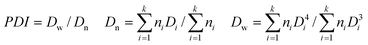Hybrid nanorattles of metal core and stimuli-responsive polymer shell for confined catalytic reactions†
Guo Liang
Li
a,
Chin An
Tai
a,
K. G.
Neoh
a,
E. T.
Kang
*a and
Xinlin
Yang
b
aDepartment of Chemical & Biomolecular Engineering, National University of Singapore, Kent Ridge, Singapore 119260. E-mail: cheket@nus.edu.sg
bKey Laboratory of Functional Polymer Materials, Ministry of Education, Institute of Polymer Chemistry, Nankai University, Tianjin, 300071, P. R. China
First published on 30th March 2011
Abstract
Narrowly-dispersed silver@silica@poly(methacrylic acid) (Ag@SiO2@PMAA) core–double shell hybrid nanoparticles (NPs) were first synthesized by distillation–precipitation polymerization, using silver@silica core–shell NPs from the sol–gel reaction as templates. Selective removal of the inorganic silica inner-shell from the Ag@SiO2@PMAA core–double shell hybrid NPs by HF etching produces the Ag@air@PMAA hybrid nanorattles with a Ag nanocore, PMAA shell and free space in between. The Ag nanocores, Ag@SiO2 core–shell NPs, Ag@SiO2@PMAA core–double shell NPs and Ag@air@PMAA hybrid nanorattles were characterized by field-emission scanning electron microscopy (FESEM), transmission electron microscopy (TEM), Fourier-transform infrared (FT-IR) spectroscopy and energy-dispersive X-ray (EDX) analysis. The as-synthesized Ag@air@PMAA hybrid nanorattles were explored as a nanoreactor system for confined catalytic reaction. The rate of catalytic reaction can be further regulated by controlling molecule diffusion in and out of the stimuli-responsive PMAA shell through the simple variation of environmental stimuli, such as salt (NaCl) concentration of the medium.
1 Introduction
Hollow functional nanostructures have been of great research interest because of their potential for advanced applications in chemistry, biotechnology, and pharmaceutical industry.1–6 A unique hollow structure, “rattle” or “yolk-shell” structure, has recently attracted considerable attention. A variety of chemical and physicochemical methods have been explored to produce the rattle-type hollow nanostructures.7–11 For instance, platinum@cobalt oxide yolk-shell nanostructures represent a novel class of nanomaterials for applications in catalysis.12 FePt@CoS2yolk-shell nanocrystals with surface-decorated cancer-targeting antibodies have found applications in nanomedicine.13 Narrowly-dispersed Au@ZrO2 nanorattles of controllable morphology have been prepared to avoid sintering of the metallic nanoparticles during CO oxidation.7,14 Thus, the inorganic “rattle-type” nanostructure has provided new opportunities for enhancing the properties of metals for advanced reactor technology and reaction design. Hollow nanorattles as reactor systems should also possess a permeable shell for controlling molecular diffusion in and out of the nanorattles.15,16Stimuli-responsive polymers can respond to external stimuli, such as pH, temperature, ionic strength and electric field, through reversible structural transitions and self-adjustment of physicochemical properties.17–19 Stimuli-responsive hollow structures have been prepared via layer-by-layer technique,20,21self-assembly22,23 and vesicular polymerization.24,25 Porous silica hollow nanostructures have also been fabricated for applications in catalysis.26,27 Combinations of stimuli-responsive polymers28 with inorganic metal nanoparticles provide a strategy for the synthesis of novel materials, such as hybrid nanorattles. In addition to the unique morphology, hybrid nanorattles with controllable functions endowed by the inorganic and polymer materials in subsequent applications are always of interest.
Herein, we describe the synthesis of Ag@air@PMAA (PMAA = poly(methacrylic acid)) hybrid nanorattles, with a silver nanocore and stimuli-responsive, cross-linked (thus solvent resistant) and yet permeable polymer shell, via combined sol–gel reaction and distillation–precipitation polymerization, for application as nanoreactors. In addition to providing a confined or controlled environment for the reaction, the catalytic reaction rate can be further regulated by controlling molecule diffusion through the polymer shell via the simple variation of environmental stimuli, such as salt concentration. Thus, the present metal@air@stimuli-responsive (and cross-linked) polymer hybrid nanorattles are uniquely different in chemical structure and physical property from those of the widely reported inorganic nanorattles and yolk-shell nanostructures,7–14 as well as from those in the case of silica@air@polymer nanorattles8 consisting mainly of an inert movable core and non-crosslinked inert polymer shell.
