Controlled stereoselective polymerization of lactide monomers by group 4 metal initiators that contain an (OSSO)-type tetradentate bis(phenolate) ligand†
Jean-Charles
Buffet
a,
Ashley N.
Martin
a,
Moshe
Kol
b and
Jun
Okuda
*a
aInstitute of Inorganic Chemistry, RWTH Aachen University, Landoltweg 1, D-52056, Aachen, Germany. E-mail: jun.okuda@ac.rwth-aachen.de; Fax: +49 241 80 92 644
bSchool of Chemistry, Raymond and Beverly Sackler Faculty of Exact Sciences, Tel Aviv University, Ramat Aviv, Tel Aviv 69978, Israel
First published on 15th August 2011
Abstract
Group 4 metal complexes Zr-1 to Zr-4, Ti-4 and Ti-4a that contain an (OSSO)-type tetradentate bis(phenolate) ligand were found to initiate the ring-opening polymerization of meso-, rac-, and L-lactides in toluene solution. The polymerizations were controlled with initiator efficiency values around one and gave polymers with low polydispersity (Mw/Mn < 1.2). The polylactides were atactic when rac-lactide was used as the monomer and heterotactic biased when meso-lactide was used. Kinetic measurements showed that meso-lactide was polymerized generally faster than rac- and L-lactide.
Introduction
Poly(lactic acids) (PLAs) have been studied intensively during the past few decades because of their biodegradability and biocompatibility.1,2PLA possesses versatile physical properties and has been used in medical applications and tissue engineering such as media for the controlled drug release.3Ring-opening polymerization (ROP) of lactide (LA) by single-site initiators is the most efficient route to PLAs with controlled molecular weight and narrow molecular weight distribution. The two stereogenic centers in one lactide molecule result in three distinct configurational isomers (S,S)-LA, (L-LA), (R,R)-LA, (D-LA) and (R,S)-LA, meso-LA. The 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of (S,S)-LA and (R,R)-LA is referred to as rac-LA (Scheme 1).
1 mixture of (S,S)-LA and (R,R)-LA is referred to as rac-LA (Scheme 1).
 | ||
| Scheme 1 Lactide monomers. | ||
Group 4 metal complexes are widely used as single-site catalysts for the polymerization of olefins.4 Currently, there is interest in the so-called post-metallocene complexes which may serve as new types of single-site polymerization catalysts.5 Polydentate ligands for metal complexation are crucial for structurally well-defined initiators for lactide polymerization as well: Davidson et al.6a and Eisen et al.6b recently reported titanium(IV) and zirconium(IV) complexes with two bidentate ligands, whilst Harada et al.7a and Mountford et al.7b introduced tridentate ligands. Recently, Ishii et al. reviewed group 4 metal complexes with (OSSO)-type bis(phenolato) ligands and their use as polymerization catalysts for olefins.8Kolet al. used an (OSSO)-type ligand that is analogous to the (ONNO)-Salan type ligand, containing a methylene group between the sulfur atoms and the phenol groups. The resulting group 4 metal complexes polymerized 1-hexene, affording stereoirregular poly(1-hexene).9 Furthermore, group 4 metal complexes containing an (OSSO)-type dithiobis(phenolate) ligand polymerized rac- and L-lactide efficiently, but with a low level of control.10 In contrast, initiators based on group 4 metal bis(phenolate) complexes catalyze the syndioselective polymerization of meso-lactide efficiently and in a controlled fashion.11
We report here the use of further group 4 metal complexes with an (OSSO)-type ligand framework as initiators for the polymerization of lactide monomers. The complexes reported are structurally classified according to their chelate ring size (5-5-5, 5-6-5 and 6-5-6) at the metal center.8,9
Results and discussion
Synthesis and characterization of complexes
To explore the steric and possible electronic influence of substituents on the phenolate rings on the stereoselective ROP of lactide, a set of proligands with and without a methylene group between the sulfur atoms and the phenol groups in the backbone were prepared. The proligands were selected to allow direct comparison with each other (Scheme 2). | ||
| Scheme 2 Group 4 metal complexes. | ||
Complexes Zr-1 to Zr-4 were synthesized by the reaction of [Zr(OtBu)4] with one equivalent of proligand H2-1 to H2-4, respectively, in n-pentane at 25 °C for 18 h. Complexes Ti-4 and Ti-4a were synthesized by the reaction of [Ti(OiPr)4] or [Ti(OiPr)3Cl] with one equivalent of H2-4 in toluene at 25 °C for 18 h. Complexes Zr-1 and Zr-3 were isolated as colorless solids after crystallization from a cold n-pentane solution (Fig. S1–S4†). Complexes Zr-1 and Zr-3 were obtained in 57% and 30% yields, respectively. The 1H NMR spectrum for complex Zr-1 shows a diagnostic doublet of doublets at 2.5 ppm for the bridging CH2 protons. In addition, a singlet at 1.85 ppm represents the methyl protons from the tert-butoxy group.
Stoichiometric reaction of proligand H2-4 with [Ti(OiPr)3Cl] at 25 °C in n-pentane afforded complex Ti-4a, as orange crystals in 78% yield after crystallization from a cold n-pentane solution (Fig. S11–S12†). A diagnostic multiplet signal is found at 5.10 ppm (1H) which corresponds to OCH(CH3)2. The bridging protons corresponding to SCH2CH2S and SCH2Ph are shifted downfield from 3.76 to 3.50 ppm and 2.57 to 1.92 ppm, respectively, upon complexation.
