Functionalized nanoscale through microscale polypeptide stabilized emulsions for display of biomolecules†
Jarrod A.
Hanson
a and
Timothy J.
Deming
*ab
aBioengineering Department, University of California, Los Angeles, CA 90095, USA. E-mail: demingt@seas.ucla.edu; Fax: +1 310-794-5956; Tel: +1 310-267-4450
bChemistry and Biochemistry Department, University of California, Los Angeles, CA 90095, USA
First published on 15th March 2011
Abstract
We have prepared stable nano- and microscale emulsion droplets using block copolypeptide surfactants that have been functionalized with biotin. We have varied the biotin density on the droplet surfaces by mixing biotinylated with non-biotinylated surfactants, and used these to optimize the level of biotinylation for binding of NeutrAvidin. Using fluorescence microscopy and dynamic light scattering we have verified specific binding of NeutrAvidin to the biotinylated droplets and developed conditions for specific binding of biotinylated ligands to the NeutrAvidin coated droplets. These experiments outline a process for preparation of a soft, nano- through microscale sized droplet platform capable of specifically conjugating and displaying biotinylated molecules.
A variety of functionalized micro- and nanoparticles have been prepared for use in applications including targeted therapeutics,1–4 ultrasound imaging,5 and mimicking cell adhesion.6,7 These materials have ranged from hard particles8–10 to soft vesicles1–4,7 and emulsion droplets,5,6,11,12 and have employed a multitude of conjugation modalities, including click chemistry13 and the biotin–avidin interaction.14 For such applications, one should be able to readily control the size and functionality of these materials to tune their properties. Recently, we reported block copolypeptide surfactants that were very effective at stabilizing oil in water emulsions and water in oil in water double emulsions.15 This system allows formation of highly stable droplets under a variety of conditions, including use of different surfactants, oils, and aqueous buffers. Consequently, the preparation of droplets with different surface chemistries (e.g. anionic, neutral or cationic) and droplet diameters ranging from tens of nanometres to tens of microns is straightforward.15 Here, we have conjugated biotin to poly(L-lysine)55-block-poly(racemic-leucine)20, K55(rac-L)20, surfactants to enable preparation of functionalized oil droplets capable of binding complimentary functionalized molecules. This system is advantageous in providing a versatile platform of droplets that can be prepared with diameters ranging from nano- through microscale and that presents easily controlled amounts of biotin groups.
For attachment and presentation of molecules to emulsion droplet surfaces, we have chosen the biotin–avidin system,14 as it is effective in a variety of media and many biotinylated molecules are readily available. Biotinylated droplets have been prepared previously,5,6,11,12 either by conjugation of biotin to intact droplets or exchange of biotinylated surfactants onto droplet interfaces. Both of these methods result in levels of biotinylation that are difficult to control and require extensive droplet washing to purify the conjugates. Following the success in using well-characterized biotinylated amphiphiles to obtain functionalized vesicles,1–4,7 we decided to prepare a biotinylated block copolypeptide surfactant, biotin–K55(rac-L)20, to better control the degree of droplet biotinylation. An attractive feature of the polypeptide surfactants employed here is their large hydrophilic segments containing many amine functional groups that allow facile derivatization. Using standard carbodiimide coupling chemistry, we conjugated 3 equivalents of biotin to each K55(rac-L)20 chain (Fig. 1), which should provide an adequate number of biotin groups while not significantly affecting the surfactant properties of the copolypeptide.