2 Experimental section
2.1 Materials
Silver nitrate (AgNO3, 99%), tetraethyl orthosilicate (TEOS, 98%), 3-(trimethoxysilyl)propyl methacrylate (MPS, 98%) and 4-nitrophenol (98%) were used as received from Sigma-Aldrich Chemical Co., Inc. Polyvinylpyrrolidone (PVP, MW: 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) was obtained from Fluka Chem. Co., hydrofluoric acid (48%) was purchased from Riedel-de Haën. Methacrylic acid (MAA, 99%) from Sigma-Aldrich Chem. Co. was purified by vacuum distillation. 2,2′-Azobisisobutyronitrile (AIBN), also from Sigma-Aldrich Chem. Co., was recrystallized in methanol. Sodium borohydride (NaBH4), ammonia (25 wt%) and acetonitrile (HPLC grade) were obtained from Merck and were used without further purification.
000) was obtained from Fluka Chem. Co., hydrofluoric acid (48%) was purchased from Riedel-de Haën. Methacrylic acid (MAA, 99%) from Sigma-Aldrich Chem. Co. was purified by vacuum distillation. 2,2′-Azobisisobutyronitrile (AIBN), also from Sigma-Aldrich Chem. Co., was recrystallized in methanol. Sodium borohydride (NaBH4), ammonia (25 wt%) and acetonitrile (HPLC grade) were obtained from Merck and were used without further purification.
2.2 Synthesis of silver nanocores
Silver nitrate was used as a precursor for the preparation of silver nanoparticles (Ag NPs).29 0.85 g of silver nitrate, 1.1 g of polyvinylpyrrolidone (molar ratio [PVP]/[AgNO3] = 2) and 1.49 mL of formaldehyde were introduced into 50 mL of deionized water in a 100 mL round bottom glass flask. About 0.3 g of anhydrous sodium hydroxide in 10 mL of water was then added into the reaction mixture. Upon the addition of sodium hydroxide, the reaction mixture turned grey immediately. The resulting silver NPs were separated from the solution by addition of four times the volume of acetone. The mixture was centrifuged (Eppendorf 5810R centrifuge) at 6000 rpm for 6 min. The black precipitate was washed twice with an acetone/water (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v:v) mixture. After washing, the collected Ag NPs were redispersed in 80 mL of ethanol for the subsequent sol–gel coating process.
1, v:v) mixture. After washing, the collected Ag NPs were redispersed in 80 mL of ethanol for the subsequent sol–gel coating process.
2.3 Synthesis of Ag@SiO2 core–shell NPs
The Ag@SiO2 core–shell NPs were synthesized by the sol–gel process on the Ag NPs.30,31 Eight mL of re-dispersed silver seeds in ethanol, 5 mL of deionized water, 14 mL of ethanol and 0.1 mL of TEOS were introduced into a 25 mL round bottom glass flask. The mixture was stirred for 10 min. About 0.8 mL of ammonia solution (25 wt%, Merck Chem. Co.) was then added drop-wise and the flask was sealed to keep the reaction mixture at the same pH value during the sol–gel reaction. The reaction mixture was stirred for 6 h at room temperature (25 °C). About 0.1 mL of MPS was then added and the mixture was stirred for another 18 h at 25 °C. At the end of reaction, the mixture was centrifuged at 8200 rpm for 10 min. The collected Ag@SiO2 core–shell NPs were washed once with THF, and twice with both acetone and ethanol. The Ag@SiO2 core–shell NPs of different shell thickness were synthesized via the sol–gel process using different initial TEOS feed concentration from 0.05 to 0.3 mL.2.4 Synthesis of Ag@SiO2@PMAA core–double shell NPs
For the preparation of Ag@SiO2@PMAA core–double shell NPs,32 0.02 g of the Ag@SiO2 core–shell seeds were dispersed into 10 mL of acetonitrile under ultrasonication. A mixture of methacrylic acid (MAA, 60 μL), divinylbenzene (DVB, a crosslinking agent, 40 μL) and 2,2′-azobisisobutyronitrile (AIBN, 2 mg) was then introduced into the flask to initiate the polymerization. The polymerization reaction was allowed to proceed under reflux conditions for 4 h. The resultant Ag@SiO2@PMAA core–double shell NPs were purified by extraction with acetonitrile and ethanol five times viacentrifugation/redispersion cycles to remove the un-reacted monomers and oligomers. The thickness of PMAA shell was tuned by adjusting the feed concentration of MAA monomer from 0.06 to 0.12 mL. The AIBN initiator was kept at 2 wt% of (MAA + DVB) and the weight ratio of MAA to DVB was at 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. For each PMAA shell thickness reported, at least three batches of the Ag@SiO2@PMAA core–double shell NPs of consistent outer shell thickness were prepared to ensure the reproducibility of synthesis procedures.
2. For each PMAA shell thickness reported, at least three batches of the Ag@SiO2@PMAA core–double shell NPs of consistent outer shell thickness were prepared to ensure the reproducibility of synthesis procedures.
