Cryostructuration as a tool for preparing highly porous polymer materials
Harald
Kirsebom
* and
Bo
Mattiasson
Department of Biotechnology, Lund University, P.O. Box 124, SE-22100, Lund, Sweden. E-mail: Harald.Kirsebom@biotek.lu.se; Tel: +46 462220881
First published on 2nd March 2011
Abstract
Cryostructuration is a technique which can be used to produce highly porous polymer materials from either monomeric or polymeric starting material. The technique utilizes freezing of dilute solutions or suspensions for the formation of porous materials. The process has been used in both aqueous and organic media for the preparation of porous materials. This mini-review highlights some recent trends for cryostructuration where it is based on a freeze/thawing approach.
 Harald Kirsebom | Harald Kirsebom received his PhD in 2010 from Lund University, Sweden. Prior to this he was working as a Marie Curie fellow at the Institute for Polymer Science and Technology in Madrid, Spain. His research areas have been focused on investigating cryostructuration for the production of macroporous materials and developing macroporous systems for improved response rate of thermosensitive polymers. |
 Bo Mattiasson | Bo Mattiasson has for more than 25 years been professor in Biotechnology at Lund University, Sweden. His research interests include bioseparation, biosensors, and environmental biotechnology. Use of polymers in biotechnology has been a theme over many years, including cryostructured polymers and particles and smart polymers. He has published more than 670 papers in peer reviewed journals and edited 10 volumes. |
Introduction
Cryostructuration for the preparation of porous materials is inspired by the natural process when seawater freezes in nature. Seawater is basically an aqueous solution of dissolved salts and during the process of freezing such a solution a heterogeneous two phase system is formed.1 Ice crystals which start forming expel any solutes or any solid impurities thus the formed crystals are of pure ice.1 Hence, a liquid non-frozen phase containing the expelled solutes and water is formed in a space surrounding the crystals.2 Since the majority of the water freezes the resulting volume of the non-frozen phase will be only a fraction of the starting volume and thus the concentration of solutes increases proportionally.2,3 The freezing temperature will determine the concentration in the non-frozen phase since the solvent will continue to crystallize until the freezing point depression in the liquid phase will reach a temperature corresponding to the actual temperature.2,3 Thus, a two phase system is formed with pure ice as one phase and a concentrated salt solution as the other. Even if ice from seawater looks solid, in reality a fraction of the material is liquid. In the case of sea ice the presence of a liquid phase results in the transportation of the brine solution through the ice. This process is being mimicked in the process of cryostructuration in which monomers, polymers or particles in solutions/suspensions are being frozen and accumulated in a non-frozen phase. Thus in this non-frozen phase chemical reactions can proceed (Fig. 1). It is however important that the temperature used is above the eutectic point of the system otherwise both phases will be solid. | ||
| Fig. 1 Schematic illustration of cryostructuration. Initially a solution or suspension is frozen causing ice crystals to form and create a non-frozen phase containing all solutes or particles. In the non-frozen phase reactions can occur which result in the formation of a stabile polymeric network. Thawing the system then leads to the melting of the ice crystals while the polymeric network retains its shape. Hence, a macroporous polymeric sample is formed in which the pores are replicas of the ice crystal network. | ||
That chemical reactions can occur in an apparently frozen sample was something that was reported by Pincock et al.. in the 1960s.4,5 It was noted that chemical reactions could proceed in a frozen state and it was attributed to the presence of a non-frozen phase in the sample. Some of the first reports of using the cryostructuration phenomena for the production of porous polymer material were by the group of Lozinsky in the 1980s.6
