Effect of several ionic liquids on the synthesis of 1,3-diphenyl-3-(phenylamino)propan-1-one in supercritical carbondioxide
E.Sultan
Giray
*a,
Cinzia
Chiappe
b,
Zeynep
Tunalı
a and
Sunita
Rajamani
b
aDepartment of Chemistry, Arts&Science Faculty Çukurova University, 01330 Adana, Turkey. E-mail: esgiray@cu.edu.tr; Fax: +90-322 338 6070; Tel: +90-322 3387411
bDepartment of Chemistry and Industrial Chemistry, University of Pisa, Via Risorgimento 35, 56126 Pisa, Italy
First published on 7th September 2011
Abstract
We found that in a scCO2-ionic liquid hybrid reaction system, Mannich reaction of benzaldehyde, aniline and acetophenone can be remarkably accelerated and the yield of Mannich base 1,3-diphenyl-3-(phenylamino)propan-1-one was significantly high. This system would be available for the green reactions with good performance.
The Mannich reaction is a classic method for the preparation of β-amino carbonyl compounds and therefore a very important carbon-carbon bond-forming reaction in organic synthesis. The Mannich reaction has successfully been employed numerous times as a key step in natural product synthesis as well as in medicinal chemistry.1 However, due to the drastic reaction conditions and the long reaction times, the classical intermolecular Mannich reaction is plagued by a number of serious disadvantages.2 The Lewis acid-catalyzed condensation of silyl enol ethers or silyl ketene acetals to pre-formed imines is an excellent variant of the classical Mannich reaction. However, this Lewis acid-catalyzed three component reaction of aldehydes, amines and silyl enolates in the same vessel has to be carried out under strict anhydrous conditions because many of the imines are unstable in water. In addition, most Lewis acids cannot be used in this one-pot reaction because of the presence of free amines and water produced in the imine formation.3–11
Green chemistry, also known as sustainable chemistry, describes the search for reducing or even eliminating the use of substances in the production of chemical products and reactions in recent years. Green chemistry searches for alternative, environmentally friendly reaction media as compared to the traditional organic solvents and at the same time aims at increased reaction rates and lower reaction temperatures as well higher selectivities.
The ideal situation for a safe and green chemical process is using no solvent, however most of the chemical processes depend on solvents. Some of these solvents are soluble in water and therefore they must be stripped from water before it leaves the process not only for ecological but also for economic reasons. Solvents must be recovered for recycle and reuse for an economically viable process. Water, perfluorinated hydrocarbons and supercritical fluids (SCFs) are alternative solvents which may be used in green chemistry. Among these, the most promising elements of green chemistry are ILs and scCO2.
Ionic liquids have attracted extensive research interest in recent years as environmentally benign solvents due to their favorable properties like non-inflammability, negligible vapour pressure, reusability and high thermal stability.12,13 They have also been referred to as ‘designer solvents’ as their physico-chemical properties (viscosity, density, conductivity, solvent polarity and so on) can be adjusted by a careful choice of cation and anion. Nevertheless, it is possible to obtain acidic or basic ILs by introducing suitable functional groups on the cation or selecting appropriate anions. Combining these unique properties, ionic liquids are emerging as a green reaction media able to act as solvent and catalyst. The use of ionic liquids as a reaction medium may offer a convenient solution to both the solvent emission and catalytic recycling problem.12,14,15Mannich reactions in [bmim][PF6] have been reported16,17 as well as Mannich reactions using Brønsted acid ionic liquids as catalysts and solvent.18,19
Study of the reactions in IL–scCO2 mixed solvent is however a new and interesting topic. For example ionic liquids as coated catalysts or additives in scCO2 tremendously alter the selectivity pattern of the heterogeneous solid catalyst in the selective hydrogenation of limonene.20 Ionic liquid–scCO2 application also has some advantages for several reactions. For example it enhanced the stereoselectivity in a racemization reaction.21 According to our literature survey, we didn't see any studies of Mannich reactions in IL–scCO2 mixed solvent. Herein, we report the possibility of an IL–scCO2 dual system as a potential new, clean and efficient method for synthesis and recovery of β-amino carbonyl compoundsviaMannich reaction.
In this study, we chose the reaction of benzaldehyde 1, acetophenone 2 and aniline 3 as a model reaction to test the catalytic activity of a series of ILs on the Mannich reaction. The Mannich reaction of compounds 1, 2 and 3 has been conducted under two different conditions (in scCO2 and at room conditions) in the presence of ILs bearing Brønsted acidic cations and/or protic anions ([Hmim]2[SO4], [Hmim][HSO4] and [N112OH][H2PO4]), basic cations ([Cndabco]Br) or basic cations and anions [Cndabco][N(CN)2]) and in [bmim][CF3SO3]. All reagents were found to be soluble in ILs except [Hmim]2[SO4] and [Hmim][HSO4] since they were sticky solids under room conditions. Hence, in the experiments at room temperature diethyl ether was used as reaction solvent and two ILs were used as catalysts.