2.5 Synthesis of Ag@air@PMAA hybrid nanorattles
The Ag@air@PMAA hybrid rattle-type hollow nanostructures were prepared by removal of the silica inner-shell from the Ag@SiO2@PMAA core–double shell NPs. Briefly, 20 mg of the Ag@SiO2@PMAA core–double shell nanospheres was first dispersed in 5 mL of ethanol, followed by addition of 17 mL of 48% HF. The etching process was allowed to proceed for 4 h. The resulting hollow metal core–polymer shell nanostructures were cleaned with at least 3 centrifugation/dispersion cycles in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v:v) mixture of ethanol and water till the pH of medium reached a constant value. The repeated cleaning process ensured the complete removal of residual HF and SiF4.
1 (v:v) mixture of ethanol and water till the pH of medium reached a constant value. The repeated cleaning process ensured the complete removal of residual HF and SiF4.
2.6 Catalytic reduction of p-nitrophenol
The reduction of aromatic nitrocompounds to amines is catalyzed by silver nanoparticles in an aqueous medium.33 For the catalytic reduction of p-nitrophenol using the synthesized Ag@air@PMAA hybrid nanorattles, 0.5 mL of the Ag@air@PMAA nanorattles suspension in deionized water (1.2 × 10−3 M with respect to the silver precursor concentration) was added to 1 mL of 0.6 M NaBH4 (Merck) in a quartz cell. The mixture was magnetically stirred for 30 min at 25 ± 2 °C. For the reaction carried out in a, for example, 0.05 M salt medium, 8.7 mg of NaCl was added into the mixture prior to the initiation of reaction. About 1.5 mL of 3.4 × 10−4 M p-nitrophenol was quickly added to the reaction mixture. The progress of the catalytic reaction was monitored by UV-visible absorption spectroscopy at an interval of 3 min. With the progress of the reaction, the original yellow solution of p-nitrophenol slowly turned colorless. For comparison purposes, silver NPs without the PMAA shells were tested for the catalytic activity under the same NaCl concentrations of 0, 0.01, 0.05 and 0.1 M. To maintain the same silver mass (concentration) for NPs with and without the PMAA shell, the initial concentration of bare silver NPs (A0) was adjusted to be the same as that of the silver nanocores in the hybrid nanorattles by UV-visible absorption spectroscopy measurements of their aqueous dispersions.2.7 Materials characterization
Field-emission scanning electron microscopy (FESEM) images were obtained on a JEOL JSM-6700 SEM. Transmission electron microscopy (TEM) images were obtained on a JEOL JEM-2010 TEM. The UV-visible absorption spectra in the wavelength range of 200 to 800 nm were measured on a Shimadzu UV-3101PC spectrophotometer. Fourier transform infrared (FT-IR) spectroscopy analysis was carried out on a Bio-Rad FTS-135 FT-IR spectrophotometer. The polydispersity index or the size distribution of the NPs was calculated from the following statistical formula:34where PDI is the polydispersity index, Dn is the number-average diameter, Dw is the weight-average diameter, Di is the diameter of the NPs and n is the number of particles used for the diameter measurement from the TEM images (Table 1). Standard deviation from the mean (δ) was determined to estimate the error in particle size (Dn) measurements.
| Sample | D n a (nm) | D w a (nm) | PDI a | Shell thickness (nm)b | n c | δ d (nm) |
|---|---|---|---|---|---|---|
| a D n is the number-average diameter, Dw is the weight-average diameter, PDI is the polydispersity index. b The shell thickness of the Ag@SiO2 core–shell and core–double shell NPs were determined from the TEM images. c n is the number of particles used for diameter determination from TEM images. d δ is the standard deviation from the mean (Dn). e The Ag@SiO2@PMAA core–double shell NPs were prepared using the Ag@SiO2 core–shell-3 NPs as seeds. | ||||||
| Ag nanocore | 38 | 56 | 1.48 | — | 100 | 15 |
| Ag@SiO2 core–shell-1 | 54 | 62 | 1.13 | 8 | 44 | 11 |
| Ag@SiO2 core–shell-2 | 67 | 72 | 1.08 | 15 | 41 | 8 |
| Ag@SiO2 core–shell-3 | 87 | 93 | 1.07 | 25 | 65 | 14 |
| Ag@SiO2 core–shell-4 | 143 | 147 | 1.03 | 53 | 59 | 14 |
| Ag@SiO2@PMAA core–double shell-1e | 141 | 145 | 1.03 | 25/27 | 38 | 13 |
| Ag@SiO2@PMAA core–double shell-2e | 170 | 181 | 1.06 | 25/42 | 44 | 25 |
| Ag@SiO2@PMAA core–double shell-3e | 221 | 225 | 1.02 | 25/67 | 60 | 15 |
3 Results and discussion