Polymerization of meso-lactide
Complexes Zr1 to Zr4, Ti-4 and Ti-4a were tested in the polymerization of meso-lactide in toluene at 100 °C (Scheme 3). The results are shown in Table 1.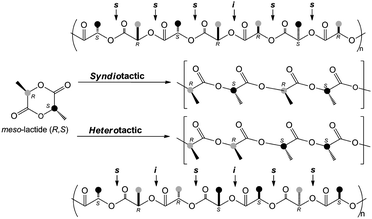 | ||
| Scheme 3 Stereoselective polymerization of meso-lactide to afford syndio- or hetero-tactic PLAs including their respective microstructures. | ||
| Entry | Init. | Time/h | Conv.b (%) | M n,exp c/g mol−1 | f d | M w/Mnc | P s e |
|---|---|---|---|---|---|---|---|
| a Polymerization conditions: [LA]0/[init]0 = 100, [LA]0 = 0.520 M, T = 100 °C, toluene, 2 mL. b Conversion of monomer (([LA]0 − [LA]t)/[LA]0). c Measured by GPC with PS standards in THF, corrected by multiplication by 0.58.12 d Efficiency calculated using f = Mn,exp/Mn,theo with Mn,theo = [LA]0/[init]0 × 144.13 × conv. e P s is the probability of a new s dyad.13 f M n,theo = [LA]0/2[init]0 × 144.13 × conv. | |||||||
| 1 | Zr-1 | 0.5 | 29 | 6500 | 1.29 | 1.03 | 0.18 |
| 2 | Zr-1 | 1 | 55 | 9800 | 1.24 | 1.02 | 0.16 |
| 3 | Zr-1 | 4 | 95 | 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
1.23 | 1.07 | 0.20 |
| 4 | Zr-2 | 4 | 67 | 9200 | 0.95 | 1.06 | 0.24 |
| 5 | Zr-3 | 48 | 84 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
1.05 | 1.16 | 0.36 |
| 6 | Zr-4 | 24 | 47 | 8100 | 1.19 | 1.03 | 0.50 |
| 7 | Ti-4 | 72 | 55 | 4800 | 1.21f | 1.14 | 0.42 |
| 8 | Ti-4a | 72 | 48 | 4500 | 0.65 | 1.08 | 0.44 |
The polymerization of meso-lactide by complex Zr-1, based on 5-5-5 (OSSO)-type ligand 1, proceeded with efficiency values f of around 1.2. The efficiency value decreased with an increase in the steric bulk of the ligand's ortho- and para-substituents from tert-butyl, Zr-1 (f = 1.23), to cumyl (CMe2Ph), Zr-2 (f = 0.95). Complex Zr-3, based on flexible (OSSO)-type ligand 3 with a 5-6-5 chelate array, polymerized meso-lactide the fastest with efficiency value f = 1.05. However, complex Zr-4, based on (OSSO)-type ligand 4 with a 6-5-6 chelate array, polymerized meso-lactide the slowest with less control over the polymerization (f = 1.19). The poly(meso-lactides) synthesized by group 4 metal complexes with an (OSSO)-type ligand showed low polydispersity (1.02 < Mw/Mn < 1.16).
Poly(meso-lactides) produced using complexes Zr-1 and Zr-2 with the 5-5-5 chelate based on (OSSO)-type ligands 1 and 2 showed heterotactic biased PLAs (0.18 < Ps < 0.24). Heterotacticity decreased from a Ps of 0.20 to 0.50 when initiators 3 and 4 with more flexible ligands were used. When the metal is changed from Zr-4 to Ti-4, complexes with the 6-5-6 chelate based on the (OSSO)-type ligand, the heterotacticity did not change (Ps of 0.50 and 0.42, respectively). Previously, we reported that the group 4 metal bis(phenolate) complex with 5-5-5 and 5-6-5 (OSSO)-chelates led to syndiotactic biased PLAs.11 The somewhat unexpected formation of heterotactic PLA from meso-lactide is ascribed to site epimerization (Λ–Δ inversion) or to chain transfer after each ring-opening step, triggered by traces of acids.17c
In Fig. 1, 13C{1H} NMR spectra of the methine region are shown which indicate the decrease of heterotacticity with increasing flexibility of the ligand.