For the cryostructuration process it is important that the freezing process and formation of an ice crystal network occur before the formation of the material.7 Thus, ice crystals form and generate, by exclusion, a non-frozen phase containing the precursors between the ice crystals and in this liquid phase the material can form. Working with dilute mixtures will cause the majority of the solvent, up to 95%, to freeze.8 Given that the sample mostly consists of ice crystals, these will thus be connected to form a connected network. When melting the crystals a porous structure is obtained with interconnected pores (where the ice was) in the size-range of 1–200 μm.9,10 The sizes of the ice crystals and thus of the pores are dependent on the freezing temperature, lower temperature results in smaller pores and higher in larger.3,11 Factors such as concentration of starting material and other additives will also influence the porosity, since more solutes will result in a larger non-frozen phase and thus larger formed polymeric material.9,12
Characteristic properties of these materials are that they are spongy and highly permeable structures due to the interconnected pore structure.13 The structure of these materials is often being studied using scanning electron microscopy (SEM). When using this technique the materials are in a dry state and thus not in the hydrated state.3,13 Some care should be taken when interpreting the results from SEM-studies since freeze drying of materials will induce pores even in non-porous hydrogels. However, proper treatment of cryostructured materials allows studying the materials using SEM and to confirm that the structure observed is similar in the wet state confocal laser scanning microscopy (CLSM) can be utilized.12,14,15 Materials which are auto-fluorescent or fluorescently labeled can be studied using CLSM in a wet state. These studies have confirmed that the observed porous structure for cryostructured materials in SEM is also observed in the wet state and hence not an artefact of the drying (Fig. 2).12
 | ||
| Fig. 2 Images from SEM (top) and CLSM (bottom) showing the pore structure of a cryostructured material from polyacrylamide. The image in the top is a gel after critical point drying and the bottom is the gel in wet state after dyeing with FITC (fluorescein isothiocyanate, isomer I). The CLSM image shows that the polyacrylamide gel exhibits the macroporous structure in the wet state which is observed in the SEM. Please note the difference in scale bar between the two images. Figure reproduced with permission from ref. 12. | ||
This mini-review focuses on highlighting the recent work in which cryostructuration is used to form materials through a freeze/thawing approach and thus not requiring freeze-drying. For materials formed by using a freeze-drying step a number of interesting reviews are available and these are not covered in this work.16,17
Polymerization reactions in a non-frozen phase
Redox initiated free radical polymerization reactions of hydrophilic monomers have been one of the most utilized systems for preparation of cryostructured materials from monomeric precursors.9,13,18,19 This has proven to be a suitable system for a wide range of monomers such as acrylamide, dimethylacrylamide, 2-hydroxyethylmethacrylate and N-isopropylacrylamide (NIPA). However, care must be taken so that the polymerization reactions proceed after the ice crystals have formed. Otherwise the ice crystals will form in a gel rather than in a solution which will affect the properties of the final material. One way to ensure that the ice crystals form prior to reaction can be to use UV-initiated polymerization. It has been shown that UV radiation of a frozen mixture of monomers or polymers and an UV-initiator results in an efficient formation of cryostructured materials.20 Potential drawbacks of this are the penetration depth of the radiation through the frozen system and heat from the radiation which might melt the ice crystals.Cryostructuration is not limited to aqueous systems, organic solvents such as dioxane, formamide and dimethyl sulfoxide have also shown to be suitable systems to utilize.7,21 Using organic solvents will change the temperature region used for the preparation (depending on the freezing point of the solvents) and it allows utilization of more hydrophobic monomers. It has been shown that it is possible to freeze an emulsion of NIPA and styrene and thus carry out the emulsion polymerization in the non-frozen phase.22 This resulted in soluble amphiphilic block copolymers but raises the possibility of using the same process to synthesise a crosslinked network using the same strategy.