Procedures for the synthesis of Ag nanocore, Ag@SiO2 core–shell, Ag@SiO2@PMAA core–double shell and Ag@air@PMAA hybrid rattle-type nanostructures are shown in Scheme 1. The first step involves coating of silver nanoparticles (NPs) with a uniform silica shell via the sol–gel process of tetraethyl orthosilicate (TEOS) and 3-(trimethoxysilyl)propyl methacrylate (MPS) to produce the Ag@SiO2 core–shell NPs.29–31 A uniform PMAA shell with controllable shell thickness is then formed viadistillation–precipitation polymerization35–37 to produce the Ag@SiO2@PMAA core-double shell NPs. Finally, the silica inner-shell between the metal nanocore and polymer outer-shell of the NPs was selectively removed by HF etching to produce the Ag@air@PMAA nanorattles.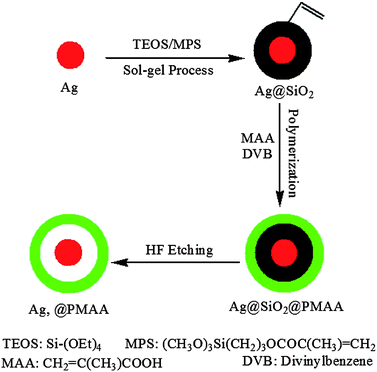 | ||
| Scheme 1 Schematic illustration of the synthesis of Ag@SiO2@PMAA core–double shell and Ag@air@PMAA rattle-type hybrid NPs. | ||
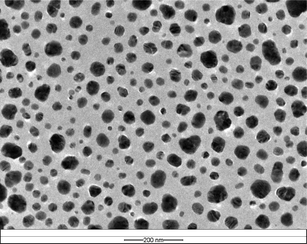
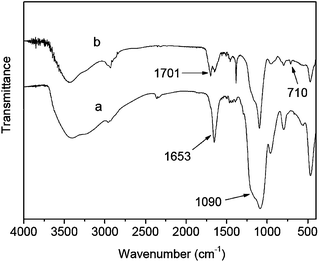
The as-synthesized silver nanocore has an average size of around 38 nm in diameter (Fig. 1). The thickness of silica shell encapsulating the metal nanocore can be controlled by using different TEOS feed concentrations. Silica shells with different thicknesses of 8, 15, 25 and 53 nm were obtained and listed in Table 1. The Ag@SiO2 core–shell NPs of different silica shell thicknesses are readily discernible in the TEM images of Fig. 2. The carbon–carbon double bonds on the surface of Ag@SiO2 core–shell NPs, introduced by the organosilicon coupling agent MPS, serve as initiation sites in the subsequent graft polymerization of methacrylic acid (MAA). The characteristic absorption peak at 1090 cm−1 in the FT-IR spectrum of the Ag@SiO2 core–shell NPs in Fig. 3a is assigned to the Si–O–Si stretching vibration.37 The absorption band at 1653 cm−1 is associated with the vinyl group of MPS on the surface of silica shell.
 | ||
| Fig. 1 TEM micrograph of the silver nanocores. The scale bar is 200 nm. | ||
 | ||
| Fig. 2 TEM micrographs of the (b) Ag@SiO2 core–shell NPs with different silica shell thickness: (a) and (a′) 8 nm, (b) and (b′) 15 nm, (c) and (c′) 53 nm. The respective scale bars for (a), (a′), (b), (b′), (c) and (c′) are 20, 10, 50, 20, 1000 and 50 nm. | ||
 | ||
| Fig. 3 FT-IR spectra of the (a) Ag@SiO2 core–shell-3 and (b) Ag@SiO2@PMAA core–double shell-2 NPs in Table 1. | ||
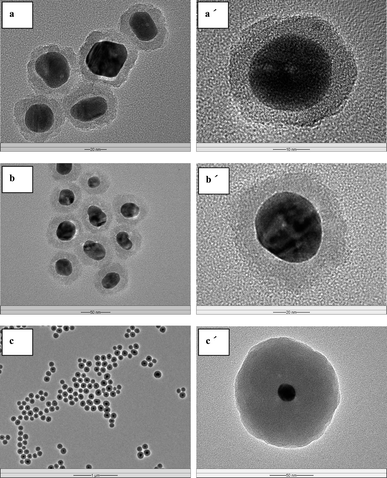
The Ag@SiO2@PMAA core–double shell hybrid NPs were prepared viadistillation–precipitation polymerization of the stimuli-responsive MAA monomer in the presence of divinylbenzene (DVB, a crosslinking agent), using the as-synthesized Ag@SiO2 core–shell NPs as seeds. Regulation of the PMAA outer shell thickness to achieve a low polydispersity index (PDI) of the NPs (Supporting Information, Fig. S1†) is an important aspect of the polymer coating process.38 The poly(methacrylic acid) (PMAA) outer-shells with different thickness of 27, 42 and 67 nm have been synthesized. The corresponding TEM images of the so-obtained Ag@SiO2@PMAA core–double shell hybrid NPs are shown in Fig. 4a–c. Core–double shell hybrid particles encapsulating more than one Ag@SiO2 core–shell seed NP were observed when an initial higher concentration of the monomers (MAA and DVB) to that of the seed NPs (w/w, 6/1) was used, as shown in Fig. 4c. The TEM images reveal a polymer shell encapsulating a dense inorganic Ag@SiO2 core–shell NP of differential contrast, forming a distinctive core–double shelled nanostructure. The crosslinked PMAA outer-shell of about 67 nm in thickness is readily discernible in the TEM image of a higher magnification (Fig. 4d). The absorption peak at 1701 cm−1 in the FT-IR spectrum of the Ag@SiO2@PMAA core–double shell hybrid NPs of Fig. 3b is associated with stretching vibration of the carbonyl groups of PMAA outer-shell. The absorption bands at 710 (δC6H6) and 1450 (ν C–H) cm−1 are associated with the characteristic vibrations of DVB units in the polymer outer shell.39 The Ag@SiO2@PMAA core–double shell hybrid NPs were also characterized by the energy-dispersive X-ray (EDX) analysis. The mass contents of silver nanocore, silica interlayer and carbon in the Ag@SiO2@PMAA core–double shell-3 microspheres (Table 1) are determined to be about 40%, 21% and 35%, respectively, from EDX analysis (of several hundred microspheres in the FESEM image, Supporting Information, Fig. S2†).