 | ||
Fig. 1
13C{1H} NMR spectra of the methine region of poly(meso-lactides) obtained at 100 °C with an initiator![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) monomer ratio of 1 monomer ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 in toluene initiated by (a) Zr-1 and (b) Zr-4. 100 in toluene initiated by (a) Zr-1 and (b) Zr-4. | ||
Polymerization of rac-lactide
Complexes Zr1, Zr4, Ti-4 and Ti-4a were tested in the polymerization of rac-lactide in toluene at 100 °C (Scheme 4). The results of the polymerization are shown in Table 2.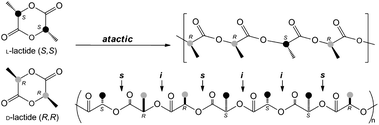 | ||
| Scheme 4 Stereoselective polymerization of rac-lactide to afford atactic PLAs including its respective microstructure. | ||
| Entry | Init. | Time/h | Conv.b (%) | M n,exp c/g mol−1 | f d | M w/Mnc | P s e |
|---|---|---|---|---|---|---|---|
| a Polymerization conditions: [LA]0/[init]0 = 100, [LA]0 = 0.520 M, T = 100 °C, C6D6, 0.5 mL. b Conversion of monomer (([LA]0 − [LA]t)/[LA]0). c Measured by GPC with PS standards in THF, corrected by multiplication by 0.58.12 d Efficiency calculated using f = Mn,exp/Mn,theo with Mn,theo = [LA]0/[init]0 × 144.13 × conv. e P s is the probability of a new s dyad.13 f M n,theo = [LA]0/2[init]0 × 144.13 × conv. | |||||||
| 1 | Zr-1 | 15 | 87 | 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
1.65 | 1.15 | 0.48 |
| 2 | Zr-4 | 141 | 90 | 8150 | 0.63 | 1.11 | 0.49 |
| 3 | Ti-4 | 255 | 69 | 4430 | 0.71f | 1.18 | 0.54 |
| 4 | Ti-4a | 255 | 86 | 5740 | 0.58 | 1.08 | 0.54 |
The polymerization of rac-lactide by complex Zr-1 led to a high efficiency value of PLA, indicating the possible occurrence of transesterification reactions (f = 1.65). The introduction of the flexible linker in complex Zr-4 decreased the efficiency of the polymerization of rac-lactide (f = 0.63). This mirrors the results found with meso-lactide. The polymerization of rac-lactide showed a slightly higher efficiency value when the metal was changed from Zr-4 to Ti-4 (f = 0.71). However, on going from the two isopropoxide groups to one (Ti-4a), the poly(rac-lactides) produced after 255 h but at a lower conversion level showed the lowest efficiency value (f = 0.58), indicating that the presence of the chloro ligand has a strong effect over the polymerization. Similar efficiency values for the ROP of rac-lactide were reported in the literature.7b,14 All the poly(rac-lactides) exhibited low polydispersities (1.08 < Mw/Mn < 1.18).
The ROP of rac-lactide using complexes Zr1, Zr4, Ti-4 and Ti-4a give an essentially atactic microstructure of poly(rac-lactides). The introduction of a methylene linker, change in the metal from zirconium to titanium, or in the number of initiating groups did not influence the microstructure (Fig. S17–S20†). The poly(rac-lactides) synthesized by using group 4 metal complexes have been shown in the literature to give isotactic,15 heterotactic,6a,14b,e or atactic polymers.6a,14c
Polymerization of L-lactide
The polymerizations of L-lactide by complexes Zr-1 and Zr-4 proceeded with high values of efficiency but low polydispersities (Mw/Mn of 1.06, f of 1.59 for Zr-1) and (Mw/Mn of 1.11, f of 1.40 for Zr-4). Kolet al. reported similar results for group 4 metal complexes in polymerizing L-lactide.16 Analysis of the microstructures by 1H{1H} and 1H NMR spectroscopy showed that the polymers produced from L-lactide exhibited high isotacticity, Pi > 0.95 (Fig. S21–S24†).Kinetic studies of the meso-lactide polymerization
Pseudo-first order kinetic data of the polymerization of meso-lactide at 100 °C in benzene-d6, with an initiator![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) monomer ratio of 1
monomer ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 using complexes Zr-1 to Zr-4, Ti-4 and Ti-4a are shown in Fig. 2 and Table S1–S6†.
100 using complexes Zr-1 to Zr-4, Ti-4 and Ti-4a are shown in Fig. 2 and Table S1–S6†.
![Semilogarithmic plots of meso-lactide conversion vs. time, [LA]0/[init]0 = 100, [LA]0 = 0.520 M, T = 100 °C, benzene-d6 (0.5 mL): (a) using zirconium complexes: Zr-1 (kobs = 423 × 10−3 h−1), Zr-2 (kobs = 271 × 10−3 h−1), Zr-3 (kobs = 535 × 10−3 h−1), and Zr-4 (kobs = 38 × 10−3 h−1); and (b) using the flexible ligand 4: Ti-4 (kobs = 16 × 10−3 h−1) and Ti-4a (kobs = 10 × 10−3 h−1).](/image/article/2011/PY/c1py00266j/c1py00266j-f2.gif) | ||
| Fig. 2 Semilogarithmic plots of meso-lactide conversion vs. time, [LA]0/[init]0 = 100, [LA]0 = 0.520 M, T = 100 °C, benzene-d6 (0.5 mL): (a) using zirconium complexes: Zr-1 (kobs = 423 × 10−3 h−1), Zr-2 (kobs = 271 × 10−3 h−1), Zr-3 (kobs = 535 × 10−3 h−1), and Zr-4 (kobs = 38 × 10−3 h−1); and (b) using the flexible ligand 4: Ti-4 (kobs = 16 × 10−3 h−1) and Ti-4a (kobs = 10 × 10−3 h−1). | ||
The C3 bridged complex, Zr-3, based on the 5-6-5 (OSSO)-type ligand, showed the highest activity in the ROP of meso-lactide (kobs = 535 × 10−3 h−1), followed by the C2 bridged complex, Zr-1, based on the 5-5-5 (OSSO)-type ligand (kobs = 423 × 10−3 h−1). This is in agreement with our previous observation that the slowest among all of the titanium complexes tested was the one that possessed a rigid trans-1,2-cyclohexanediyl backbone and the fastest was the titanium complex with a C3 bridged backbone.11
Higher activity is observed with the complexes containing tert-butyl substituents, Zr-1 (kobs = 423 × 10−3 h−1), rather than cumyl (CMe2Ph), Zr-2 (kobs = 271 × 10−3 h−1). This is to be expected as coordination of the monomer to the Zr-1 complex with tert-butyl substituents is less sterically hindered, allowing easier monomer addition.
