DOI:
10.1039/C1PY00192B
(Paper)
Polym. Chem., 2011,
2, 2102-2106
A novel poly(thienylenevinylene) derivative for application in polymer solar cells
Received
4th May 2011
, Accepted 13th June 2011
First published on 25th June 2011
Abstract
A narrow bandgap conjugated polymer poly(2-(5-(5,6-bis(octyloxy)-4-(thiophen-2yl)benzo[c]) [1,2,5]thiadiazol-7-yl)thiophen-2-yl)-vinylene), POTBTV, was synthesized by a Pd-catalyzed Stille-coupling method, for application as donor material in polymer solar cells (PSCs). The polymer possesses good thermal stability and reasonable solubility. The absorption edge of POTBTV film is at 750 nm, indicating a narrow band gap of 1.65 eV. The HOMO and LUMO energy levels of POTBTV are −4.97 eV and −2.99 eV, respectively. The power conversion efficiency (PCE) of the PSC based on POTBTV as the donor PC70BM as the acceptor reached 1.53% with a short circuit current density of 6.83 mA cm−2, an open circuit voltage of 0.6V and a fill factor of 0.374 under the illumination of AM1.5, 100 mW cm−2, which is among the highest PCE values for PSCs based on PTV derivatives.
1. Introduction
In recent years, bulk-heterojunction polymer solar cells (PSCs) have received considerable attention because of their advantages of low cost, light weight, easy fabrication and compatibility with flexible substrates.1 The photoactive layer of the PSCs is composed of the blend of a conjugated polymer donor and a fullerene derivative (such as [6,6]-phenyl-C61-butyric acid methyl ester (PC60BM)) acceptor. To increase the power conversion efficiency (PCE) of PSCs, the key issue is to design and synthesize high efficiency donor and acceptor materials with broad visible absorption, high charge carrier mobility, and suitable HOMO and LUMO energy levels.2,3
For conjugated polymer donor materials, broad and strong absorption and high hole mobility are very important. Poly(thienylene vinylene) derivatives (PTVs) possess a broad absorption covering the whole visible region and higher hole mobility,4 which is attractive for the application as donor materials in PSCs. However, the power conversion efficiency (PCE) of the PSCs based on PTVs with alkyl chain as donor is only ca. 0.2∼0.3%,5 which is probably due to nonluminescent nature of the polymers.6
Recently, Heet al. synthesized a PTV derivative with D–A structure, (PTBTV) (see Scheme 1), which showed broad absorption and weak photoluminescence (PL). The PSC based on PTBTV as the donor demonstrated a PCE of 0.51%,7 which is obviously improvement in comparison with that of the common PTVs. More interestingly Huo et al. synthesized poly(3-carboxylated thienylenevinylene) (P3CTV) with an electron-withdrawing carboxylate substituent, which possesses a lower HOMO energy level and weak PL. The PCE of the PSCs based on P3CTV as the donor reached 2.01%, which is one order increase compared to the PSCs based on the common PTVs.8 These results indicate that the photovoltaic performance of the PTV derivatives could be greatly improved by appropriate molecular structure modification. In addition, planar polymers tend to pack in films to facilitate charge carrier transport for photovoltaic applications. The (5,6-bis(octyloxy)-4-(thiophen-2yl)benzo[c])[1,2,5]thiadiazol-7-yl)thiophen-2-yl (OTBT) unit has been shown to have a better planar structure in the D–A copolymer copolymerized with carbazole units.9 In order to further explore new PTV derivatives for application as the donor in PSCs, in this work we designed a novel PTV derivative, poly(2-(5-(5,6-bis(octyloxy)-4-(thiophen-2-yl)benzo[c])[1,2,5]thiadiazol-7-yl)thiophen-2-yl)-vinylene) (POTBTV, as shown in Scheme 1) based on a planar OTBT unit to improve the photovoltaic properties of the PTBTV. POTBTV was designed with two octyloxy chains on the benzothiazole ring to achieve more planar polymer conformation than PTBTV reported by Heet al. and increase the dissolution of polymer. The structure of POTBTV is shown in Scheme 1.
