Synthesis of paramagnetic polymers using ionic liquid chemistry†
Markus
Döbbelin
*a,
Vasko
Jovanovski
ab,
Irantzu
Llarena
c,
Luis J.
Claros Marfil
d,
Germán
Cabañero
a,
Javier
Rodriguez
a and
David
Mecerreyes
*a
aNew Materials Department, CIDETEC, Center for Electrochemical Technologies, Parque Tecnológico de San Sebastián, Paseo Miramón 196, Donostia-San Sebastián, 20009, Spain. E-mail: mdobbelin@cidetec.es; dmecerreyes@cidetec.es; Fax: +34 943309136; Tel: +34 943309022
bAnalytical Chemistry Laboratory, National Institute of Chemistry, Hajdrihova 19, 1000, Ljubljana, Slovenia
cCIC BIOMAGUNE, Parque Tecnológico de San Sebastián, Paseo Miramón 182 C, Donostia-San Sebastián, 20009, Spain
dACCIONA Infraestructuras, Centro Tecnológico I+D+i, Valportillo II, no. 8, Alcobendas, 28108, Spain
First published on 24th March 2011
Abstract
Herein, the synthesis of polymeric ionic liquids (PILs) with paramagnetic anions based on tetrachloroferrate(III) (FeCl4−) and tetrabromoferrate(III) (FeBr4−) is presented. The formation of the novel polymers encompassing the paramagnetic anions is proven by UV-VIS and Raman spectroscopy. Paramagnetic properties are analyzed using a SQUID measuring system. Some of the obtained PILs show higher magnetic susceptibility values than previously measured for paramagnetic ionic liquids. It is further found that the magnetic susceptibility can be tailored by controlling the iron content in the anion. Interestingly, casted films of the PILs show a strong response to a neodymium magnet. As a potential application, a microgel based on a paramagnetic PIL is used as a reusable catalyst in Friedel–Crafts alkylation. The paramagnetic PIL microgel can be easily separated and reused after the reaction making these new materials interesting for green chemistry processes.
Introduction
Ionic liquids (ILs) are room-temperature liquid salts, composed mostly of organic ions that have been the focus of many investigations because of their low melting point (often below room temperature), good chemical and thermal stability, low flammability, negligible vapour pressure, high ionic conductivity and wide electrochemical potential window.1 Today, ionic liquids are finding a way into industrial applications as recyclable (green) solvents for organic synthesis,2 separation processes3 and in electrochemical technologies such as solar cells,4lithium batteries,5 supercapacitors6 and others.The ionic nature of ILs provides great possibilities in the design of new ILs. Hence, by combining different cations and anions ILs may undergo almost unlimited structural variations.7 The nature of cation and anion has crucial influence on the properties of ILs and characteristics like solubility, hydro-phobicity/-philicity, melting point, magnetic responsiveness, etc. can be selectively tailored. The latter characteristic has been first reported by Hayashi and Hamaguchi8 showing that imidazolium-based ILs with the paramagnetic anion FeCl4− show a strong magnetic field response. Furthermore, they found that these ILs have a good magnetic susceptibility which opened up a new research area in the ionic liquid chemistry. Another interesting feature of magnetic ILs is its potential use in organic synthesis. For example, it was reported that ILs with FeCl4− anions show very good performances as a “reusable catalyst” in Friedel–Crafts reactions.9 This feature presents a great advantage to conventional catalysts (AlCl3, FeCl3 and ZnCl2) as these Lewis acids possess several drawbacks such as difficulties in separation/recovery, environmental hazards, disposal problems, etc. Other groups described the incorporation of magnetic ionic liquids into a PMMA matrix for the obtainment of soft, flexible and transparent ionogels10 or the use of polymeric ionic liquids with FeCl4− anion as a microwave-absorbing material.11
Albeit several studies have been devoted to paramagnetic ILs, the synthesis, characterization and application of paramagnetic polymeric ionic liquids (PILs) are still in their infancy. PILs are usually derived from IL monomers with polymerizable groups, e.g. vinyl, diallyl or methacrylic.12 PILs are very interesting materials as they retain some of the intrinsic properties of ILs such as ionic conductivity, thermal and chemical stability while offering very good solubility and processing properties.13
In this study several PILs with paramagnetic anions were synthesized by mixing imidazolium or pyrrolidinium-based PILs with the Lewis acids FeCl3 or FeBr3. In this way, PILs bearing FeCl4−, Fe2Cl7−, Fe3Cl10−, FeBr4− or mixed anions such as FeBr3Cl− or FeBrCl3− were obtained. All polymers showed excellent magnetic susceptibility, in some cases even higher values than those reported for paramagnetic ILs. Furthermore, films of paramagnetic PILs showed a strong response to a neodymium magnet. To show a potential application of these paramagnetic/Lewis acid materials a polymeric microgel was synthesised and used as a reusable catalyst in an aromatic Friedel–Crafts alkylation.