Reversible addition fragmentation chain transfer (RAFT) polymerization has also been utilized both in aqueous and organic systems for the preparation of cryostructured polymers.23,24 This highlights that a number of other polymerization techniques can be utilized for the preparation of porous polymers by using cryostructuration. Recently we have shown that it is possible to combine cryostructuration with a reaction induced phase separation.12 Freezing an aqueous mixture of acetone, acrylamide and methylenebisacrylamide results in the formation of a material with bimodal pore size distribution (Fig. 3). Macropores in the range 10–80 μm are formed by the ice crystals and the pore walls of the material exhibit pores less than 1 μm caused by a reaction induced phase separation. Acetone and monomers are enriched in the non-frozen solvent phase, and when polymerization starts phase separation will take place as a consequence of the poor solubility of the growing polymer chain in the acetone enriched phase. This results in a reaction induced phase separation in the non-frozen phase creating porous pore walls.12
 | ||
| Fig. 3 Scanning electron microscopy image of polyacrylamide cryogel prepared in an aqueous 0.6 M acetone solution. The macropores are formed due to the formation of ice crystals and the porosity in the pore walls is caused by the reaction induced phase separation. Figure reproduced with permission from ref. 12. | ||
Recent studies which have been highlighted here show that polymerization reactions during cryostructuration are not limited to the classical redox initiated reactions but rather that more systems can be applicable. The cryoconcentration effect causing high local concentrations of monomers and the low temperatures used for the reactions can make cryostructuration an interesting route for investigating other reactions.
Chemical crosslinking of polymers
Cryostructuration of polymers through chemical crosslinking has been investigated for a wide range of polymers both synthetic and natural, for example, polyacrylamide,25 polyvinylalcohol,26 chitosan27 and proteins.28Glutaraldehyde has frequently been utilized for covalent crosslinking of polymers to produce a stable material.27 However, it is not limited to water soluble polymers but also polyisobutylene in cyclohexene has been studied.29 Our group has recently explored the possibility of cryostructuration of suspensions in which particles are linked together through covalent stable bonds.30 Freezing of suspensions causes the formation of a densely packed particle network and simultaneously inter-particle crosslinking. After this simply thawing the material produces a macroporous structure and thus freeze-drying is not necessary which has been used previously when structuring suspensions.31,32 We showed that crosslinking of pNIPA particles containing amino groups using glutaraldehyde resulted in a cryostructured material wherein the walls consist of densely packed polymer particles (Fig. 4). | ||
| Fig. 4 Images illustrating the structure of a material formed from pNIPA particles. The upper image shows the macroporous structure formed by the ice crystals in the cryostructuration process and the image on the bottom shows that the pore wall is built from packed and crosslinked polymer particles. Figure reproduced with permission from ref. 30. | ||
Similar results were obtained when using microorganisms instead of polymeric particles.30 Using bacteria for the process resulted in the formation of a material composed of densely packed and crosslinked bacterial cells (Fig. 5). This might provide a new way of whole cell immobilization in which the cells are not encapsulated in a polymeric matrix but rather the cells themselves constitute the material. Hence, the resulting material will provide a high cell density and low diffusion resistance between the cells and the surrounding medium. Whole cell immobilization is currently being exploited for biocatalysis in which the intracellular enzymes are utilized.
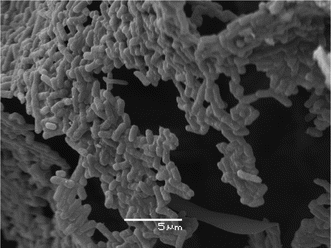 | ||
| Fig. 5 Scanning electron microscopy image of the pore wall of a material composed of cryostructured E. colicells. The image shows that the wall consists of densely packed and crosslinked bacterial cells. Image reproduced with permission from ref. 30. | ||
However, this cryostructuration process can also be done by freezing a suspension of NIPA-co-N-hydroxymethylacrylamide particles in hydrochloric acid.33 The cryoconcentration of particles and acid causes the formation of inter-particle bonds between the N-hydroxymethylacrylamide through a condensation reaction. Water formed in the reaction can be frozen and thus drive the condensation reaction, while when the particles are in suspension without freezing no gelation takes place. The structure formed from cryostructuration of thermoresponsive pNIPA resulted in a fast responsive swelling/deswelling of the structure.33 This can be attributed both to the porous structure allowing fast transfer of water in and out of the structure and that the structure consists of microgels which exhibit individual fast responses. Another property of the technique is that it allows the incorporation of porous adsorbent particles within the pore walls.34 Structuring polymer particles with a diameter larger than the pore size of the adsorbent material prevents any filling of the pores of the adsorbent. This problem was encountered when a solution of monomers together with carbon particles were subjected to cryostructuration.34 The monomers filled up the pores of the carbon and blocked the internal porosity.