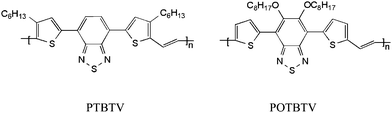 |
| | Scheme 1 Molecular structure of PTBTV and POTBTV. | |
2. Experimental section
2.1 Materials
The monomer 4,7-bis(5-bromothiophen-2-yl)-5,6-bis(octyloxy)benzo[c][1,2,5]thiadiazole was synthesized as reported in the literature,9 and (E)-1,2-bis(tributylstannyl) was obtained from J&K. Other reagents and solvents were commercial grade and used as received without further purification. All reactions were performed under nitrogen atmospheres.
2.2 Measurements and characterization
The permeation chromatography (GPC) measurements were performed on a Waters 717-2410 with polystyrenes as reference standard and THF as an eluent. The Nuclear Magnetic Resonance (NMR) spectra were taken on a Bruker AV 600 spectrometer in CDCl3 at room temperature. UV-vis absorption spectra were recorded on a shimadzu spectrometer model UV-3150. Photoluminescence (PL) spectra were measured using a Hitachi F-4500. Both absorption and PL spectra were measured for the polymer solutions in chloroform (analytical reagent) at room temperature. Thermogravimetric analysis (TGA) analyses were conducted with a TA instrument 2500VB2 + PC with heating rate of 10 °C min−1 under nitrogen gas flow. Differential scanning calorimetry (DSC) analysis was performed on a TA instrumentation DSC-1 in a nitrogen atmosphere. The electrochemical cyclic voltammetry was conducted on a Zahner IM6e Electrochemical Workstation with Pt disk, Pt plate, and Ag/Ag+ electrode as working electrode, counter electrode, and reference electrode respectively in a 0.1 mol L−1tetrabutylammonium hexafluorophosphate (Bu4NPF6) acetonitrile solution. Polymer thin films were formed by drop-casting 0.001 mL of polymer solutions in o-dichlorobenzene (analytical reagent, 1 mg mL−1) onto the working electrode, and then drying in air.
2.3 Synthesis
The synthesis routes for monomer 3 and POTBTV are shown in Scheme 2 and 3, respectively. All starting materials, reagents, and solvents were carefully purified, and all procedures were performed under an air-free environment.
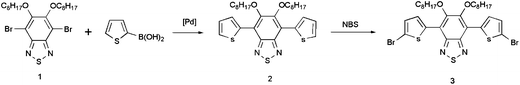 |
| | Scheme 2 Synthetic steps involved in the preparation of monomer 3. | |
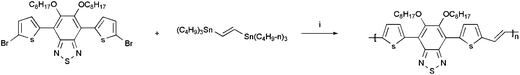 |
| | Scheme 3 The synthetic route for POTBTV. (i) Pd (PPh3)4, toluene, argon, reflux, 12 h. | |
5,6-Bis(octyloxy)-4,7-di(thiophen-2-yl)benzo-[c][1,2,5]-thiadiazole (2).
A mixture of 4,7-dibromo-5,6-bis(octyloxy)benzo-[c][1,2,5]-thiadiazole1 (5.5 g, 10 mmol) and 4,4,5,5-tetramethyl-2-(thiophen-2-yl)-1,3,2-dioxaborolane (3.2 g, 25 mmol), sat. Na2CO3(aq) (150 mL), and THF (300 mL) was carefully degassed before and after Pd(PPh3)4 (462 mg, 0.4 mmol) was added. The reaction mixture was heated to reflux for 48 h under nitrogen atmosphere. The organic layer was separated; the aqueous one was extracted with CH2Cl2 (3 × 100 mL); the combined organic layers were dried over anhydrous Na2SO4 and evaporated to dryness. The residue was chromatographically purified on silica gel column eluting with CH2Cl2/hexane (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100, v
100, v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v) to afford 2 as a yellow oil (3.34 g, 60%). 1H NMR (600 MHz, CDCl3): δ 8.46 (d, 2H),7.48 (d, 2H), 7.23 (t, 2H), 4.0 (t, 4H), 1.88 (m, 4H), 1.4 (m, 4H), 1.20–1.31 (m, 16H), 0.81–0.90 (t, 6H).