Reactions in scCO2
The use of the IL–scCO2 dual system had a significant effect on the synthesis of compound 4 (Scheme 1). In the case of the IL–scCO2 dual system, the reaction times were considerably decreased (4 h in contrast to 24 h) and yields of reaction increased (54–87% relative to nil-58%). scCO2 probably promotes the association of the reactants in a solvent cavity during the activation process inducing an acceleration of Mannich reaction in comparison to ILs only.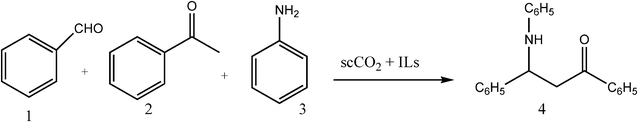 | ||
| Scheme 1 | ||
The results are summarized in Table 1. No Mannich base is observed in the absence of ionic liquids (entry 1). Moreover, ILs can catalyse the Mannich reaction more efficiently in scCO2 than under room conditions (Table 1, entries 2–5, 7,11).
| Entry | IL (1 mmol) | Yield (%) | |
|---|---|---|---|
| scCO2 | Room cond. | ||
| a The product started to form after 3 h. b The product started to form after 6 h. | |||
| 1 | Non | n.o. | n.o. |
| Basic ILs | |||
| 2 | [C4dabco][N(CN)2] | 63 | trace, 96 h |
| 3 | [C6dabco][N(CN)2] | 71 | 58, 12 ha |
| 4 | [C8dabco][N(CN)2] | 87 | 51, 4 h |
| 5 | [C10dabco][N(CN)2] | 61 | trace, 48 h |
| 6 | [C6dabco]Br | Trace | 16, 72 h |
| 7 | [C10dabco]Br | 36 | trace, 96 h |
| Acidic Ils | |||
| 8 | [Hmim]2[SO4] | 5 | 45, 24 hb |
| 9 | [Hmim][HSO4] | Trace | n.o. |
| 10 | [N1112OH][H2PO4] | 16 | 44, 48 h |
| Neutral IL | |||
| 11 | [bmim][CF3SO3] | 76 | 53, 24 h |
Almost all of the ionic liquids used were found to be suitable for the Mannich reaction. However, monoquaternarized diazabicyclo[2.2.2]octane (dabco) based ILs were more effective in terms of Mannich product yield than acidic ILs. Higher yields (87, 71, 63, 54%) were obtained with dabco based ILs in comparison with acidic ILs as catalysts; in particular, using [C8dabco][N(CN)2] in scCO2 a yield of 87% was obtained (Table 1, entry 4). This class of ILs associates a basic cation, characterized by a tertiary nitrogen atom, to a basic anion (dicyanamide). It is noteworthy that the same cation associated with bromide did not have the same impact on the Mannich product yield (i.e. entries 4 and 6) suggesting that the [N(CN)2] anion is directly involved in the catalytic performance or, much more that the bromide anion, it is able to favor the interaction of the basic IL cation with the reagents in the rate determining transition state. ILs are indeed equimolar mixtures of positively and negatively charged species (cations and anions) which interact with each other to give three-dimensional networks whose structure depends on cation and anion nature; anion and cation nature determines interaction strength and space disposition.22 Starting from this consideration, since the bromide anion generally gives stronger interactions with the IL cations than dicyanamide23 we cannot exclude the possibility that the bromide effect may be related at least partially to the increased network ability that reduces the interaction of IL components with dissolved species.
Nevertheless, data reported in Table 1 show that the Mannich reaction is also affected by the alkyl chain length on the cation (entries 2–5); catalytic amounts of ionic liquids based on long chain mono alkylated DABCO cations and [N(CN)2]− anions catalyse the Mannich reaction in relatively shorter times and give high isolated yields. The alkyl chain length determines the possibility of formation and the entity of polar and unpolar regions inside the IL network, a feature which can be particularly important in the case of IL-scCO2 system. Therefore, it can be concluded that both the anion and the cation in the ionic liquid play an important role as the catalyst toward the Mannich reaction.
