RSC.
- I. Streeter and R. G. Compton, Sens. Actuators, B, 2008, 130, 620 CrossRef.
- R. Scipioni, M. Pumera, M. Boero, Y. Miyahara and T. Ohno, J. Phys. Chem. Lett., 2010, 1, 122 CrossRef CAS.
- M. Wooten and W. Gorski, Anal. Chem., 2010, 82, 1299 CrossRef CAS.
- J. R. Cooper, F. E. Bloom, R. H. Roth, The Biochemical Basis of Neuropharmacology, Oxford University Press, Oxford, 1982. Search PubMed.
- P. Damier, E. C. Hirsch, Y. Agid and A. M. Graybiel, The substantia nigra of the human brain: II. Patterns of loss of dopamine containing neurons in Parkinson's disease, Brain, 1999, 122, 1437 CrossRef.
- J. W. Mo and B. Ogorevc, Anal. Chem., 2001, 73, 1196 CrossRef CAS.
- R. M. Wightman, L. J. May and A.C. Michael, Anal. Chem., 1998, 60, 769 CrossRef.
- S.-H. Huang, H.-H. Liao and D.-H. Chen, Biosens. Bioelectron., 2010, 25, 2351 CrossRef CAS.
- V. V. S. E. Dutt and H. A. Mottola, Anal. Chem., 1974, 46, 1777 CrossRef CAS.
- J. Ping, J. Wu and Y. Ying, Electrochem. Commun., 2010, 12, 1738 CrossRef CAS.
- R. G. E. Baldrich, G. Gabriel and F. X. Muñoz, Biosens. Bioelectron., 2011, 26, 1876 CrossRef.
- C. B. Jacobs, M. J. Peairs and B. J. Venton, Anal. Chim. Acta, 2010, 662, 105 CrossRef CAS.
- S. Thiagarajan and S. M. Chen, Talanta, 2007, 74, 212 CrossRef CAS.
- J.-C. C. K. S. Prasad, C. Ay and J.-M. Zen, Sens. Actuators, B, 2007, 123, 715 CrossRef.
- C. Deng, J. Chen, X. L. Chen, C. Xiao, Z. Nie and S. Yao, Electrochem. Commun., 2008, 10, 907 CrossRef CAS.
- M. G. Zhang, A. Smith and W. Gorski, Anal. Chem., 2004, 76, 5045 CrossRef CAS.
- L. Wu, X. Zhang and H. Ju, Anal. Chem., 2007, 79, 453 CrossRef CAS.
- L. Zu, J. Zhai, R. Yang, C. Tian and L. Guo, Biosens. Bioelectron., 2007, 22, 2768 CrossRef.
- J. Zeng, W. Wei, L. Wu, X. Liu, K. Liu and Y. Li, J. Electroanal. Chem., 2006, 595, 152 CrossRef CAS.
- D. C. A. Radoi, M. A. Valcarcel, P. Placidi, S. Materazzi, D. Moscone and G. Palleschi, Electrochim. Acta, 2008, 53, 2161 CrossRef.
- C. M. Maroneze, L. T. Arenas, R. C. S. Luz, E. V. Benvenutti, R. Landers and Y. Gushikem, Electrochim. Acta, 2008, 53, 4167 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2011 |