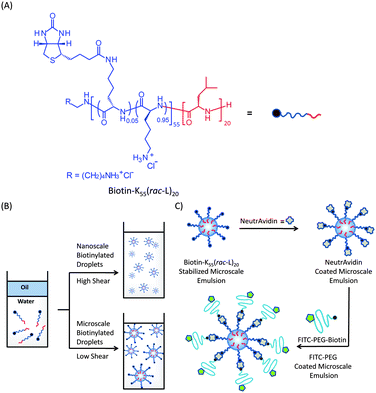 | ||
| Fig. 1 (A) Structure of biotin–K55(rac-L)20 containing an average of 3 biotin molecules per chain (5% of lysine amines functionalized). (B) Nano- and microscale formation of biotin–K55(rac-L)20 stabilized droplets. (C) Schematic illustration showing binding of NeutrAvidin onto emulsion surface and subsequent addition of FITC–PEG–Biotin (Mn = 3400) onto NeutrAvidin coated droplets. | ||
Our first experiments studied the ability of biotin–K55(rac-L)20 to stabilize nano- and microscale emulsions. Droplets were prepared by mixing solutions of different concentrations of biotin–K55(rac-L)20 with polydimethylsiloxane oil (PDMS, 10 cSt, ϕ = 0.2) using a handheld homogenizer. Samples emulsified using ultrasonic homogenization formed smaller droplets, with mean diameters of 390 nm as determined by dynamic light scattering.15 Both the nano- and microscale emulsions contained a range of droplet diameters, with polydispersities of ca. 0.22. As shown previously, these droplets can be size fractionated by centrifugation to obtain samples with a narrow range of droplet diameters.15 Both the nano- and microscale emulsions were stable over time at ambient temperature with droplet sizes remaining unchanged after 24 days (see Fig. S1 and S2 in the ESI†). These results show that these highly biotinylated droplets are similar to the unmodified surfactant by being able to form stable emulsions with a range of droplet sizes. Our previous work with the parent surfactant showed that such droplets can be formed with a variety of oil phases.15
To study and optimize specific binding to the biotinylated droplets by NeutrAvidin, a non-glycosylated variant of avidin with a pI of 6.3 that minimizes nonspecific interactions,16 we prepared a series of emulsions with different ratios of biotinylated to unmodified K55(rac-L)20 surfactants to vary the amount of biotin presented on the droplet surfaces (see Fig. S3 and S4 in the ESI†). Our goal was to minimize the amount of biotin needed to obtain good NeutrAvidin binding and droplet coverage, as well as limit the amount of excess biotin as it could saturate NeutrAvidin binding sites or bind to other NeutrAvidin coated droplets. Microscale emulsions of PDMS (10 cSt, ϕ = 0.2) stabilized with 1 mM solutions of different biotin–K55(rac-L)20/K55(rac-L)20 mixtures in deionized water were prepared using a handheld homogenizer. After formation, the droplets were centrifuged multiple times to remove the free biotinylated surfactant and each time resuspended in a 0.01 M PBS solution containing 0.5 mM K55(rac-L)20 and 0.05% Triton X-100. Unmodified K55(rac-L)20 was added during resuspension to maintain droplet stability since the surfactant was removed during each centrifugation. Small amounts of the nonionic surfactant, Triton X-100, were also added to help reduce nonspecific adsorption of NeutrAvidin to the droplets, and were found to have no effect on either droplet formation or stability.
The resulting emulsion droplets containing different degrees of biotinylation were next incubated with NeutrAvidin. To monitor this process, fluorescently labeled NeutrAvidin, FITC–NeutrAvidin, and fluorescence microscopy were used to image the droplets before and after binding. A large excess of FITC–NeutrAvidin was added to the emulsions to encourage complete coverage of available biotin groups and to prevent NeutrAvidin mediated droplet–droplet coupling. Droplets containing low amounts of biotinylated surfactant (25 mol%) were found to bind little FITC–NeutrAvidin, while those containing 50 mol% biotinylated surfactant showed good NeutrAvidin coverage (Fig. 2, see Fig. S3 in the ESI†). Higher levels of biotinylation (75 and 100 mol%) gave droplets densely coated with NeutrAvidin, which appeared to aggregate on the droplet surface (see Fig. S3 in the ESI†). Hence, emulsions containing 50 mol% of biotin–K55(rac-L)20 were chosen as the optimal surfactant composition for NeutrAvidin binding to microscale droplets.
