Novel electrochemical and pH stimulus-responsive supramolecular polymer with disparate pseudorotaxanes as relevant unimers†
Xiang
Ma
,
Ruyi
Sun
,
Weifeng
Li
and
He
Tian
*
Key Labs for Advanced Materials and Institute of Fine Chemicals, East China University of Science & Technology, Shanghai, 200237, P. R. China. E-mail: tianhe@ecust.edu.cn; Fax: (+86)-21-64252288
First published on 8th March 2011
Abstract
The assembly/disassembly process of a reversible supramolecular polymer, based on homoditopic calixarene and a viologen dication connector decorated with dimethylamino portion and fluorophore, can be reversibly controlled electrochemically and by protonation/deprotonation with two disparate pseudo[2]rotaxanes worked as unimers.
Supramolecular polymers are obtained, when monomeric units in a macromolecule are held together by highly directional and reversible noncovalent interactions,1 such as hydrogen bonds, metal–ligand interaction, and Van der Waals forces.2 The properties of supramolecular polymers can be adjusted by external stimuli. In addition, the growing diversity of artificial supramolecular nanomaterials3 provides choices and references for devising new style polymers formed from interlocked macromolecular unimers, which can take advantage of the unimers which are responsive to different stimuli.
The host–guest interaction based on calixarene derivatives are relatively less explored in supramolecular polymers, while crown ethers and cyclodextrins as relatively common host monomer units has been studied systematically.4–6 We report here the formation of a novel fluorescent supramolecular polymer formed from two complimentary monomers which contain p-sulfonatocalix[4]arene based homoditopic receptors and the viologen and dimethylamino connectors decorated with a fluorophore.7 This fluorescent supramolecular polymer can respond to both electrochemical redox and pH variance, allowing the two kinds of pseudorotaxanes formed as unimers to be induced by respective stimuli. Despite the fact that some calixarene-based supramolecular polymers have been reported in previous and more recent works, for example, Liu recently reported an electrochemical stimulus-responsive supramolecular polymer based on sulfonatocalixarene and viologen dimers,8 no such reversibly controlled electrochemical and pH dual-responsive fluorescent systems have been reported before. Moreover, it involves two disparate pseudorotaxanes as unimers in the reversible assembly/disassembly process of the supramolecular polymer. The assembly/disassembly process of polymer, reversibly controlled electrochemically and by protonation/deprotonation was accompanied by fluorescence emission variance and induced circular dichroism (ICD) signals.
Supramolecular polymers with a high molecular weight can only be obtained with high association constant.1P-sulfonatocalix[4]arene and viologen were selected as host–guest pairs because of their high complexation stability,9 and the dimethylamino unit for its high association constant with sulfonatocalix[4]arene after protonation10 (Scheme 1). On the other hand, the complex stability constants of viologen and sulfonatocalix[4]arene are dramatically sensitive to the redox process of viologen, decreasing sharply from dication to radical cation, to neutral form which can insure the reversible assembly/disassembly process between them. Before protonation, the dimethylamino unit can hardly associate with the host. The assembly/disassembly process between two pseudorotaxane unimers and the supramolecular polymer can be manipulated by both redox control and protonation/deprotonation process separately and it turns out to be a reversibly controlled electron and pH dual-responsive fluorescent supramolecular polymer (Scheme 1).
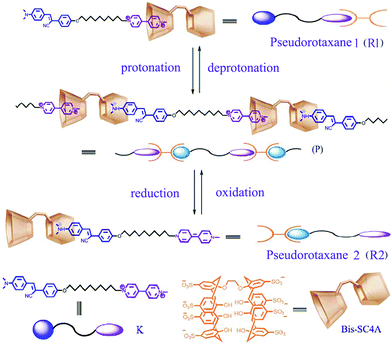 | ||
| Scheme 1 Structural illustration of Bis-SC4A, K, pseudorotaxaneR1, R2 and the reversible assembly/disassembly process of supramolecular polymerP induced by the respective stimuli of protonation/deprotonation and electrochemical redox. The 8Na+ of Bis-SC4A and 2Br−, 2I− of K are omitted in all following figures for clarity. | ||
The supramolecular polymers were formed from interlocked unimers. The pseudorotaxane as a unimer was formed by a ditopic p-sulfonatocalix[4]arene (Bis-SC4A) as host and a viologen and dimethylamino unit containing connector decorated with a fluorophore as guest K (Scheme 1). Bis-SC4A and viologen dication can form highly stable 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 cagelike complexes.9 The formation of the inclusion pseudorotaxaneR1 between Bis-SC4A and K is evident in 1HNMR spectroscopic experiments (Fig. 1). In the presence of 1 equiv. of Bis-SC4A, all the protons of viologen dication units Ha–d and Hk exhibit upfield shift (Δδ, 2.31 ppm for Ha, 1.65 ppm for Hb, 0.35 ppm for Hc, 0.21 ppm for Hd and 2.34 ppm for Hk) owing to the inclusion complexation behaviors, which suggests that the viologen dication part of K is encapsulated by Bis-SC4A with the methyl-pyridium portion immersed in the cavity.