v) to afford 2 as a yellow oil (3.34 g, 60%). 1H NMR (600 MHz, CDCl3): δ 8.46 (d, 2H),7.48 (d, 2H), 7.23 (t, 2H), 4.0 (t, 4H), 1.88 (m, 4H), 1.4 (m, 4H), 1.20–1.31 (m, 16H), 0.81–0.90 (t, 6H).
4,7-Bis(5-bromothiophen-2-yl)-5,6-bis(octyloxy)benzo-[c][1,2,5]-thiadiazole (3).
N-Bromosuccimide (NBS) (6.146 mg, 34.5 mmol) was added to a solution of 2 (9.6 g, 17.27 mmol) in CHCl3 (400 mL) and acetic acid (400 mL) in one portion. The mixture was stirred at room temperature for 48 h in the dark. The solvent was removed under reduced pressure, the residue was chromatographically purified on silica gel column eluting with hexane to afford 3 as an orange crystal (7.5 g, 60.9%). 1H NMR (600 MHz, CDCl3): δ 8.37 (d, 2H), 7.15–7.18 (d, 2H), 4.08–4.14 (t, 4H), 1.95–1.97 (m, 4H), 1.44–1.47 (m, 4H), 1.25–1.34 (16, H), 0.88–0.90 (t, 6H).
Poly(2-(5-(5,6-bis(octyloxy)-4-(thiophen-2yl)benzo[c])[1,2,5]thiadiazol-7-yl)thiophen-2-yl)-vinylene) (POTBTV).
Under the protection of nitrogen atmosphere, monomer (1 mmol) and (E)-1,2-bis(tributylstannyl) ethane (1 mmol) were dissolved in 20 ml dried toluene, and then 10 mg of Pd(pph3)4 was added. The reactant was heated to reflux for 24 h and allowed to cool to room temperature. Then methanol (200 mL) was added. A precipitate was collected and subjected to Soxhlet extraction with methanol, hexane, and chloroform, in turn. A solid with copper-like luster was obtained after chloroform was removed in vacuum. 1H NMR (600 MHz, CDCl3): δ (ppm) 7.08–7.43 (br, 6H), 4.19 (t, 4H), 2.0 (t,4H), 0.9–1.6 (br, 26H), Elemental Analysis Calcd for C32H40N2O2S3: C: 66.20%; H, 6.89%; N, 4.82%. Found (C: 66.5%; H, 6.89%, N, 4.43%).
2.4 Fabrication of photovoltaic devices
Polymer solar cells were fabricated with ITO glass as a positive electrode, Ca/Al as a negative electrode and the blend film of the polymer/PC70BM between them as a photosensitive layer. The ITO glass was pre-cleaned and modified by a thin layer of PEDOT:PSS which was spin-cast from a PEDOT:PSS aqueous solution (Clevious P VP AI 4083 H. C. Stark, Germany) on the ITO substrate, and the thickness of the PEODT:PSS layer was about 60 nm. The photosensitive layer was prepared by spin-coating a blend solution of the polymer and PC70BM with a weight ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 in o-dichlorobenzene at 1000 rpm on the ITO/PEDOT:PSS electrode. Then the Ca/Al cathode was deposited on the polymer layer by vacuum evaporation under 3 × 10−4 pa. The device was thermally annealed at 130 °C for 15 min. The thickness of the photosensitive layer is ca. 60 nm, measured on an Ambios Tech. XP-2 profilometer. The effective area of one cell is 4 mm2.The current–voltage (I–V) measurement of the devices was conducted on a computer-controlled Keithley 236 Source Measure Unit. A xenon lamp with AM1.5 filter was used as the white light source, and the optical power at the sample was 100 mW cm−2.