Despite the fact that the Mannich reaction may be catalyzed also by acids, [Hmim]2[SO4]-scCO2 dual system gave lowest yields when compared to the other ionic liquids presently studied (Table 1, entry 8). On the other hand, the comparison between the cholinium phosphate ([N112OH][H2PO4]) IL bearing the amphoteric diprotic anion, H2PO4−, and the ILs based on the Brønsted acid methylimidazolium cation, [Hmim]+, and a neutral or monoprotic sulfate anion ([Hmim]2[SO4] and [Hmim][HSO4]) shows that these latter acid catalysts give the lowest yields in scCO2 conditions (Table 1, entries 8, 9 and 10). The low efficiency of the methylimidazolium based ILs can be explained considering that the acidity of these salts, in particular in scCO2 conditions, is sufficiently high to bind the amine and reduce the formation of Mannich base.
Finally, to mention the catalytic effect of [bmim][CF3SO3], which does not bear acidic or basic moieties, which is able to give the Mannich product in relatively high yield. This behavior could be due to some specific effects of this IL; [bmim]+ is able to act as a hydrogen bond donor whereas the triflate anion shows a significant hydrogen bond acceptor ability, comparable to that of the more basic dicyanamide anion: the β values being around 0.5 in both cases.24,25 Both these properties could sufficiently stabilize the rate determining transition state and favor product formation.
Reactions at room temperature
Generally, in room temperature reactions where ILs are used as both solvents and catalysts, the reaction rate of Mannich process was lower. Also in this case a dabco-based IL having dicyanamide as counteranion, [C6dabco][N(CN)2], gave highest Mannich product yield; the product started to form after 3 h and reached 58% over a period of 12 h. On the contrary to the scCO2–IL dual system, the Brønsted acidic IL [Hmim]2[SO4] gave a higher Mannich product yield under room conditions. The yield was 45% over a period of 24 h (the product started to form after 6 h). When we compare our results with literature data,21 we have to conclude that some sultone-based Brønsted acidic ionic liquids are able to give higher Mannich products in shorter reaction times than the acidic ILs used in this study. On the other side, the lower Mannich product yield and longer reaction times observed in the experiments carried out at room temperature show that scCO2 can act as a means to favor the interaction of the substrates with the ionic liquid.Experimental
Chemicals
All the benzaldehyde, acetophenone, aniline and [bmim][CF3SO3] were obtained from Merck. All other ionic liquids were synthesized as described below.Synthesis of ionic liquids
Ionic liquids (Scheme 2) based on monoquaternarized diazabicyclo[2.2.2]octane (dabco) cations [Cndabco]+ associated with dicyanamide anions ([N(CN)2]−) have been obtained by metathesis of the corresponding bromide salts ([Cndabco][N(CN)2] with silver dicyanamide, freshly prepared by reacting AgNO3 and Na[N(CN)2] in equimolar quantities, as previously reported.26Methylimidazolium sulfate ([Hmim]2[SO4]) and methylimidazolium hydrogensulfate ([Hmim][HSO4] have been prepared by a dropwise addition of the proper amount of H2SO4 (97%) to N-methylimidazole. The resulting mixture was stirred at 60° for 5 h. Cholinium phosphate ([N1112OH[H2PO4]) was prepared by the neutralisation of a commercially available aqueous solution of [N1112OH][OH] with H3PO4.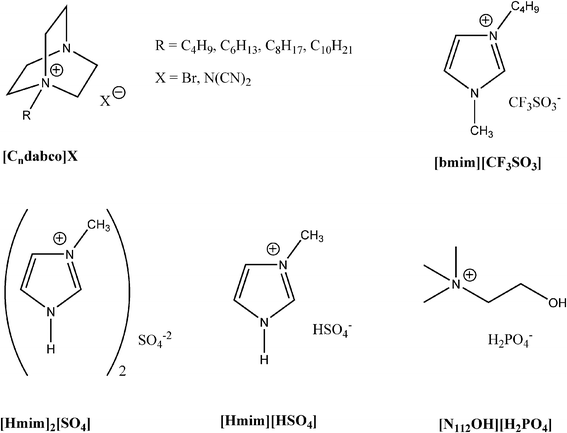 | ||
| Scheme 2 | ||
Synthesis of 1,3-diphenyl-3-(phenylamino)propan-1-one: scCO2-IL dual system
In a typical reaction, benzaldehyde (3 mmol), aniline (5 mmol), acetophenone (5 mmol), and ionic liquids (1 mmol) as catalysts were placed in to a stainless steel reactor with 20 ml capacity and furnished with a thermocouple and pressure gauge. 70 atm CO2 pumped into reactor with an ISCO pump. The reaction temperature was 60 °C and the reaction time was 4 h. During the experiments, the pressure increased to 250 atm. After the reaction, the ionic liquid was separated from the reaction mixture by extraction with copious amount of water (5 × 3 ml). The ionic liquid being soluble in water comes in the water layer. The solid was separated by filtration and the product was recrystallized from ethanol. The product was identified using FTIR on a Perkin-Elmer spectro-photometer using KBr plates in a frequency range.Synthesis of 1,3-diphenyl-3-(phenylamino)propan-1-one: typical procedure at room condition
In a typical reaction, benzaldehyde (3 mmol), aniline (5 mmol), acetophenone (5 mmol), and ionic liquids (1 mmol) as catalysts and solvent (except the reaction with [hmim]2[SO4] and [hmim][HSO4], where ether was used as solvent) were stirred at room temperature (25 °C) in a round-bottomed flask fitted with a condenser. After a certain time the reaction mixture became viscous and solidified. At this stage the time was noted and the ionic liquid was separated from the reaction mixture by extraction with copious amounts of water (5 × 3 ml). The ionic liquid, being soluble in water, comes in the water layer. The solid was separated by filtration and the product was recrystallized from ethanol. The product was identified using FTIR on a Perkin-Elmer spectro-photometer using KBr plates in a frequency range.References
- N. Risch, M. Arend and B. Westermann, Angew. Chem., Int. Ed., 1998, 37, 1044 CrossRef.