The introduction of a flexible linker (SCH2Ph) between the phenol ring and the sulfur bridge of the ligand has a significant effect on the polymerization rates of meso-lactide. Complex Zr-1, based on the 5-5-5 (OSSO)-type ligand, has 11 times higher activity (kobs = 423 × 10−3 h−1) than Zr-4, based on the 6-5-6 chelate array (kobs = 38 × 10−3 h−1).
This slow rate with the flexible ligand can also be observed by changing the metal center to titanium using complex Ti-4 (kobs = 16 × 10−3 h−1) which has twice as many initiating OiPr groups than Ti-4a which showed ca. 1.6 times faster rate for the polymerization of meso-lactide (kobs = 10 × 10−3 h−1). This is attributed to a combination of steric and strong electronic effects of the chloro ligand in comparison with those from a second growing chain (i.e. starting from the bis(isopropoxide) titanium initiator Ti-4).
The polymerization of meso-lactide using Zr-1 in toluene (kobs = 1360 × 10−3 h−1) showed a faster rate than in benzene-d6 (kobs = 423 × 10−3 h−1) (Fig. S25†). This rate for the polymerization of meso-lactide in toluene is comparable to the fastest rate reported in the literature by Feijen et al. with salan aluminium complexes.17c The polymerization rates for the polymerization of meso-lactide in benzene-d6 are the highest for group 4 metal initiators,11 but lower than those of other initiator metals reported in the literature.17
Kinetic studies of the polymerization of rac- and L-lactides
Kinetic studies of the polymerization of rac-lactide at 100 °C in benzene-d6 with an initiator![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) monomer ratio of 1
monomer ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 using complex Zr-1 and complexes bearing the flexible linkers Zr-4, Ti-4, and Ti-4a are shown in Fig. S26 and Table S7–S10†.
100 using complex Zr-1 and complexes bearing the flexible linkers Zr-4, Ti-4, and Ti-4a are shown in Fig. S26 and Table S7–S10†.
As with the ROP of meso-lactide, Zr-1 is faster than Zr-4 towards the ROP of rac-lactide (kobs = 142 × 10−3 h−1 and kobs = 21 × 10−3 h−1 respectively). Likewise, Zr-4 is faster than Ti-4. However, the rate of the polymerization of rac-lactide using Ti-4 showed an initiation period with a higher rate constant (kobs = 14 × 10−3 h−1) followed by a slower period after around 50% conversion (kobs = 5 × 10−3 h−1). Interestingly, the rate for the slower second phase was equal to that of propagation using Ti-4a (kobs = 5 × 10−3 h−1). This could be due to the growing polymer chains becoming too large for initiation by both isopropoxide groups. A rate similar to that of the mono(isopropoxide) complex Ti-4a may result. The titanium and zirconium complexes polymerized rac-lactide more slowly than other group 4 metal complexes reported in the literature.6a,7b,14b
Kinetic studies of the polymerization of L-lactide at 100 °C in benzene-d6 with an initiator![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) monomer ratio of 1
monomer ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 using Zr-1 and Zr-4 are shown in Fig. S27 and Table S11–S14†. As was the case for meso- and rac-lactides, Zr-1 polymerizes L-lactide faster (kobs = 164 × 10−3 h−1) than does Zr-4 (kobs = 24 × 10−3 h−1). In addition, zirconium initiators polymerized L-lactide faster than the titanium homologs (kobs = 3 × 10−3 h−1 for Ti-4, kobs = 7 × 10−3 h−1 for Ti-4a) (Fig. S28–S29†).
100 using Zr-1 and Zr-4 are shown in Fig. S27 and Table S11–S14†. As was the case for meso- and rac-lactides, Zr-1 polymerizes L-lactide faster (kobs = 164 × 10−3 h−1) than does Zr-4 (kobs = 24 × 10−3 h−1). In addition, zirconium initiators polymerized L-lactide faster than the titanium homologs (kobs = 3 × 10−3 h−1 for Ti-4, kobs = 7 × 10−3 h−1 for Ti-4a) (Fig. S28–S29†).
The kinetic results of the polymerization of meso-, rac- and L-lactides using Zr-1 and Zr-4 are given in Fig. 3. meso-Lactide is polymerized faster than rac- and L-lactides, when using Zr-1 and Zr-4 (Table 3). This can be attributed to the fact that the RS configuration of meso-lactide is more strained in comparison to the RR and SS configuration found in rac-lactide.18 Furthermore, rac- and L-lactide are polymerized with similar propagation rates relative to each other (Table 3).