Experimental part
Materials
Poly(1-vinyl-3-ethyl-imidazolium bromide), poly(1-vinyl-3-butyl-imidazolium chloride) and poly(diallyldimethylammonium chloride) were synthesised following the procedures described in the literature.13,14Iron(III) chloride (98%) and iron(III) bromide (98%), benzyl chloride (≥99.0%) and all solvents were purchased from Aldrich. Poly(1-vinyl-3-butyl-imidazolium chloride) spherical, cross-linked microparticles (microgel, average particle size: 6 µm) were prepared as previously described.15Friedel–Crafts alkylation
A paramagnetic microgel of poly(1-vinyl-3-butyl-imidazolium FeCl4−) was used as a reusable catalyst in Friedel–Crafts alkylation. The alkylation of toluene with benzyl chloride was performed according to the following procedure: 0.05 g of catalyst, 1.5 g of toluene (16.3 mmol) and 0.2 g of benzyl chloride (1.63 mmol) were placed into a 10 mL round-bottom flask equipped with a stirrer and a reflux condenser. After refluxing for 20 min, the microgel was separated by centrifugation or by a magnet, thoroughly washed with toluene, dried under vacuum and subsequently reused. The isomers of benzylmethylbenzene were obtained by removal of excess toluene on the rotary evaporator. The isolated products were identified by 1H-NMR analysis (ESI†).Characterisation
All obtained polymers were freeze-dried with a Cryodos-50 freeze-dryer from IMA-Telstar. Ultraviolet-visible spectroscopy (UV-Vis) was performed on a Shimadzu UV-1603 spectrophotometer. Raman measurements were carried out on a Renishaw inVia Raman microscope at a wavelength of 785 nm. 1H NMR measurements were conducted on a Bruker Avance III NMR spectrometer. Magnetic measurements were conducted on a SQUID Quantum Design MPMS 5S magnetometer at 300 K. The SEM image was taken with a JSM-5500LV from JEOL.Results and discussion
For the synthesis of paramagnetic polymeric ionic liquids, one equivalent (with respect to monomer units) of FeCl3 and FeBr3, respectively, were dissolved in water or methanol and then added drop-wise and under stirring to the polymeric ionic liquids (PILs) which were previously dissolved in water or methanol. To synthesize materials with anions with higher Fe content, 2 and 3 equivalents of FeCl3 were added to the PIL, respectively. After addition of the iron halogenides, most of the newly formed compounds precipitated from the medium. The final products were obtained by removing the solvents on the rotary evaporator followed by freeze-drying to constant weight. In all cases the product yield lay above 99%. A total of seven PILs were prepared. Interestingly, the different materials showed variations in their colours: poly(diallyldimethylammonium FeCl4−) (pDaDmAm+ FeCl4−), yellow, poly(1-vinyl-3-butyl-imidazolium FeCl4−) (pViBuIm+ FeCl4−), light