 | ||
| Fig. 4 TEM micrographs of the Ag@SiO2@PMAA core–double shell NPs with different PMAA outer shell thickness: (a) 27 nm, (b) 42 nm and (c) and (d) 67 nm. The respective scale bars for (a), (b), (c) and (d) are 50, 100, 500 and 50 nm. | ||
Well-defined Ag@air@PMAA hybrid nanorattles are obtained by selective removal of the inorganic silica interlayer by HF treatment. The size and shell thickness of the PMAA outer shell can be regulated though the simple adjustment of initial MAA monomer concentration. TEM images of the Ag@air@PMAA hybrid nanorattles with shell thicknesses of 27, 42 and 67 nm are shown in Fig. 5a–c, respectively. The TEM images clearly reveal the hybrid nanorattle structure with a free-moving metal nanocore encapsulated in a hollow polymer shell. No deformation of the polymer shell was observed, suggesting that the crosslinked PMAA shell is stable and rigid enough to sustain the cavity upon etching of the silica inner shell.
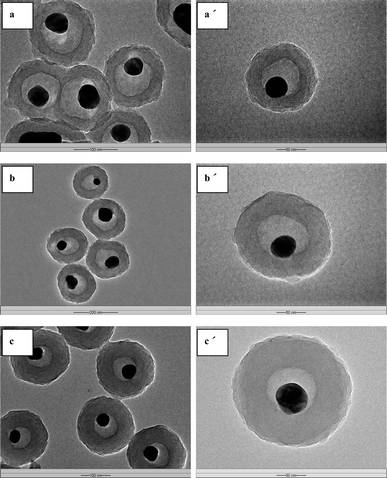 | ||
| Fig. 5 TEM micrographs of the Ag@air@PMAA hybrid nanorattles with different PMAA shell thickness: (a) and (a′) 27 nm, (b) and (b′) 42 nm, (c) and (c′) 67 nm. The respective scale bars for (a), (a′), (b), (b′), (c) and (c′) are 100, 50, 200, 50, 100 and 50 nm. | ||
Fig. 6 shows the UV-visible absorption spectra of the Ag nanocores, Ag@SiO2 core–shell NPs and Ag@air@PMAA hybrid nanorattles in aqueous dispersions. For the Ag NPs, the absorption maximum occurs at 415 nm. The absorption peak of the Ag@SiO2 core–shell NPs is shifted to 441 nm from that of the Ag NPs at 415 nm. The red-shift is due to an increase in the local refractive index of the medium surrounding the Ag NPs, i.e. from water to silica (refractive index of 1.33 to 1.46).8,9 The absorption peak of the Ag@air@PMAA hybrid nanorattles at 428 nm is located between those of the Ag and Ag@SiO2 core–shell NPs, consistent with the fact that PMAA has a refractive index of 1.41, between those of water and silica.40
 | ||
| Fig. 6 UV-visible absorption spectra of Ag nanocores, Ag@SiO2 core-shell NPs and Ag@air@PMAA hybrid nanorattles in aqueous dispersions. | ||
To achieve good catalytic performance, the metal core in the nanorattles should be highly accessible to the reactants. Thus, the outer-shell of nanorattles should allow diffusion of molecules in and out of the nanorattles for confined catalytic reactions.41 The so-obtained Ag@air@PMAA hybrid nanorattles thus provide a unique nanoreactor system for spatially confined catalytic reactions. The catalytic reduction of p-nitrophenol by sodium borohydride (NaBH4) to p-aminophenol in the nanorattles was studied.