2 in o-dichlorobenzene at 1000 rpm on the ITO/PEDOT:PSS electrode. Then the Ca/Al cathode was deposited on the polymer layer by vacuum evaporation under 3 × 10−4 pa. The device was thermally annealed at 130 °C for 15 min. The thickness of the photosensitive layer is ca. 60 nm, measured on an Ambios Tech. XP-2 profilometer. The effective area of one cell is 4 mm2.The current–voltage (I–V) measurement of the devices was conducted on a computer-controlled Keithley 236 Source Measure Unit. A xenon lamp with AM1.5 filter was used as the white light source, and the optical power at the sample was 100 mW cm−2.
3. Results and discussion
3.1 Synthesis and thermal stability of POTBTV
The synthesis of the polymer was carried out using palladium-catalyzed Stille-coupling between monomer 4,7-dibromo-5,6-bis(octyloxy)benzo-2,1,3-thiadiazole and (E)-1,2-bis(tributylstannyl)ethane, as shown in Scheme 3.
The weight average molecular weight of the polymer is 4.5 kg mol−1 with a PDI of 1.4, determined by gel permeation chromatography (GPC). POTBTV displayed good solubility at elevated temperature in chloroform, 1,2-dichlorobenzene (DCB), THF, etc.
Fig. 1 shows the thermogravimetric analysis (TGA) plot for POTBTV. The onset temperature with 5% weight loss of the polymer is at 330 °C, indicating that POTBTV has good thermal stability for the application in PSCs.
 |
| | Fig. 1
TGA plot of POTBTV with a heating rate of 10 °C min−1 under inert atmosphere. | |
3.2 Electrochemical properties
The electrochemical cyclic voltammetry was performed to determine the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) energy levels of the conjugated polymers.10Fig. 2 shows the cyclic voltammogram of POTBTV film on Pt electrode in acetonitrile solution containing 0.1 M Bu4NPF6 at a potential scan rate of 100 mV s−1. It can be seen that there are reversible p-doping/dedoping (oxidation/re-reduction) processes at positive potential range and n-doping/dedoping (reduction/re-oxidation) processes at the negative potential range. The HOMO and LUMO energy of the polymer were calculated from the onset oxidation potentials (Eoxonset) and the onset reduction potentials (Eredonset) according the following equations:11
| HOMO = −e(Eoxonset + 4.71) (eV); |
| LUMO = −e (Eredonset + 4.71) (eV) |
Where the units of Eoxonset and Eredonset are V vs.Ag/Ag+. The Eoxonset and Eredonset of POTBTV were 0.26 V and −1.72 V vs.Ag/Ag+ respectively, accordingly, the HOMO and LUMO energy levels of POTBTV were calculated to be −4.97 eV and −2.99 eV respectively.
3.3 Film morphology
The surface morphology of the polymer film was investigated by atomic force microscope (AFM). The phase image (see Fig. 3) shows a crystalline structure with extensive nanocrystalline domains of parallel rod-like structures. The rod-like structure was likely due to a π–π stacking order composed of face-to-face stacking lamellae with the stacking direction along the length of the rod. This demonstrates that POTBTV is a planar structure.12 Two octyloxy chains on the benzothiazole ring make the copolymer form an order structure, which possesses closing packing chains of the D–A copolymer, and thus could facilitate charge carrier transport for photovoltaic application.