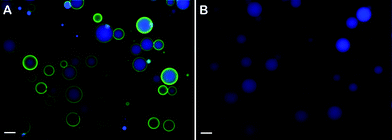 | ||
| Fig. 2 Fluorescence microscopy images showing FITC–NeutrAvidin interactions with microscale emulsion droplets containing either 50 mol% biotin–K55(rac-L)20 or 100 mol% unmodified K55(rac-L)20. (A) Fluorescence microscopy overlay image of biotinylated droplets after addition of FITC–NeutrAvidin followed by centrifugation and resuspension. Surfactant composition: 0.5 mM biotin–K55(rac-L)20 + 0.5 mM K55(rac-L)20; oil phase: 10 cSt PDMS containing 50 mM pyrene; ϕ = 0.20; emulsification: handheld homogenizer for 30 seconds. (B) Fluorescence microscopy overlay image of non-biotinylated droplets after addition of FITC–NeutrAvidin followed by centrifugation and resuspension. Surfactant composition: 1.0 mM K55(rac-L)20; oil phase: 10 cSt PDMS containing 50 mM pyrene; ϕ = 0.20; emulsification: handheld homogenizer for 30 seconds. Blue = pyrene, green = FITC–NeutrAvidin. Droplets were diluted approximately tenfold prior to imaging. Scale bar = 20 μm. | ||
To confirm that NeutrAvidin attachment to these droplets was due solely to specific binding to biotin, the interaction of non-biotinylated emulsions with NeutrAvidin was also studied. For fluorescence imaging, the emulsion oil phases were labeled with pyrene yielding blue fluorescence. While the biotinylated droplets were found to bind NeutrAvidin well, no NeutrAvidin was found to bind to the non-functionalized emulsions indicating good specific binding of NeutrAvidin to the biotin groups (Fig. 2). Similar experiments using nanoscale emulsion droplets showed similar results (data not shown), except that a lower ratio of biotinylated surfactant (ca. 10 mol%) was found to be optimal for NeutrAvidin binding.
The ability to prepare stable biotinylated emulsion droplets that can be specifically coated with NeutrAvidin offers potential for their use as a platform for binding and display of biotinylated molecules. Success in this application requires the NeutrAvidin proteins to be bound to the droplets in a manner where some of the biotin binding sites (four per protein) remain unoccupied and available.1–7 To check if this was the case for our system, we attempted to attach biotinylated poly(ethylene glycol), PEG–biotin, to the NeutrAvidin coated droplets described above. To aid visualization by fluorescence imaging, biotinylated droplets were coated with non-labeled NeutrAvidin, followed by addition of excess fluorescently labeled PEG–biotin, FITC–PEG–biotin. FITC–PEG–biotin was also added to biotinylated droplets that had not been coated with NeutrAvidin as a control. The results of these studies showed that FITC–PEG–biotin binds strongly to the NeutrAvidin coated droplets, and does not bind to the biotinylated droplets lacking NeutrAvidin (Fig. 3). These data indicate that the emulsion bound NeutrAvidin is capable of binding biotinylated molecules, and that there is no nonspecific binding of FITC–PEG–biotin.
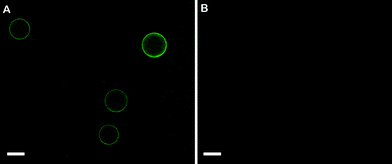 | ||
| Fig. 3 Fluorescence microscopy images showing FITC–PEG–biotin interactions with microscale biotinylated emulsion droplets with or without bound NeutrAvidin. (A) Fluorescence microscopy image of NeutrAvidin coated biotinylated droplets after addition of FITC–PEG–biotin followed by centrifugation and resuspension. (B) Fluorescence image of biotinylated droplets without NeutrAvidin after addition of FITC–PEG–biotin followed by centrifugation and resuspension. Surfactant composition of both emulsions: 0.5 mM biotin–K55(rac-L)20 + 0.5 mM K55(rac-L)20; oil phase: 10 cSt PDMS; ϕ = 0.20; emulsification: handheld homogenizer for 30 seconds. Green = FITC–PEG–biotin. Droplets were diluted approximately tenfold prior to imaging. Scale bar = 20 μm. | ||
Overall, the attachment of biotin to the hydrophilic domains in block copolypeptide stabilized emulsions was shown to result in no adverse effects on emulsion formation or stability. These biotinylated emulsions were prepared with diameters ranging from nano- to microscale, and retained physical properties similar to unmodified polypeptide stabilized emulsions. By analogy to our work on these unmodified emulsions, we expect that similar biotinylated emulsions could be prepared using surfactants containing different hydrophilic functionalities (e.g. cationic, anionic or neutral segments) or with different oil phases as needed for different uses. The ability to mix biotinylated and unmodified surfactants also offers a practical advantage for simple adjustment of droplet biotinylation to tune their properties. Future studies will focus on development of this emulsion platform for specific applications.