The catalytic reduction process occurring in the Ag@air@PMAA hybrid nanorattle is illustrated in Scheme 2. Reactants, such as NaBH4 and p-nitrophenol, diffuse through the PMAA shell into the hollow cavity. After adsorption of both reactants onto the silver NP surface, the catalytic reduction is initiated by electron transfer from the donor BH4− to the substrate p-nitrophenolate ion (acceptor).42 The product, p-aminophenol, desorbs from the silver catalyst surface and diffuses out of the PMAA shell. No reduction reaction was observed in the absence of Ag NPs or Ag@air@PMAA hybrid nanorattles. Fig. 7a and 8a shows the changes in UV-visible absorption spectra of the aqueous reaction mixtures in the presence of Ag NPs and Ag@air@PMAA hybrid hollow nanorattles, respectively. As the reduction reaction proceeds, p-nitrophenol is converted into p-aminophenol, resulting in a decrease in the absorption intensity at 400 nm.43 In comparison, the reaction catalyzed by the silver NPs without the PMAA shells proceeds more rapidly than that catalyzed by Ag@air@PMAA hybrid nanorattles with the same mass of silver.
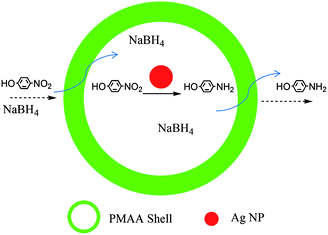 | ||
| Scheme 2 The Ag@air@PMAA hybrid nanorattle as a nanoreactor for the confined catalytic reduction of p-nitrophenol by NaBH4. | ||
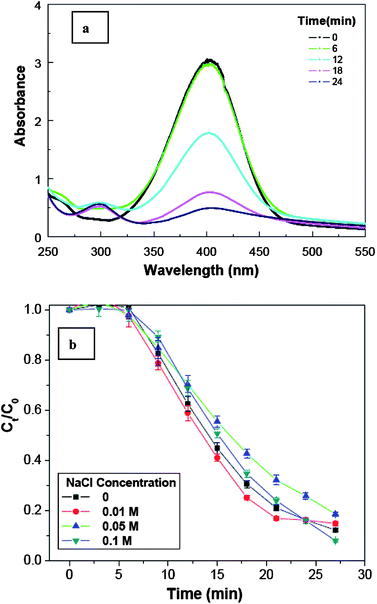 | ||
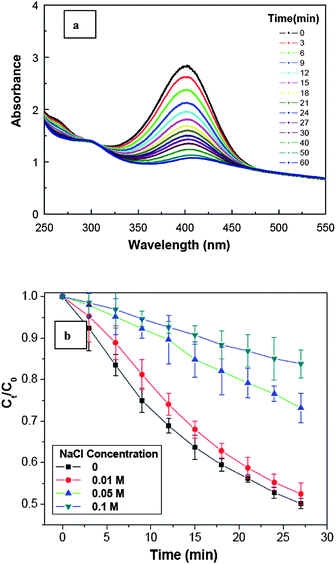
| Fig. 7 (a) Catalytic reduction of p-nitrophenol (C0 = 3.4 × 10−4 M) by the Ag NPs (∼1.2 × 10−3 M) as monitored by time-dependent UV-visible absorption. (b) Reaction kinetics of p-nitrophenol reduction by the Ag NPs under the effect of salt concentration of the medium (pH 9.2, C0 and Ct are the initial and instantaneous concentration of p-nitrophenol, respectively). | ||
 | ||
| Fig. 8 (a) Catalytic reduction of p-nitrophenol (C0 = 3.4 × 10−4 M) by the Ag@air@PMAA nanorattles (from Ag@SiO2@PMAA core–double shell-3 NPs in Table 1, ∼1.2 × 10−3 M with respect to the Ag concentration, PMAA shell thickness = 67 nm) as monitored by time-dependent UV-visible absorption. (b) Reaction kinetics of p-nitrophenol reduction by the Ag@air@PMAA nanorattles under the effect of salt concentration of the medium (pH 9.2, C0 and Ct are the initial and instantaneous concentration of p-nitrophenol, respectively). | ||
It is known that the PMAA outer-shell is pH-responsive. It is ionic at a high pH value, and is highly sensitive to the ionic strength of surrounding medium.20,44 The effect of ionic strength of the medium, and thus the permeability of reactants and product through the PMAA shell, on the reaction rate was evaluated. Fig. 7b and 8b show the changes in reactant concentration with time for the reactions catalyzed by Ag NPs and Ag@air@PMAA hybrid nanorattles (PMAA shell thickness of 67 nm), respectively, in media of increasing NaCl salt concentrations. For the hybrid nanorattle system, the reaction rate decreases significantly as the NaCl concentration increases from 0 to 0.1 M. For the silver NPs without the stimuli-responsive PMAA shell, the reaction rate does not show a significant dependence on the salt concentration of the medium. Thus, the catalytic reaction is not inhibited by the salt addition. The pH of the reaction mixture was maintained at around 9.2. At pH 9.2, the PMAA shell is fully ionized. Electrostatic repulsion among the negatively charged carboxylic groups allows the PMAA shell to adopt a highly swollen or expanded conformation. With the increase in salt concentration (ionic strength), more Na+ counter-ions become available to shield the charges in the PMAA network. The charge shielding effect reduces the internal osmotic pressure by reducing the internal electrostatic repulsion, leading to an associated state of the PMAA shell according to the Donnan equilibrium effect.16 The condensed PMAA layer is likely to present a higher steric barrier for the diffusion of reactant and product molecules, resulting in a lower reaction rate. Thus, the rate of catalytic reaction can be effectively controlled by the diffusion rate of reactant through the stimuli (NaCl concentration)-responsive polymer shell to the surface of Ag NP catalyst.