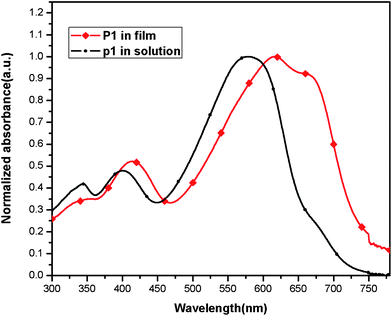
3.4 Optical properties of the polymer
The UV-vis absorption spectra of the POTBTV in chloroform solution and as thin film were shown in Fig. 4. The polymer solution in chloroform exhibited broad absorption between 300 and 700 nm, which is the characteristic of the PTV derivatives.5,7 The maximal absorption of POTBTV solution was at 579 nm. The thin film absorption of POTBTV was broadened and red-shifted in comparison with that of the polymer solution. The absorption maximum of the polymer film was at 618 nm, red-shifted by ca. 39 nm compared to that of the polymer solution, and the absorption edge of the polymer film is at ca. 750 nm corresponding to a bandgap of 1.65 eV. The broad and red-shifted absorption of the polymer film indicates that strong intermolecular interaction and aggregation exist in the solid-state of this polymer. In addition, the absorption spectrum of the polymer film exhibited a well-defined vibronic splitting at 667 nm (an absorption shoulder), which is likely to be because of the high structural order in the thin film.13
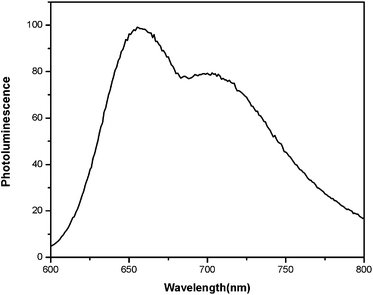
Fig. 5 shows the photoluminescence (PL) spectrum of POTBTV solution in chloroform. POTBTV shows photoluminescence in the wavelength range from 600 nm to 800 nm with a peak at ca. 655 nm. The PL emission of POTBTV could be benefited from the thiophene-benzothiadiazole-thiophene unit in the polymer, which is highly important for improving the photovoltaic properties of the PTVs.
3.5 Photovoltaic properties
The photovoltaic property of POTBTV was evaluated in bulk-heterojunction (BHJ) polymer solar cell (PSCs). The polymer POTBTV was used as donor and PC70BM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, w/w) was used as acceptor in the active layer of the devices. Fig. 6. shows the I–V curve of the PSC under the illumination of AM1.5, 100 mW cm−2. The Voc, Isc and PCE of the device were 0.6 V, 6.83 mA cm−2 and 1.53% respectively. The PCE of 1.53% is significant improved in comparison with the PSCs based on the PTVs, and it is three times increased than that of the device based on PTBTV with a similar structure.7
2, w/w) was used as acceptor in the active layer of the devices. Fig. 6. shows the I–V curve of the PSC under the illumination of AM1.5, 100 mW cm−2. The Voc, Isc and PCE of the device were 0.6 V, 6.83 mA cm−2 and 1.53% respectively. The PCE of 1.53% is significant improved in comparison with the PSCs based on the PTVs, and it is three times increased than that of the device based on PTBTV with a similar structure.7
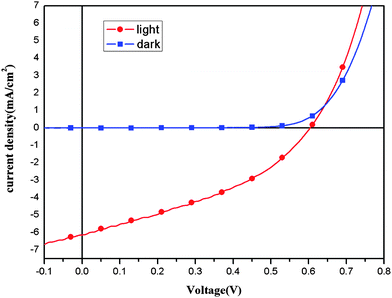 |
| | Fig. 6 Current–voltage characteristics of PSC based on POTBTV/PC70BM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, w/w) under the illumination of AM 1.5G, 100 mW cm−2. 2, w/w) under the illumination of AM 1.5G, 100 mW cm−2. | |
Fig. 7 shows the external quantum efficiency (EQE) plot of the PSCs based on the POTBTV/PC70BM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, w/w). It can be seen that the photoresponse curve of the PSC covers a wide wavelength range from 300 nm up to 780 nm, which coincides with the absorption spectrum of the photoactive film. The maximum EQE value of the device based on POTBTV was 38% at ca. 460 nm. The broad coverage of the solar spectrum of the PSC guarantees a high Jsc value of the device. These results indicate that poly(thienylenevinylene) derivatives with planar and relatively lower HOMO energy level could be a promising photovoltaic material after further structural modification.