![Semilogarithmic plots of lactide monomer conversion vs. time, [LA]0/[init]0 = 100, [LA]0 = 0.520 M, T = 100 °C, benzene-d6 (0.5 mL): (a) Zr-1meso-lactide (kobs = 423 × 10−3 h−1), l-lactide (kobs = 164 × 10−3 h−1), and rac-lactide (kobs = 142 × 10−3 h−1); and (b) Zr-4meso-lactide (kobs = 38 × 10−3 h−1), l-lactide (kobs = 24 × 10−3 h−1), and rac-lactide (kobs = 21 × 10−3 h−1).](/image/article/2011/PY/c1py00266j/c1py00266j-f3.gif) | ||
| Fig. 3 Semilogarithmic plots of lactide monomer conversion vs. time, [LA]0/[init]0 = 100, [LA]0 = 0.520 M, T = 100 °C, benzene-d6 (0.5 mL): (a) Zr-1meso-lactide (kobs = 423 × 10−3 h−1), L-lactide (kobs = 164 × 10−3 h−1), and rac-lactide (kobs = 142 × 10−3 h−1); and (b) Zr-4meso-lactide (kobs = 38 × 10−3 h−1), L-lactide (kobs = 24 × 10−3 h−1), and rac-lactide (kobs = 21 × 10−3 h−1). | ||
| Entry | Initiator | Monomer | Solvent | k obs/×10−3 h−1 |
|---|---|---|---|---|
| a Polymerization conditions: [LA]0/[init]0 = 100, [LA]0 = 0.520 M, T = 100 °C. | ||||
| 1 | Zr-1 | meso | C6D6 | 423 |
| 2 | Zr-2 | meso | C6D6 | 271 |
| 3 | Zr-3 | meso | C6D6 | 535 |
| 4 | Zr-4 | meso | C6D6 | 38 |
| 5 | Ti-4 | meso | C6D6 | 16 |
| 6 | Ti-4a | meso | C6D6 | 10 |
| 7 | Zr-1 | meso | Toluene | 1360 |
| 8 | Zr-1 | rac | C6D6 | 142 |
| 9 | Zr-4 | rac | C6D6 | 21 |
| 10 | Ti-4 | rac | C6D6 | 5 |
| 11 | Ti-4a | rac | C6D6 | 5 |
| 12 | Zr-1 | L | C6D6 | 164 |
| 13 | Zr-4 | L | C6D6 | 24 |
| 14 | Ti-4 | L | C6D6 | 3 |
| 13 | Ti-4a | L | C6D6 | 7 |
Conclusions
Structurally defined initiators based on group 4 metal bis(phenolate) complexes with an (OSSO)-type ligand catalyzed the polymerization of lactide monomers with initiator efficiency values f of around 1 to give polymers with low polydispersity (Mw/Mn < 1.2). All zirconium complexes used during this study led to heterotactic poly(meso-lactides). The titanium complexes with a flexible CH2 linker between the phenolate ring and the sulfur-containing bridge gave heterotactic biased PLAs. In contrast, those without the methylene linker gave syndiotactic biased poly(meso-lactides).11Complexes with the 5-6-5 chelate derived from the C3-bridged (OSSO)-type ligand 3 led to higher rates of the polymerization of the lactide monomers than those based on C2 bridged ligands 1 and 2 with the 5-5-5 chelate array. The introduction of the methylene linker between the sulfur donor and the phenolate group in the complexes based on C2 bridged ligand 4 with the 6-5-6 chelate resulted in lower polymerization rates.
Experimental
General details
All operations were performed under an inert atmosphere of argon using standard Schlenk-line or glove box techniques. Toluene, n-hexane and THF were distilled under argon from sodium/benzophenone ketyl prior to use. n-Pentane was purified by distillation from sodium/triglyme benzophenone ketyl. Benzene-d6, chloroform-d1 and other reagents were carefully dried and stored in a glove box. [Ti(OiPr)4] (Sigma Aldrich), [Zr(OtBu)4] (ABCR), and [Ti(OiPr)3Cl] (Sigma Aldrich) were purchased and used as received. meso-Lactide was recrystallized from isopropanol at −30 °C, washed with diethylether and dried under vacuum. L-Lactide and rac-lactide were purchased from Sigma-Aldrich and purified as meso-lactide. Glassware and vials used in the polymerization were dried overnight in an oven at 120 °C and exposed to a vacuum–argon cycle three times. Ligands were synthesized following the procedures in the literature.4c,d,9,19,20Chemical shifts for 1H and 13C{1H} NMR spectra were recorded on a Bruker Avance 400 MHz spectrometer or a Varian NMR 200 MHz spectrometer at room temperature in 5 mm NMR tubes. Chemical shifts were reported in parts per million and referenced against TMS using the residual proton signal of the solvent (1H: benzene-d6, δ = 7.16 ppm; chloroform-d1, δ = 7.26 ppm), (13C{1H}: benzene-d6, δ = 128.06 ppm; chloroform-d1, δ = 77.00 ppm).