Notes and references
- H. C. Loughrey, L. S. Choi, K. F. Wong, P. R. Cullis and M. B. Bally, Liposome Technology, CRC Press, 1993 Search PubMed.
- B. Rivney, E. A. Bayer and M. Wilchek, Methods Enzymol., 1987, 149, 119–123 Search PubMed.
- H. Loughrey, M. B. Bally and P. R. Cullis, Biochim. Biophys. Acta, 1987, 901, 157–160 CAS.
- R. J. Mart, K. P. Liem and S. J. Webb, Pharm. Res., 2009, 26, 1701–1710 CAS.
- J. M. M. Simons, L. M. Kornmann, K. D. Reesink, A. P. G. Hoeks, M. F. Kemmere, J. Meuldijk and J. T. F. Keurentjes, J. Mater. Chem., 2010, 20, 3918–3923 RSC.
- J. Fattaccioli, J. Baudry, N. Henry, F. Brochard-Wyart and J. Bibette, Soft Matter, 2008, 4, 2434–2440 RSC.
- D. A. Hammer, G. P. Robbins, J. B. Haun, J. J. Lin, W. Qi, L. A. Smith, P. P. Ghoroghchian, M. J. Therien and F. S. Bates, Faraday Discuss., 2008, 139, 129–141 RSC.
- J. Beeg, S. Klumpp, R. Dimova, R. S. Gracia, E. Unger and R. Lipowsky, Biophys. J., 2008, 94, 532–541 CrossRef CAS.
- R. Yokokawa, M. C. Tarhan, T. Kon and H. Fujita, Biotechnol. Bioeng., 2008, 101, 1–8 CrossRef CAS.
- G. Muthukrishnan, B. M. Hutchins, M. E. Williams and W. O. Hancock, Small, 2006, 2, 626–630 CrossRef CAS.
- J. Fattaccioli, J. Baudry, J.-D. Emerard, E. Bertrand, C. Goubault, N. Henry and J. Bibette, Soft Matter, 2009, 5, 2232–2238 RSC.
- C. Bottier, J. Fattaccioli, M. C. Tarhan, R. Yokokawa, F. O. Morin, B. Kim, D. Collarda and H. Fujita, Lab Chip, 2009, 9, 1694–1700 RSC.
- P. Wu, A. K. Feldman, A. K. Nugent, C. J. Hawker, A. Scheel, B. Voit, J. Pyun, J. M. Frechet, K. B. Sharpless and V. V. Fokin, Angew. Chem., Int. Ed., 2004, 43, 3928–3932 CrossRef CAS.
- N. M. Green, Methods Enzymol., 1990, 184, 51–67 CAS.
- J. A. Hanson, C. Chang, S. Graves, Z. Li, T. G. Mason and T. J. Deming, Nature, 2008, 455, 85–88 CrossRef CAS.
- Y. Hiller, J. M. Gershoni, E. A. Bayer and M. Wilchek, Biochem. J., 1987, 248, 167–171 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures and Fig. S1 (dynamic light scattering data), Fig. S2 (image showing emulsion stability), Fig. S3 (images of NeutrAvidin binding to droplets containing different levels of biotin) and Fig. S4 (droplet sizes as a function of level of biotinylation). See DOI: 10.1039/c1py00045d |
| This journal is © The Royal Society of Chemistry 2011 |