Cryostructuration of particles combined with inter-particle crosslinking provides a simple one-step process for the preparation of porous structures built from particles. Varying the particles will give a way of changing the properties of the final material. Furthermore the chemistry used for the crosslinking can be tailored.
Concluding remarks
Cryostructuration is proving to be an interesting route of preparing highly porous polymer systems. The method highlighted in this mini-review is focused on a freeze/thawing approach which avoids freeze-drying for the sample preparation. The highly porous structure of these materials has so far been applied in for example: tissue engineering,27proteinpurification13 and waterpurification.35 It has proven to be a versatile technique which can be employed for soluble systems (monomers, polymers) or suspensions (particle, microorganisms). Furthermore the technique is not limited to aqueous systems but can also be used for a number of organic solvents (depending on the freezing point). However, so far the polymerization reactions used in cryostructuration have not to any larger extent involved any controlled polymerization reactions. There are still a number of chemical reactions or interactions which could be interesting to exploit for the formation of cryostrucutured materials.Acknowledgements
The authors would like to thank the Swedish Research Council (VR) and Ångpanneföreningens Forskningsstiftelse for the financial support.References
- M. G. Worster and J. S. Wettlaufer, J. Phys. Chem. B, 1997, 101, 6132–6136 CrossRef CAS.
- J. Wolfe, G. Bryant and K. L. Koster, CryoLetters, 2002, 23, 157–166 Search PubMed.
- H. Kirsebom, G. Rata, D. Topgaard, B. Mattiasson and I. Y. Galaev, Macromolecules, 2009, 42, 5208–5214 CrossRef CAS.
- T. E. Kiovsky and R. E. Pincock, J. Am. Chem. Soc., 1966, 88, 4704–4710 CrossRef CAS.
- R. E. Pincock, Acc. Chem. Res., 1969, 2, 97–103 CrossRef CAS.
- E. S. Vainerman, V. I. Lozinsky and S. V. Rogozhin, Colloid Polym. Sci., 1981, 259, 1198–1201 CrossRef CAS.
- F. Plieva, H. T. Xiao, I. Y. Galaev, B. Bergenstahl and B. Mattiasson, J. Mater. Chem., 2006, 16, 4065–4073 RSC.
- H. Kirsebom, G. Rata, D. Topgaard, B. Mattiasson and I. Y. Galaev, Polymer, 2008, 49, 3855–3858 CrossRef CAS.
- M. M. Ozmen and O. Okay, Polymer, 2005, 46, 8119–8127 CrossRef CAS.
- F. M. Plieva, J. Andersson, I. Y. Galaev and B. Mattiasson, J. Sep. Sci., 2004, 27, 828–836 CrossRef CAS.
- F. M. Plieva, I. N. Savina, S. Deraz, J. Andersson, I. Y. Galaev and B. Mattiasson, J. Chromatogr., B: Anal. Technol. Biomed. Life Sci., 2004, 807, 129–137 CrossRef CAS.
- H. Kirsebom, D. Topgaard, I. Y. Galaev and B. Mattiasson, Langmuir, 2010, 26, 16129–16133 CrossRef CAS.
- P. Arvidsson, F. M. Plieva, V. I. Lozinsky, I. Y. Galaev and B. Mattiasson, J. Chromatogr., A, 2003, 986, 275–290 CrossRef CAS.
- F. M. Plieva, P. Ekstrom, I. Y. Galaev and B. Mattiasson, Soft Matter, 2008, 4, 2418–2428 RSC.