brown, poly(1-vinyl-3-butyl-imidazolium Fe2Cl7−) (pViBuIm+ Fe2Cl7−), brown, poly(1-vinyl-3-butyl-imidazolium Fe3Cl10−) (pViBuIm+ Fe3Cl10−), dark brown, poly(1-vinyl-3-ethyl-imidazolium FeBr4−) (pViEtIm+ FeBr4−), dark brown, poly(diallyldimethylammonium FeBr3Cl−) (pDaDmAm+ FeBr3Cl−), carmine red and poly(1-vinyl-3-ethyl-imidazolium FeBrCl3−) (pViEtIm+ FeBrCl3−), brown. Scheme 1 displays the synthetic procedure for obtaining paramagnetic PILs. A similar procedure was employed to synthesize paramagnetic polymer microgels. A microgel of poly(1-vinyl-3-butyl-imidazolium chloride) was stirred for 12 h in a 1 M FeCl3 aqueous solution. The particles were subsequently washed with water and separated by centrifugation. Residual water was removed by freeze drying.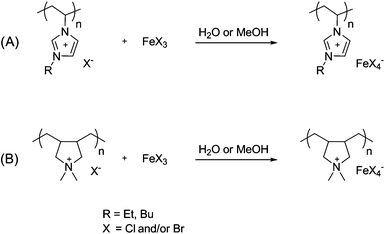 | ||
| Scheme 1 Synthesis of polymeric ionic liquids (PILs) with paramagnetic anions where (A) imidazolium-based PILs and (B) pyrrolidinium-based PILs. | ||
First, the paramagnetic PILs were characterized by UV-Vis spectroscopy. UV-Vis spectra of paramagnetic PILs could be obtained either from polymeric films or solutions. As an example, Fig. 1(a) shows the spectrum of a film of poly(1-vinyl-3-butyl-imidazolium FeCl4−). As previously reported in the literature, the typical three absorption bands of FeCl4− can be observed at around 533, 615 and 688 nm.8,16 The spectrum in Fig. 1(b) was taken from an acetonitrile solution of poly(1-vinyl-3-ethyl-imidazolium FeBr4−). Also here, typical bands for FeBr4− are seen at about 605, 708, 778 and 845 nm.16 It can therefore be concluded that PILs with FeCl4− and FeBr4− anions were correctly formed.
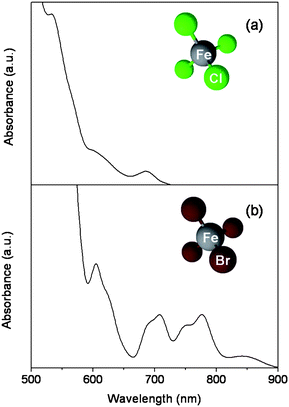 | ||
| Fig. 1 UV-Vis absorption spectra of (a) a polymeric film of poly(1-vinyl-3-butyl-imidazolium FeCl4−) and (b) a poly(1-vinyl-3-ethyl-imidazolium FeBr4−) acetonitrile solution. Molecular structures of FeCl4− and FeBr4−. | ||
To study the compositions of the synthesized PILs more in detail, Raman Spectroscopy was used. At room temperature, iron (III) chloride formed paramagnetic polymeric ionic liquids with poly(1-vinyl-3-butyl-imidazolium chloride) and poly(diallyldimethylammonium chloride). In this way, corresponding tetrachloroferrate (FeCl4−) is formed when the ratio between Cl−/FeCl3 is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.17 Analogously, tetrabromoferrate (FeBr4−) was formed from poly(1-vinyl-3-ethyl-imidazolium bromide) and FeBr3. Poly(diallyldimethylammonium FeBr3Cl−) and poly(1-vinyl-3-ethyl-imidazolium FeBrCl3−) were also prepared. Fig. 2 shows Raman spectra of paramagnetic polymeric ionic liquids containing different Cl− to FeCl3 ratios, as well as poly(1-vinyl-3-ethyl-imidazolium FeBr4−), poly(1-vinyl-3-ethyl-imidazolium FeBrCl3−) and poly(diallyldimethylammonium FeBr3Cl−).