Finally, comparison of the catalytic reaction kinetics of pristine Ag NPs to that of the Ag NPs derived from HF etching of the Ag@SiO2 NPs (Fig. S3, Supporting Information†) suggests that the F− ions have only limited effect on the catalytic activity of the Ag NPs. The Ag@air@PMAA hybrid nanorattles could be easily collected by centrifugation after the catalytic reaction and recycled for another round of catalytic reaction in the confined space of the nanorattles. The TEM image of recycled Ag@air@PMAA hybrid nanorattles (Fig. S4, Supporting Information†) suggests that the synthesized hybrid nanorattles remain intact and retain their well-defined hollow core–shell morphology after the catalytic reaction.
4 Conclusions
Functional Ag@air@PMAA hybrid nanorattles, comprising of a silver nanocore and a crosslinked PMAA shell with an internal void, have been synthesized by selective etching of the silica inner-shell of Ag@SiO2@PMAA core–double shell NPs prepared from combined sol–gel reaction and distillation–precipitation polymerization. The permeable and stimuli-responsive PMAA outer shell allows the easy and controlled access of reactant molecules to the silver nanocore. The silver-catalyzed reduction of p-nitrophenol and the effect of ionic strength on the permeability of the PMAA shell (and thus reaction rate) in this hybrid nanoreactor system were demonstrated. The Ag@air@PMAA nanoreactors can be easily separated from the reaction mixture by centrifugation. It is envisioned that this versatile nanoreactor system can serve as a useful platform for studying various heterogeneous catalytic reactions in a confined and controlled manner, as the metallic nanocore and stimuli-responsive polymer shell can be tailored to specific reactions and environmental stimuli using the synthesis strategy developed.References
- Y. Wang, H. P. Hu and X. Zhang, Adv. Mater., 2009, 21, 2849–2864 CrossRef CAS.
- X. W. Lou, L. A. Archer and Z. Yang, Adv. Mater., 2008, 20, 3987–4019 CrossRef CAS.
- Y. Lan, L. Yang, M. C. Zhang, W. Q. Zhang and S. N. Wang, ACS Appl. Mater. Interfaces, 2010, 2, 127–133 CrossRef CAS.
- A. Khanal, Y. Inoue, M. Yada and K. Nakashima, J. Am. Chem. Soc., 2007, 129, 1534–1535 CrossRef CAS.
- G. L. Li, C. Lei, C. Wang, K. G. Neoh, E. T. Kang and X. Yang, Macromolecules, 2008, 41, 9487–9490 CrossRef CAS.
- C. I. Zoldesi and A. Imhof, Adv. Mater., 2005, 17, 924–928 CrossRef CAS.
- P. Arnal, M. Comotti and F. Schüth, Angew. Chem., Int. Ed., 2006, 45, 8224–8227 CrossRef CAS.
- K. Kamata, Y. Lu and Y. Xia, J. Am. Chem. Soc., 2003, 125, 2384–2385 CrossRef CAS.
- J. Lee, J. C. Park, J. U. Bang and H. Song, Chem. Mater., 2008, 20, 5839–5844 CrossRef CAS.
- E. V. Shevchenko, M. I. Bodnarchuk, M. V. Kovalenko, D. V. Talapin, R. K. Smith, S. Aloni, W. Heiss and A. P. Alivisatos, Adv. Mater., 2008, 20, 4323–4329 CrossRef CAS.
- X. J. Wu and D. Xu, Adv. Mater., 2010, 22, 1516–1520 CrossRef CAS.
- Y. Yin, R. M. Rious, C. K. Erdonmez, S. G. Hughes, A. Somorjai and A. P. Alivisatos, Science, 2004, 282, 711–714 CrossRef.
- J. Gao, G. Liang, B. Zhang, Y. Kuang, X. Zhang and B. Xu, J. Am. Chem. Soc., 2007, 129, 1428–1433 CrossRef CAS.
- R. Güttel, M. Paul and F. Schüth, Chem. Commun., 2010, 46, 895–897 RSC.