Molecular weights and polydispersities were determined by size exclusion chromatography (SEC) in THF at 35 °C, at a flow rate of 1 mL min−1 utilizing an Agilent 1100 Series HPLC, a G1310A isocratic pump, an Agilent 1100 Series refractive index detector and 8 × 600 mm, 8 × 300 mm, 8 × 50 mm PSS SDV linear M columns. Calibration standards were commercially available narrowly distributed linear polystyrene samples that cover a broad range of molar masses (103 < M < 2 × 106 g mol−1). The molecular weights were multiplied by 0.58 according to a method in the literature.12
Polymerization procedure
A solution of a certain amount of the initiator Zr-1 in 0.5 mL of toluene (according to table entry) was added to a solution of 150 mg of meso-lactide which was dissolved in 1.5 mL of toluene. After the desired time the polymerization mixture was quenched with drops of moist hexanes and was added slowly to a cooled, quickly stirred hexanes solution. The polymer was filtered using a Büchner funnel, washed with diethyl ether, and dried in vacuo. The polymer was dissolved in a minimum quantity of CH2Cl2 and ran through a flash silica gel column (in a Pasteur pipette) to afford a colorless solution, which was added slowly to a cooled, quickly stirred hexanes solution. The colorless polymer was filtered and dried under vacuum.Kinetic measurement of polymerization
From a stock solution of complex Zr-1 in benzene-d6, a certain amount was added to a solution of lactide monomer in benzene-d6 and was monitored regularly using 1H NMR spectroscopy. Finally, after the desired time of the polymerization, the polylactide was worked-up as previously.Synthesis and characterization of Zr-2, Zr-4 and Ti-4
These complexes were synthesized following literature procedures.9,11Synthesis and characterization of complex Zr-1
To a solution of 1 equivalent of [Zr(OtBu)4] (270 mg, 0.70 mmol) in n-pentane (2 mL) was added one equivalent of a solution of H2-1 (350 mg, 0.70 mmol) in n-pentane (2 mL) and the reaction mixture was stirred overnight at room temperature. The solvent was removed in vacuo to afford an off-white solid that was dissolved in the minimum amount of n-pentane, filtered and left overnight at −30 °C. The solvent was removed to afford a white solid which was dried in vacuo to give a yield of 305 mg (59%). 1H NMR (400 MHz, C6D6, 25 °C): δ (ppm) 7.52 (d, 2H, 4JHH = 2.5 Hz, 3-H), 7.23 (d, 2H, 4JHH = 2.5 Hz, 5-H), 2.40 (dd, 4H, 2JHH = 10.8 Hz, SCH2), 1.70 (s, 18H, OC(CH3)3), 1.36 (s, 18H, Ph–C(CH3)3), 1.20 (s, 18H, C(CH3)3). 13C{1H} NMR (100.1 MHz, C6D6, 25 °C): δ (ppm) 163.88 (C-1), 140.63 (C-2), 137.64 (ipso, C-6), 127.16 (C-3), 125.44 (C-5), 121.46 (ipso, C-4), 77.57 (O–C(CH3)3), 36.64 (SCH2), 35.79 (Ph–C(CH3)3), 34.37 (Ph–C(CH3)3), 32.61 (Ph–C(CH3)3), 31.71 (Ph–C(CH3)3), 29.69 (O–C(CH3)3). Elemental analysis, calculated for C38H62S2O4Zr (738.25 g mol−1): C 61.82%, H: 8.46%; found: C 61.13%, H 8.8%.Synthesis and characterization of complex Zr-3
To a solution of [Zr(OtBu)4] (190 mg, 0.49 mmol) in n-pentane (2 mL) was added one equivalent of H2-3 (250 mg, 0.49 mmol) and the reaction mixture was stirred overnight at room temperature. The solvent was removed in vacuo to afford a white solid which was not soluble in n-pentane; this was dissolved in the minimum amount of n-pentane with a few drops of tetrahydrofuran, filtered and then left overnight at −30 °C. The solvent was then removed, washed with n-pentane and dried in vacuo which afforded a white solid in a yield of 110 mg (30%). 1H NMR (400 MHz, C6D6, 25 °C): δ (ppm) 7.51 (d, 2H, 4JHH = 4.0 Hz, 3-H), 7.31 (d, 2H, 4JHH = 4.0 Hz, 5-H), 2.43 (t, 4H, 3JHH = 8.0 Hz, SCH2), 1.72 (s, 18H, OC(CH3)3), 1.34 (s, 18H, C(CH3)3), 1.29 (m, 2H, SCH2), 1.26 (s, 18H, C(CH3)3). 13C{1H} NMR (100.1 MHz, C6D6, 25 °C): δ (ppm) 163.90 (C-1), 140.65 (C-2), 137.66 (ipso, C-6), 127.16 (C-3), 125.44 (C-5), 121.46 (ipso, C-4), 77.60 (O–C(CH3)3), 36.67 (Ph–C(CH3)3), 35.73 (Ph–C(CH3)3), 34.33 (SCH2), 32.61 (Ph–C(CH3)3), 31.71 (Ph–C(CH3)3), 29.69 (O–C(CH3)3), 23.43 (SCH2). Elemental analysis, calculated for C39H64S2O4Zr (766.3 g mol−1), C 62.27%, H 8.58%; found: C: 61.55%, H 6.35%.Synthesis and characterization of complex Ti-4a