1.17 Analogously, tetrabromoferrate (FeBr4−) was formed from poly(1-vinyl-3-ethyl-imidazolium bromide) and FeBr3. Poly(diallyldimethylammonium FeBr3Cl−) and poly(1-vinyl-3-ethyl-imidazolium FeBrCl3−) were also prepared. Fig. 2 shows Raman spectra of paramagnetic polymeric ionic liquids containing different Cl− to FeCl3 ratios, as well as poly(1-vinyl-3-ethyl-imidazolium FeBr4−), poly(1-vinyl-3-ethyl-imidazolium FeBrCl3−) and poly(diallyldimethylammonium FeBr3Cl−).
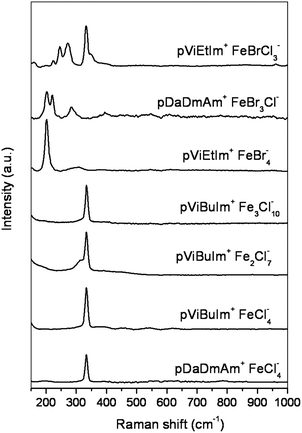 | ||
| Fig. 2 Raman spectra of paramagnetic polymeric ionic liquids. | ||
The band at 334 cm−1 corresponded very well with literature values for FeCl4− as a solid or in solution.18 When the Cl−/FeCl3 ratio was increased in favour of FeCl3, the peak at 334 cm−1 remained predominant due to FeCl4− and additional weak features at about 315 and 420 cm−1 belonging to Fe2Cl7− were observed. This was rather surprising because a band at 370 cm−1 was expected according to the literature. The 370 cm−1 feature has been assigned to an A symmetry vibration of Fe2Cl7−. However, the band at 370 cm−1 was observed in a water solution and not for a solid. Therefore, Raman spectroscopy was also performed in water solution. Raman spectra of water solution of both Fe2Cl7− and Fe3Cl10− containing polymeric ionic liquids exhibited a shoulder at 370 cm−1. Raman spectrum of poly(1-vinyl-3-ethyl-imidazolium FeBr4−) exhibited an intense band at 202 cm−1, assigned to FeBr4−, and a weak broad band at 306 cm−1 assigned to A symmetry vibration of Fe2Br7−.
When an equimolar amount of FeBr3 was added to poly(diallyldimethylammonium chloride) two new bands at 221 and 284 cm−1 appeared besides the band at 202 cm−1 due to the formation of asymmetric FeBr3Cl− anion. Similarly, when an equimolar amount of FeCl3 was added to poly(1-vinyl-3-ethyl-imidazolium bromide), the formation of FeBrCl3− was accompanied with the appearance of new bands at 224, 245, 273 (Fe–Br), 334, 349 and a shoulder at 400 (Fe–Cl) cm−1.
The magnetic properties of the PILs were examined using the SQUID method. For the measurements, a small amount (typically 20–500 mg) of the PILs was located in a gelatine capsule and the magnetic moment was determined in a magnetic field range between −50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and 50
000 and 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Oe. Fig. 3 shows the obtained results. As expected, all PILs showed a linear response to the magnetic field which is typical for paramagnetic materials and has been reported previously for ionic liquids with metallic halogenide anions.8
000 Oe. Fig. 3 shows the obtained results. As expected, all PILs showed a linear response to the magnetic field which is typical for paramagnetic materials and has been reported previously for ionic liquids with metallic halogenide anions.8
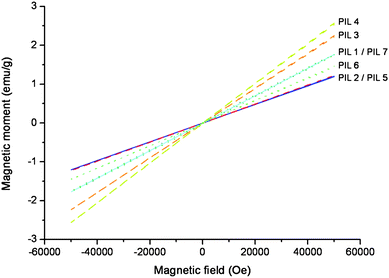 | ||
| Fig. 3 SQUID magnetization of paramagnetic PILs where PIL1 = pDaDmAm+FeCl4−, PIL2 = pViBuIm+ FeCl4−, PIL3 = pViBuIm+ Fe2Cl7−, PIL4 = pVibuIm Fe3Cl10−, PIL 5 = pViEtIm+ FeBr4−, PIL6 = pDaDmAm+FeBr3Cl−, PIL7 = pViEtIm+ FeBrCl3−. (PIL2, PIL3, and PIL4 = dashed line; PIL5 and PIL7 = solid line; PIL1 and PIL6 = dotted line). | ||
The magnetic susceptibility is an indicator for the paramagnetic strength of a material and can be calculated from the gradients of the magnetic field dependence. From Fig. 3 the magnetic susceptibility of the paramagnetic PILs was determined. The PILs pViBuIm+ FeCl4− and pViEtIm+ FeBr4− revealed a similar magnetic susceptibility of 24.5 × 10−6 and 24.1 × 10−6 emu g−1. For the pyrrolidinium-based PILs pDaDmAm+FeBr3Cl− and pDaDmAm+ FeCl4− slightly higher values of 29.3 × 10−6 and 35.3 × 10−6 emu g−1 were calculated. These values were similar to the one of pViEtIm+ FeBrCl3− with 35.3 × 10−6 emu g−1.