2, w/w). It can be seen that the photoresponse curve of the PSC covers a wide wavelength range from 300 nm up to 780 nm, which coincides with the absorption spectrum of the photoactive film. The maximum EQE value of the device based on POTBTV was 38% at ca. 460 nm. The broad coverage of the solar spectrum of the PSC guarantees a high Jsc value of the device. These results indicate that poly(thienylenevinylene) derivatives with planar and relatively lower HOMO energy level could be a promising photovoltaic material after further structural modification.
 |
| | Fig. 7 External quantum efficiency (EQE) of the PSC device based on POTBTV/PC70BM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, w/w) with an active layer thickness of 60 nm. 2, w/w) with an active layer thickness of 60 nm. | |
4. Conclusions
A planar poly(thienylenevinylene) derivative, POTBTV, was synthesized by the Stille coupling reaction. POTBTV exhibited reasonable solubility and good thermal stability, broad absorption in the visible region and narrow bandgap of 1.65 eV. The LUMO and HOMO energy levels of POTBTV were −4.97 eV and −2.99 eV, respectively, measured by electrochemical cyclic voltammetry. The PCE of the PSC based on POTBTV/PC70BM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, w/w) reached 1.53% with Jsc = 6.83 mA cm−2, Voc = 0.60 V, and FF = 0.374, which is three times higher than that of the device based on PTBTV with a similar structure. Further investigation of POTBTV is still ongoing, and a higher PCE is anticipated. The results reveal that PTV derivatives with a planar structure and an electron-withdrawing unit could be a promising photovoltaic materials. More importantly, this work offered a useful and important structure modification guideline for PTV derivatives for high performance PSCs.
2, w/w) reached 1.53% with Jsc = 6.83 mA cm−2, Voc = 0.60 V, and FF = 0.374, which is three times higher than that of the device based on PTBTV with a similar structure. Further investigation of POTBTV is still ongoing, and a higher PCE is anticipated. The results reveal that PTV derivatives with a planar structure and an electron-withdrawing unit could be a promising photovoltaic materials. More importantly, this work offered a useful and important structure modification guideline for PTV derivatives for high performance PSCs.
Acknowledgements
This work was supported by NSFC (Nos. 20874106, 20821120293 and 50933003), and Chinese Academy of Sciences.
References
-
(a) J. W. Chen and Y. Cao, Acc. Chem. Res., 2009, 42, 1709–1718 CrossRef CAS;
(b) G. Dennler, M. C. Scharber and C. J. Brabec, Adv. Mater., 2009, 21, 1323 CrossRef CAS;
(c) Y.-J. Chen, S.-H. Yang and C.-S. Hsu, Chem. Rev., 2009, 109, 5868 CrossRef;
(d) B. C. Thompson and J. M. J. Fréchet, Angew. Chem., Int. Ed., 2008, 47, 58–77 CrossRef CAS;
(e) Y. F. Li and Y. P. Zou, Adv. Mater., 2008, 20, 2952–2958 CrossRef CAS;
(f) X. W. Zhan and D. B. Zhu, Polym. Chem., 2010, 1, 409–419 RSC.
- H. Y. Chen, J. H. Hou, S. Q. Zhang, Y. Y. Liang, G. W. Yang, Y. Yang, L. P. Yu, Y. Wu and G. Li, Nat. Photonics, 2009, 3, 649–654 CrossRef CAS.
-
(a) Y. J. He, H.-Y. Chen, J. H. Hou and Y. F. Li, J. Am. Chem. Soc., 2010, 132, 1377–1382 CrossRef CAS;
(b) Y. J. He and Y. F. Li, Phys. Chem. Chem. Phys., 2011, 13, 1970–1983 RSC.
- H. Fuchigami, A. Tsumura and H. Koezuka, Appl. Phys. Lett., 1993, 63, 1372 CrossRef CAS.