To a solution of one equivalent of [Ti(OiPr)3Cl] (98 mg, 0.38 mmol) in n-pentane (2 mL) was added a solution of 1 equivalent of H2-4 (200 mg, 0.38 mmol) in n-pentane (2 mL) and the solution instantly turned a dark orange colour at room temperature. The solvent was removed in vacuo to afford an orange solid, which was dissolved in the minimum amount of n-pentane, filtered and then left over the weekend at −30 °C. The solvent was removed to afford an orange solid which was dried in vacuo to give a yield of 196 mg (78%). 1H NMR (400 MHz, C6D6, 25 °C): δ (ppm) 7.55 (br d, 2H, 3-H), 6.64 (br d, 2H, 4-H), 5.20 (m, CH(CH3)2), 4.41 (br s, 1H, SCH2CH2S), 3.66 (br s, 1H, SCH2CH2S), 3.12 (br s, 2H, SCH2CH2S), 1.89 (br s, 6H, Ph–C(CH3)3), 1.87 (br s, 18H, Ph–C(CH3)3), 1.45 (br s, 4H, SCH2Ph), 1.30 (s, 18H, Ph–C(CH3)3). 13C{1H} NMR (100.1 MHz, C6D6, 25 °C): δ (ppm) 161.00 (C-1), 142.27 (C-2), 138.50 (ipso, C-6), 129.04 (C-3), 125.78 (C-5), 121.46 (ipso, C-4), 83.98 (O–C(CH3)2), 36.84 (Ph–C(CH3)3), 36.17 (Ph–C(CH3)3), 34.78 (SCH2CH2CH2S), 32.18 (Ph–C(CH3)3), 31.15 (Ph–C(CH3)3), 25.53 (O–C(CH3)3), 23.06 (SCH2CH2CH2S), 14.61 (SCH2Ph). Elemental analysis, calculated for C35H55ClS2O3Ti (671.26 g mol−1): C 62.62%, H 8.26%; found: C 63.19%, H 7.28%.Acknowledgements
We thank the German-Israeli Foundation, Deutsche Forschungsgemeinschaft, the Fonds der Chemischen Industrie for financial support and Uhde Inventa-Fischer for the generous gift of meso-lactide.Notes and references
- (a) R. E. Drumright, P. R. Gruber and D. E. Henton, Adv. Mater., 2000, 12, 1841 CrossRef CAS; (b) S. Inkinen, M. Hakkarainen, A.-C. Albertsson and A. Södergard, Biomacromolecules, 2011, 12, 523 CrossRef CAS.
- (a) P. J. Dijkstra, H. Du and J. Feijen, Polym. Chem., 2011, 2, 520 RSC; (b) C. M. Thomas, Chem. Soc. Rev., 2010, 39, 165 RSC; (c) M. J. Stanford and A. P. Dove, Chem. Soc. Rev., 2010, 39, 486 RSC; (d) C. A. Wheaton, P. G. Hayes and B. J. Ireland, Dalton Trans., 2009, 4832 RSC; (e) R. H. Platel, L. M. Hodgson and C. K. Williams, Polym. Rev., 2008, 48, 11 Search PubMed; (f) A. Amgoune, C. M. Thomas and J.-F. Carpentier, Pure Appl. Chem., 2007, 79, 2013 CrossRef CAS; (g) J. Wu, T.-.L. Yu, C.-T. Chen and C.-C. Lin, Coord. Chem. Rev., 2006, 250, 602 CrossRef CAS; (h) O. Dechy-Cabaret, B. Martin-Vaca and D. Bourissou, Chem. Rev., 2004, 104, 6147 CrossRef; (i) B. J. O'Keefe, M. A. Hillmyer and W. B. Tolman, J. Chem. Soc., Dalton Trans., 2001, 2215 RSC.
- (a) L. Huang, X. Zhuang, J. Hu, L. Lang, P. Zhang, Y. Wang, X. Chen, Y. Wei and X. Jing, Biomacromolecules, 2008, 9, 850 CrossRef CAS; (b) E. T. H. Vink, K. R. Rábago, D. A. Glassner and P. R. Gruber, Polym. Degrad. Stab., 2003, 80, 403 CrossRef CAS; (c) J. Park, M. Ye and K. Park, Molecules, 2005, 10, 146 Search PubMed.
- (a) Z. Janas, Coord. Chem. Rev., 2010, 254, 2227 CrossRef CAS; (b) M. Lamberti, M. Mazzeo, D. Pappalardo and C. Pellecchia, Coord. Chem. Rev., 2009, 253, 2082 CrossRef CAS; (c) C. Capacchione, R. Manivannan, M. Barone, K. Beckerle, R. Centore, L. Oliva, A. Proto, A. Tuzi, T. P. Spaniol and J. Okuda, Organometallics, 2005, 24, 2971 CrossRef CAS; (d) C. Capacchione, A. Proto, H. Ebeling, R. Mülhaupt, K. Möller, T. P. Spaniol and J. Okuda, J. Am. Chem. Soc., 2003, 125, 4964 CrossRef CAS.
- (a) L. S. Baugh and J. A. M. Canich, Stereoselective Polymerization with Single-Site Catalysts, CRC Press, Boca Raton, FL, 2008 Search PubMed; (b) V. C. Gibson and S. K. Spitzmesser, Chem. Rev., 2003, 103, 283 CrossRef CAS.