Better performances were observed for pViBuIm+ Fe2Cl7− and pViBuIm Fe3Cl10− with 45.1 × 10−6 and 51.5 × 10−6 emu g−1. These values are higher than those reported for paramagnetic ILs which average values of 40.6 × 10−6 emu g−1.8
No clear influence of the nature of the polymer, either imidazolium or diallyldimethylammonium-based, or the anion type, chloride or bromide containing or both, on the paramagnetic strength was seen.
Though, the magnetic susceptibility increased with the iron content in the anion for the same polymer following the trend FeCl4− < Fe2Cl7− < Fe3Cl10−. Hence, the paramagnetic performance of PILs can be tailored by controlling the iron content in the anion.
The pictures in Fig. 4 show the response of a polymeric film of pViEtIm+ FeBr4− (obtained by casting from acetone solution) to a neodymium magnet. The film showed a good response to the magnetic field and eventually stuck to the magnet when close enough. Similar behaviour was observed for films of the other paramagnetic PILs.
 | ||
| Fig. 4 Response of a film of pViEtIm+ FeBr4− to a neodymium magnet. | ||
The paramagnetic/Lewis acid character of the presented PILs makes them interesting candidates to be applied as a “reusable catalyst” in Friedel–Crafts reactions. For this purpose, a microgel of paramagnetic PIL was synthesized. The magnetic response can be beneficial to overcome difficulties in separation/recovery of the catalyst as it can be simply isolated by a magnet or by centrifugation, washed and reused. Therefore, a microgel of poly(1-vinyl-3-butyl-imidazolium FeCl4−) was used. Fig. 5 shows the reaction scheme of the aromatic Friedel–Crafts alkylation and a SEM micrograph of the reusable polymeric microgel. The isomers of benzylmethylbenzene were obtained from toluene and benzyl chloride. The procedure was repeated 10 times without any loss in catalytic performance of the paramagnetic microgel. The product yield ranged constantly above 99%.
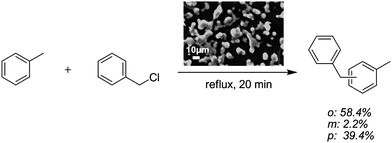 | ||
| Fig. 5 Synthesis scheme of Friedel–Crafts alkylation. A SEM micrograph shows the paramagnetic microgel. | ||
Conclusions
Novel polymeric ionic liquids with paramagnetic anions have been synthesized and characterized. UV-Vis studies revealed the formation of FeCl4− and FeBr4− anions. Moreover, Raman spectroscopy was used as an anion characterization method.An increase in the magnetic susceptibility was observed with increasing iron content in the anion. Interestingly, polymeric films of the PILs showed strong response to a magnetic field. As a potential application, a microgel based on a paramagnetic PIL was applied as a reusable catalyst for Friedel–Crafts alkylation. The microgel could be easily removed after the reaction and reused several times making these new materials attractive candidates for green chemistry processes. This type of mild reusable catalyst is currently under investigation for other carbon–carbon bond forming reactions.