- K. T. Kim, J. L. M. Cornelissen, R. J. M. Nolte and J. C. M. van Hest, Adv. Mater., 2009, 21, 2787–2791 CrossRef CAS.
- X. X. Wang, H. F. Ji, X. Zhang, H. Zhang and X. L. Yang, J. Mater. Sci., 2010, 45, 3981–3989 CrossRef CAS.
- M. Motornov, Y. Roiter, I. Tokarev and S. Minko, Prog. Polym. Sci., 2010, 35, 174–211 CrossRef CAS.
- S. Bhattacharya, F. Eckert, V. Boyko and A. Pich, Small, 2007, 3, 650–657 CrossRef CAS.
- G. L. Li, G. Liu, E. T. Kang, K. G. Neoh and X. L. Yang, Langmuir, 2008, 24, 9050–9055 CrossRef CAS.
- C. S. Peyratout and L. Dähne, Angew. Chem., Int. Ed., 2004, 43, 3762–3783 CrossRef.
- G. Sukhorukov, A. Fery and H. Mőhwald, Prog. Polym. Sci., 2005, 30, 885–897 CrossRef CAS.
- A. Klaikherd, C. Nagamani and S. Thayumanavan, J. Am. Chem. Soc., 2009, 131, 4830–3195 CrossRef CAS.
- G. Li, L. Shi, R. Ma, Y. An and N. Huang, Angew. Chem., Int. Ed., 2006, 45, 4959–4962 CrossRef CAS.
- M. Sauer and W. Meier, Chem. Commun., 2001, 55–56 RSC.
- M. Sauer, D. Streich and W. Meier, Adv. Mater., 2001, 13, 1649–1651 CrossRef CAS.
- Q. Zhang, T. R. Zhang, J. P. Ge and Y. D. Yin, Nano Lett., 2008, 8, 2867–2871 CrossRef CAS.
- G. L. Li, E. T. Kang, K. G. Neoh and X. L. Yang, Langmuir, 2009, 25, 4361–4364 CrossRef CAS.
- G. L. Li, L. Q. Xu, X. Z. Tang, K. G. Neoh and E. T. Kang, Macromolecules, 2010, 43, 5797–5803 CrossRef CAS.
- K. S. Chou and Y. S. Lai, Mater. Chem. Phys., 2004, 83, 82–88 CrossRef CAS.
- W. Stöber, A. Fink and E. Bohn, J. Colloid Interface Sci., 1968, 26, 62–69 CrossRef.
- E. Bourgeat-Lami and J. Lang, J. Colloid Interface Sci., 1998, 197, 293–308 CrossRef CAS.
- G. L. Li, D. L. Zeng, L. Wang, B. Y. Zong, K. G. Neoh and E. T. Kang, Macromolecules, 2009, 42, 8561–8565 CrossRef CAS.
- N. Pradhan, A. Pal and T. Pal, Colloids Surf., A, 2002, 196, 247–257 CrossRef CAS.
- F. Bai, X. L. Yang and W. Q. Huang, Macromolecules, 2004, 37, 9746–9752 CrossRef CAS.
- G. L. Li, D. Wan, K. G. Neoh and E. T. Kang, Macromolecules, 2010, 43, 10275–10282 CrossRef CAS.
- G. L. Li and X. L. Yang, J. Phys. Chem. B, 2007, 111, 12781–12786 CrossRef CAS.
- G. L. Li, Q. Shi, S. J. Yuan, E. T. Kang, K. G. Neoh and X. L. Yang, Chem. Mater., 2010, 22, 1309–1317 CrossRef CAS.
- P. M. Arnal, F. Schüth and F. Kleitz, Chem. Commun., 2006, 1203–1205 RSC.
- G. L. Li, X. L. Yang and F. Bai, Polymer, 2007, 48, 3074–3081 CrossRef CAS.
- D. Pristinski, V. Kozlovskaya and S. A. Sukhishvili, J. Opt. Soc. Am. A, 2006, 23, 2639–2644 CrossRef.
- Y. Kobayashi, H. Katakami, E. Mine, D. Nagao, M. Konno and L. M. Liz-Marzan, J. Colloid Interface Sci., 2005, 283, 392–396 CrossRef.
- P. Narayan, P. Anjali and P. Tarasankar, Colloids Surf., A, 2002, 196, 247–257 CrossRef CAS.
- N. Pradhan, A. Pal and T. Pal, Langmuir, 2001, 17, 1800–1802 CrossRef CAS.
- T. Mauser, C. Déjugnat, H. Möhwald and G. B. Sukhorukov, Langmuir, 2006, 22, 5888–5893 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: FESEM micrograph, EDX spectrum of the Ag@SiO2@PMAA core–double shell hybrid NPs, and catalytic reaction kinetics of Ag NPs under the effect of F−1 ions. See DOI: 10.1039/c1py00054c |
| This journal is © The Royal Society of Chemistry 2011 |