-
(a) A. P. Smith, R. R. Smith, B. E. Taylor and M. F. Durstock, Chem. Mater., 2004, 16, 4687 CrossRef CAS;
(b) J. H. Hou, Z. A. Tan, Y. J. He, C. H. Yang and Y. F. Li, Macromolecules, 2006, 39, 4657 CrossRef CAS.
-
(a) A. J. Brassett, N. F. Colaneri, D. D. C. Bradley, R. A. Lawrence, R. H. Friend, H. Murata, S. Tokito, T. Tsutsui and S. Saito, Phys. Rev. B: Condens. Matter, 1990, 41, 10586 CrossRef CAS;
(b) M. Lies, S. Jeglinski, P. A. Lane and Z. V. Vardeny, Synth. Met., 1997, 84, 891 CrossRef;
(c) I. W. Hwang, Q. H. Xu, C. Soci, B. Q. Chem, A. K. Y. Jen, D. Moses and A. J. Heeger, Adv. Funct. Mater., 2007, 17, 563 CrossRef CAS.
- Y. J. He, G. J. Zhao, J. Min, M. J. Zhang and Y. F. Li, Polymer, 2009, 50, 5055–5058 CrossRef CAS.
- L. J. Huo, T. L. Chen, Y. Zhou, J. H. Hou, H. Y. Chen, Y. Yang and Y. F. Li, Macromolecules, 2009, 42, 4377–4380 CrossRef CAS.
- R. P. Qin, W. W. Li, C. H. Li, C. Du, C. Veit, H.-F. Schleiermacher, M. Andersson, Z. S. Bo, Z. P. Liu, O. Inganas, U. Wuerfel and F. L. J. Zhang, J. Am. Chem. Soc., 2009, 131, 14612–14613 CrossRef CAS.
- Y. F. Li, Y. Cao, J. Gao, D. L. Wang, G. Yu and A. J. Heeger, Synth. Met., 1999, 99, 243–248 CrossRef CAS.
- Q. J. Sun, H. Q. Wang, C. H. Yang and Y. F. Li, J. Mater. Chem., 2003, 13, 800–806 RSC.
- N. Blouin, A. Michaud, D. Gendron, S. Wakim, B. Blair, R. Neaguplesu, M. Belletete, G. Durocher, Y. Tao and M. Leclerc, J. Am. Chem. Soc., 2008, 130, 732–742 CrossRef CAS.
- B. S. Ong, Y. Wu, P. Liu and S. Gardner, Adv. Mater., 2005, 17, 1141–1144 CrossRef CAS.
|
| This journal is © The Royal Society of Chemistry 2011 |
Click here to see how this site uses Cookies. View our privacy policy here. 


![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100, v
100, v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v) to afford 2 as a yellow oil (3.34 g, 60%). 1H NMR (600 MHz, CDCl3): δ 8.46 (d, 2H),7.48 (d, 2H), 7.23 (t, 2H), 4.0 (t, 4H), 1.88 (m, 4H), 1.4 (m, 4H), 1.20–1.31 (m, 16H), 0.81–0.90 (t, 6H).
v) to afford 2 as a yellow oil (3.34 g, 60%). 1H NMR (600 MHz, CDCl3): δ 8.46 (d, 2H),7.48 (d, 2H), 7.23 (t, 2H), 4.0 (t, 4H), 1.88 (m, 4H), 1.4 (m, 4H), 1.20–1.31 (m, 16H), 0.81–0.90 (t, 6H).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 in o-dichlorobenzene at 1000 rpm on the ITO/PEDOT:PSS electrode. Then the Ca/Al cathode was deposited on the polymer layer by vacuum evaporation under 3 × 10−4 pa. The device was thermally annealed at 130 °C for 15 min. The thickness of the photosensitive layer is ca. 60 nm, measured on an Ambios Tech. XP-2 profilometer. The effective area of one cell is 4 mm2.The current–voltage (I–V) measurement of the devices was conducted on a computer-controlled Keithley 236 Source Measure Unit. A xenon lamp with AM1.5 filter was used as the white light source, and the optical power at the sample was 100 mW cm−2.