- (a) A. J. Chmura, D. M. Cousins, M. G. Davidson, M. D. Jones, M. D. Lunn and M. F. Mahon, Dalton Trans., 2008, 1437 RSC; (b) F. Gornshtein, M. Kapon, M. Botoshansky and M. S. Eisen, Organometallics, 2007, 26, 497 CrossRef CAS.
- (a) Y. Takashima, Y. Nakayama, K. Watanabe, T. Itono, N. Ueyama, A. Nakamura, H. Yasuda and A. Harada, Macromolecules, 2002, 35, 7538 CrossRef CAS; (b) A. D. Schwarz, K. R. Herbert, C. Paniagua and P. Mountford, Organometallics, 2010, 29, 4171 CrossRef CAS.
- N. Nakata, T. Toda and A. Ishii, Polym. Chem., 2011, 2, 1597 RSC.
- A. Cohen, A. Yeori, I. Goldberg and M. Kol, Inorg. Chem., 2007, 46, 8114 CrossRef CAS.
- E. Sergeeva, J. Kopilov, I. Goldberg and M. Kol, Inorg. Chem., 2010, 49, 3977 CrossRef CAS.
- J.-C. Buffet and J. Okuda, Chem. Commun., 2011, 47, 4796 RSC.
- M. Save, M. Schappacher and A. Soum, Macromol. Chem. Phys., 2002, 203, 889 CrossRef CAS.
- Probability of tetrad sequences in PLA based on Bernoullian statistics: [sss] = Ps2 + PsPi/2, [sis] = (Pi2 + PiPs)/2, [ssi] = [iss] = (PsPi)/2 and [isi] = Pi2/2.
- (a) S. L. Hancock, M. F. Mahon and M. D. Jones, Dalton Trans., 2011, 40, 2033 RSC; (b) E. L. Whitelaw, M. D. Jones and M. F. Mahon, Inorg. Chem., 2010, 49, 7176 CrossRef CAS; (c) E. L. Whitelaw, M. D. Jones, M. F. Mahon and G. Kociok-Köhn, Dalton Trans., 2009, 9020 RSC; (d) A. D. Schwarz, A. L. Thompson and P. Mountford, Inorg. Chem., 2009, 48, 10442 CrossRef CAS; (e) A. J. Chmura, M. G. Davidson, C. J. Frankis, M. D. Jones and M. D. Lunn, Chem. Commun., 2008, 1293 RSC; (f) S. K. Russell, C. L. Gamble, K. J. Gibbins, K. C. S. Juhl, W. S. Mitchell, A. J. Tumas and G. E. Hofmeister, Macromolecules, 2005, 38, 10336 CrossRef CAS.
- (a) M. Hu, M. Wang, H. Zhu, L. Zhang, H. Zhang and L. Sun, Dalton Trans., 2010, 39, 4440 RSC; (b) F. Zhang, H. Song and G. Zi, J. Organomet. Chem., 2010, 695, 1993 CrossRef CAS.
- S. Gendler, S. Segal, I. Goldberg, Z. Goldschmidt and M. Kol, Inorg. Chem., 2006, 45, 4783 CrossRef CAS.
- For kinetic studies on the polymerization of meso-lactide see: (a) J.-C. Buffet and J. Okuda, Polym. Chem., 10.1039/c1py00206f; (b) A. Pietrangelo, S. C. Knight, A. K. Gupta, L. J. Yao, M. A. Hillmyer and W. B. Tolman, J. Am. Chem. Soc., 2010, 132, 11649 CrossRef CAS; (c) H. Du, A. H. Velders, P. J. Dijkstra, J. Sun, Z. Zhong, X. Chen and J. Feijen, Chem.–Eur. J., 2009, 15, 9836 CrossRef CAS; (d) T. M. Ovitt and G. W. Coates, J. Am. Chem. Soc., 2002, 124, 1316 CrossRef CAS; (e) B. M. Chamberlain, M. Cheng, D. R. Moore, T. M. Ovitt, E. B. Lobkovsky and G. W. Coates, J. Am. Chem. Soc., 2001, 123, 3229 CrossRef CAS; (f) H. Du, A. H. Velders, P. J. Dijkstra, Z. Zhong, X. Chen and J. Feijen, Macromolecules, 2009, 42, 1058 CrossRef CAS.
- (a) M. H. Chisholm, N. W. Eilerts, J. C. Huffman, S. S. Iyer, M. Pacold and K. Phomphrai, J. Am. Chem. Soc., 2000, 122, 11845 CrossRef CAS; (b) I. A. Shuklov, H. Jiao, J. Schulze, W. Tietz, K. Kühlein and A. Börner, Tetrahedron Lett., 2011, 52, 1027 CrossRef CAS.
- Y.-L. Wong, C.-Y. Mak, H. S. Kwan and H. K. Lee, Inorg. Chim. Acta, 2010, 363, 1246 CrossRef CAS.
- J. Klosin, L. Ackerman, X. Bei, G. M. Diamond, J. Longmire, V. Murphy, V. Nava-Salgado and J. A. W. Shoemaker, WO. Pat., 200707529, 2007.
Footnote |
| † Electronic supplementary information (ESI) available: NMR data of complexes Zr-1 to Zr4, Ti-4 and Ti-4a; NMR of the microstructures of polylactides and tables for the kinetic of polymerization data. See DOI: 10.1039/c1py00266j |
| This journal is © The Royal Society of Chemistry 2011 |