Acknowledgements
This work was supported by the Spanish Ministry of Science and Education: Grant No. TEC2007-66506-C02-02, CTQ2007-60459, Project HOPE CSD2007-0007 (Consolider-Ingenio 2010), the Basque Government through i-NANOGUNE Etortek Program, Project TECNOCAI No. CEN-20091010 (CENIT 2009) and ACCIONA Infraestructuras S.A. V.J. acknowledges the financial support of the European Commission through a Marie Curie IEF program. Partial financial support of the Slovenian Research Agency (Program P1-0034) is also gratefully acknowledged.Notes and references
- H. Ohno, Electrochemical Aspects of Ionic Liquids, Wiley-VCH, Hoboken, NJ, 2005 Search PubMed.
- J. D. Holbrey, W. M. Reichert, R. G. Reddy and R. D. Rogers, Ionic Liquids as Green Solvents: Progress and Prospects, ACS Symposium Series, American Chemical Society, Washington, DC, 2003 Search PubMed.
- T. Letcher, Chemical Thermodynamics for Industry, Royal Society of Chemistry, 2004 Search PubMed.
- N. Papageorgiou, Y. Athanassov, M. Armand, P. Bonhôte, H. Pettersson, A. Azam and M. Grätzel, J. Electrochem. Soc., 1996, 143, 3099 CAS.
- S. Seki, Y. Kobayashi, H. Miyashiro, Y. Ohno, A. Usami, Y. Mita, M. Watanabe and N. Terada, Chem. Commun., 2006, 544 RSC.
- T. Sato, G. Masuda and K. Takagi, Electrochim. Acta, 2004, 49, 3603 CrossRef CAS.
- M. Armand, F. Endres, D. R. MacFarlane, H. Ohno and B. Scrosati, Nat. Mater., 2009, 8, 621 CrossRef CAS.
- S. Hayashi and H. Hamaguchi, Chem. Lett., 2004, 33, 1590 CrossRef CAS.
- G. Wang, N. Yu, L. Peng, R. Tan, H. Zhao, D. Yin, H. Qiu, Z. Fu and D. Yin, Catal. Lett., 2008, 123, 252 CrossRef CAS.
- Z.-L. Xie, A. Jeličić, F.-P. Wang, P. Rabu, A. Friedrich, S. Beuermann and A. Taubert, J. Mater. Chem., 2010, 20, 9543 RSC.
- J. Tang, M. Radosz and Y. Shen, Macromolecules, 2008, 41, 493 CrossRef CAS.
- (a) O. Green, S. Grubjesic, S. Lee and M. A. Firestone, Polym. Rev., 2009, 49, 339 Search PubMed; (b) D. M. Green and T. E. Long, Polym. Rev., 2009, 49, 291 Search PubMed; (c) W. Jaeger, J. Bohrisch and A. Laschewsky, Prog. Polym. Sci., 2010, 35, 511 CrossRef CAS.
- R. Marcilla, J. A. Blazquez, J. Rodriguez, J. A. Pomposo and D. Mecerreyes, J. Polym. Sci., Part A: Polym. Chem., 2004, 42, 208 CrossRef CAS.
- A. L. Pont, R. Marcilla, I. De Meatza, H. Grande and D. Mecerreyes, J. Power Sources, 2009, 188, 558 CrossRef CAS.
- R. Marcilla, M. Sanchez-Paniagua, B. Lopez-Ruiz, E. Lopez-Cabarcos, E. Ochoteco, H. Grande and D. Mecerreyes, J. Polym. Sci., Part A: Polym. Chem., 2006, 44, 3958 CrossRef CAS.
- Y. Yoshida and G. Saito, J. Mater. Chem., 2006, 16, 1254 RSC.
- M. S. Sitze, E. R. Schreiter, E. V. Patterson and R. Griffith Freeman, Inorg. Chem., 2001, 40, 2298 CrossRef CAS.
- J. S. Avery, C. D. Burbridge and D. M. L. Goodgame, Spectrochim. Acta, Part A, 1968, 24, 1721 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: 1H-NMR spectrum. See DOI: 10.1039/c1py00044f |
| This journal is © The Royal Society of Chemistry 2011 |