2 in o-dichlorobenzene at 1000 rpm on the ITO/PEDOT:PSS electrode. Then the Ca/Al cathode was deposited on the polymer layer by vacuum evaporation under 3 × 10−4 pa. The device was thermally annealed at 130 °C for 15 min. The thickness of the photosensitive layer is ca. 60 nm, measured on an Ambios Tech. XP-2 profilometer. The effective area of one cell is 4 mm2.The current–voltage (I–V) measurement of the devices was conducted on a computer-controlled Keithley 236 Source Measure Unit. A xenon lamp with AM1.5 filter was used as the white light source, and the optical power at the sample was 100 mW cm−2.



![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, w/w) was used as acceptor in the active layer of the devices. Fig. 6. shows the I–V curve of the PSC under the illumination of AM1.5, 100 mW cm−2. The Voc, Isc and PCE of the device were 0.6 V, 6.83 mA cm−2 and 1.53% respectively. The PCE of 1.53% is significant improved in comparison with the PSCs based on the PTVs, and it is three times increased than that of the device based on PTBTV with a similar structure.7
2, w/w) was used as acceptor in the active layer of the devices. Fig. 6. shows the I–V curve of the PSC under the illumination of AM1.5, 100 mW cm−2. The Voc, Isc and PCE of the device were 0.6 V, 6.83 mA cm−2 and 1.53% respectively. The PCE of 1.53% is significant improved in comparison with the PSCs based on the PTVs, and it is three times increased than that of the device based on PTBTV with a similar structure.7

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, w/w) under the illumination of AM 1.5G, 100 mW cm−2.
2, w/w) under the illumination of AM 1.5G, 100 mW cm−2.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, w/w). It can be seen that the photoresponse curve of the PSC covers a wide wavelength range from 300 nm up to 780 nm, which coincides with the absorption spectrum of the photoactive film. The maximum EQE value of the device based on POTBTV was 38% at ca. 460 nm. The broad coverage of the solar spectrum of the PSC guarantees a high Jsc value of the device. These results indicate that poly(thienylenevinylene) derivatives with planar and relatively lower HOMO energy level could be a promising photovoltaic material after further structural modification.
2, w/w). It can be seen that the photoresponse curve of the PSC covers a wide wavelength range from 300 nm up to 780 nm, which coincides with the absorption spectrum of the photoactive film. The maximum EQE value of the device based on POTBTV was 38% at ca. 460 nm. The broad coverage of the solar spectrum of the PSC guarantees a high Jsc value of the device. These results indicate that poly(thienylenevinylene) derivatives with planar and relatively lower HOMO energy level could be a promising photovoltaic material after further structural modification.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, w/w) with an active layer thickness of 60 nm.
2, w/w) with an active layer thickness of 60 nm.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, w/w) reached 1.53% with Jsc = 6.83 mA cm−2, Voc = 0.60 V, and FF = 0.374, which is three times higher than that of the device based on PTBTV with a similar structure. Further investigation of POTBTV is still ongoing, and a higher PCE is anticipated. The results reveal that PTV derivatives with a planar structure and an electron-withdrawing unit could be a promising photovoltaic materials. More importantly, this work offered a useful and important structure modification guideline for PTV derivatives for high performance PSCs.
2, w/w) reached 1.53% with Jsc = 6.83 mA cm−2, Voc = 0.60 V, and FF = 0.374, which is three times higher than that of the device based on PTBTV with a similar structure. Further investigation of POTBTV is still ongoing, and a higher PCE is anticipated. The results reveal that PTV derivatives with a planar structure and an electron-withdrawing unit could be a promising photovoltaic materials. More importantly, this work offered a useful and important structure modification guideline for PTV derivatives for high performance PSCs.