- S.J. Danishefsky, S. Chackalamannil, P. Harrison, M. Silvestri and P. Cole, J. Am. Chem. Soc., 1985, 107, 2474 CrossRef CAS.
- C. Gennari, I. Venturini, G. Gislon and G. Schimperna, Tetrahedron Lett., 1987, 28, 227 CrossRef CAS.
- G. Guanti, E. Narisano and R.L. Ban, Tetrahedron Lett., 1987, 28, 4331 CrossRef CAS.
- T. Mukaiyama, K. Kashiwagi and S. Matsui, Chem. Lett., 1989, 18, 1397 CrossRef.
- T. Mukaiyama, H. Akamatsu and J. S. Han, Chem. Lett., 1990, 19, 889 CrossRef.
- M. Onaka, R. Ohno, N. Yanagiya and Y. Izumi, Synlett, 1993, 141 CrossRef CAS.
- O. Ishihra, M. Funahashi, N. Hanaki, M. Miyata and H. Yamamoto, Synlett, 1994, 963 CrossRef.
- S. Kobayashi, M. Araki and M. Yasuda, Tetrahedron Lett., 1995, 36, 5773 CAS.
- P.G. Cozzi, B.D. Simone and A. Umani-Ronchi, Tetrahedron Lett., 1996, 37, 1691 CrossRef CAS.
- T. Welton, Chem. Rev., 1999, 99, 2071 CrossRef CAS.
- P. Wasserscheid and W. Keim, Angew. Chem., Int. Ed., 2000, 39, 3773 CrossRef.
- M. J. Earle, P. B. McCormac and K. R. Seddon, Chem. Commun., 1998, 2245 RSC.
- F. Liu, M. B. Abrams, R.T. Baker and W. Tumas, Chem. Commun., 2001, 433 RSC.
- E. D. Bates, R. D. Mayton, I. Ntai and J. H. Davis, J. Am. Chem. Soc., 2002, 124, 926 CrossRef CAS.
- X.-F. Yang, M. Wang, R. S. Varma and C.-J. Li, J. Mol. Catal. A: Chem., 2004, 214, 147 CrossRef CAS.
- S.-Gi. Lee and J. H. Park, Bull. Korean Chem. Soc., 2002, 23, 1367 CrossRef CAS.
- G. Zhao, T. Jiang, H. Gao, B. Han, J. Huang and D. Sun, Green Chem., 2004, 6, 75 RSC.
- S. Sahoo, T. Joseph and S. B. Halligudi, J. Mol. Catal. A: Chem., 2006, 244, 179 CrossRef CAS.
- E. Bogel-Łukasika, S. Santosa, R. Bogel-Łukasika and M. Nunes da Ponte, J. Supercrit. Fluids, 2010, 54, 210 CrossRef.
- P. Lozano, T. De Diego, M. Larnicol, M. Vaultier and J. I. Iborra, Biotechnol. Lett., 2006, 28, 1559 CrossRef CAS.
- R. Bini, C. Chiappe, V. L. Mestre, C. S. Pomelli and T. Welton, Org. Biomol. Chem., 2008, 6, 2522 CAS.
- C. Chiappe, Monatsh. Chem., 2008, 138, 1035 CrossRef.
- R. Bini, O. Bortolini, C. Chiappe, D. Pieraccini and T. Siciliano, J. Phys. Chem. B, 2007, 111, 598 CrossRef CAS.
- T. P. Wells, J. P. Hallett, C. K. Williams and T. Welton, J. Org. Chem., 2008, 85, 5585 CrossRef.
- C. Chiappe, B. Melai, A. Sanzone and G. Valentini, Pure Appl. Chem., 2009, 81, 2035 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2011 |