- I. N. Savina, M. Tuncel, A. Tuncel, I. Y. Galaev and B. Mattiasson, eXPRESS Polym. Lett., 2007, 1, 189–196 Search PubMed.
- M. C. Gutierrez, M. L. Ferrer and F. del Monte, Chem. Mater., 2008, 20, 634–648 CrossRef CAS.
- H. Zhang and A. I. Cooper, Adv. Mater., 2007, 19, 1529–1533 CrossRef CAS.
- I. N. Savina, V. Cnudde, S. D'Hollander, L. Van Hoorebeke, B. Mattiasson, I. Y. Galaev and F. Du Prez, Soft Matter, 2007, 3, 1176–1184 RSC.
- G. A. Komarova, S. G. Starodubtsev, V. I. Lozinsky, E. V. Kalinina, K. Landfester and A. R. Khokhlov, Langmuir, 2008, 24, 4467–4469 CrossRef CAS.
- P. Petrov, E. Petrova and C. B. Tsvetanov, Polymer, 2009, 50, 1118–1123 CrossRef CAS.
- M. M. Ozmen, M. V. Dinu and O. Okay, Polym. Bull., 2008, 60, 169–180 CrossRef CAS.
- C. Zheng, W. D. He, W. J. Liu, J. Li and J. F. Li, Macromol. Rapid Commun., 2006, 27, 1229–1232 CrossRef CAS.
- X. L. Sun, W. D. He, J. Li, L. Y. Li, B. Y. Zhang and T. T. Pan, J. Polym. Sci., Part A: Polym. Chem., 2009, 47, 6863–6872 CrossRef CAS.
- X. L. Sun, W. D. He, T. T. Pan, Z. L. Ding and Y. J. Zhang, Polymer, 2010, 51, 110–114 CrossRef CAS.
- R. V. Ivanov, V. I. Lozinsky, S. K. Noh, S. S. Han and W. S. Lyoo, J. Appl. Polym. Sci., 2007, 106, 1470–1475 CrossRef CAS.
- F. M. Plieva, M. Karlsson, M. R. Aguilar, D. Gomez, S. Mikhalovsky, I. Y. Galaev and B. Mattiasson, J. Appl. Polym. Sci., 2006, 100, 1057–1066 CrossRef CAS.
- H. Kirsebom, M. R. Aguilar, J. S. Roman, M. Fernandez, M. A. Prieto and B. Bondar, J. Bioact. Compat. Polym., 2007, 22, 621–636 CrossRef CAS.
- C. D. Mu, F. Liu, Q. S. Cheng, H. L. Li, B. Wu, G. Z. Zhang and W. Lin, Macromol. Mater. Eng., 2010, 295, 100–107 CAS.
- D. C. Tuncaboylu and O. Okay, Langmuir, 2010, 26, 7574–7581 CrossRef CAS.
- H. Kirsebom, B. Mattiasson and I. Y. Galaev, Langmuir, 2009, 25, 8462–8465 CrossRef CAS.
- M. L. Ferrer, R. Esquembre, I. Ortega, C. R. Mateo and F. del Monte, Chem. Mater., 2006, 18, 554–559 CrossRef CAS.
- C. A. L. Colard, R. A. Cave, N. Grossiord, J. A. Covington and S. A. F. Bon, Adv. Mater., 2009, 21, 2894–2898 CrossRef CAS.
- H. Kirsebom, B. Mattiasson and I. Y. Galaev, Macromol. Rapid Commun., 2010, 31, 1095–1100 CrossRef CAS.
- S. Hajizadeh, H. Kirsebom and B. Mattiasson, Soft Matter, 2010, 6, 5562–5569 RSC.
- M. Le Noir, F. Plieva, T. Hey, B. Guieysse and B. Mattiasson, J. Chromatogr., A, 2007, 1154, 158–164 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2011 |