1 cagelike complexes.9 The formation of the inclusion pseudorotaxaneR1 between Bis-SC4A and K is evident in 1HNMR spectroscopic experiments (Fig. 1). In the presence of 1 equiv. of Bis-SC4A, all the protons of viologen dication units Ha–d and Hk exhibit upfield shift (Δδ, 2.31 ppm for Ha, 1.65 ppm for Hb, 0.35 ppm for Hc, 0.21 ppm for Hd and 2.34 ppm for Hk) owing to the inclusion complexation behaviors, which suggests that the viologen dication part of K is encapsulated by Bis-SC4A with the methyl-pyridium portion immersed in the cavity.
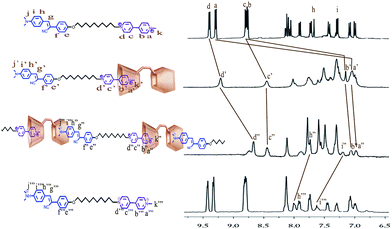 | ||
Fig. 1
1H NMR spectra of K, R1 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of Bis-SC4A and K), P (protonation of R1 with CF3COOH), and K-H (protonation of K with CF3COOH) in DMSO-d6 (400 MHz, 293 K). 1 mixture of Bis-SC4A and K), P (protonation of R1 with CF3COOH), and K-H (protonation of K with CF3COOH) in DMSO-d6 (400 MHz, 293 K). | ||
1H NMR spectropy also presents obvious evidence for the host–guest complexation between protonated dimethylamino part of K and the cavity of the p-sulfonatocalix[4]arene of R1. Upon addition of CF3COOH, the protons Hh′′′–j′′′ of K-H undergo pronounced downfield shifts (Δδ, 0.34 ppm for Hi′′, 0.3 ppm for Hh′′ and 0.37 ppm for Hj′′). It is therefore indicated that K is captured by Bis-SC4A with protonated dimethylamino portion immersed into one of the cavity and the methyl pyridium portion immersed into the other one, inferring that a polymerP based on the pseudorotaxaneR1 unimer was formed.
To further investigate the shape, size and size distribution of the supramolecular polymerP, atomic force microscopy (AFM) amplitude images are shown in Fig. 2. The cast film was prepared on a mica plate. The AFM images were measured by tapping mode. As expected, P shows the morphology of a 1D linear array with a length over 515.63 nm. The morphology of the supramolecular polymer is also validated by scanning electron microscopy (SEM) measurements (Fig. 3), further indicating the fiber-like aggregation of P. Dynamic light scattering (DLS) was also performed and the result shows a relatively high broad peak at the average diameter of 2 μm, and there was a small portion of particles at the average diameter of 167 nm, indicating that the formation of highly polymerized supramolecular assemblies make up the main part in the solution (ESI, Fig. S6†).
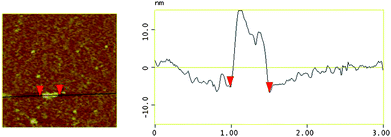 | ||
| Fig. 2 AFM amplitude images of linear supramolecular polymerP formed by protonation induced host–guest reaction of pseudorotaxaneR1. | ||
 | ||
| Fig. 3 Scanning electron micrograph (SEM) of cast film of the supramolecular polymerP on a glass plate. | ||
Fluorescent spectra experiments11 on the reversible transformation process between supramolecular polymerP and pseudorotaxaneR1 upon stimulation by protonation and deprotonation were carried out. As shown in Fig. 4, the fluorescence intensity at about 368 nm increases when the homoditopic receptor, which decorated with a fluorophore, mixed with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 equiv of Bis-SC4A. This is indicative of the host–guest complexation between the methyl-pyridium portion and the cavity of Bis-SC4A. As expected, the fluorescent intensity of polymerP decreases upon protonation of pseudorotaxaneR1 with CF3COOH, whose intensity is much lower than that of the prononated K-H. The quenching of fluorescence is mainly due to the photoinduced electron transfer (PET) from the calixarene moieties to the excited fluorophore.12 When deprotonated by tributylamine (P-DH), the supramolecular polymer disassembles to pesudorotaxane R1, and the fluorescent intensity nearly turns back reversibly.
1 equiv of Bis-SC4A. This is indicative of the host–guest complexation between the methyl-pyridium portion and the cavity of Bis-SC4A. As expected, the fluorescent intensity of polymerP decreases upon protonation of pseudorotaxaneR1 with CF3COOH, whose intensity is much lower than that of the prononated K-H. The quenching of fluorescence is mainly due to the photoinduced electron transfer (PET) from the calixarene moieties to the excited fluorophore.12 When deprotonated by tributylamine (P-DH), the supramolecular polymer disassembles to pesudorotaxane R1, and the fluorescent intensity nearly turns back reversibly.
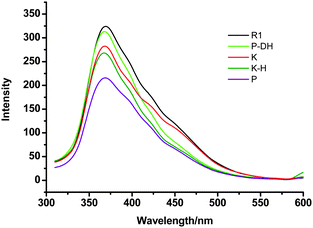 | ||
| Fig. 4 Fluorescence spectra of K, K-H (protonation with CF3COOH), R1, P, P-DH (deprotonation of P with tributylamine, similar to R1). | ||
The ICD13 measurements were used to observe the polymerassembly/disassembly process induced by protonation/deprotonation and electrochemical stimuli. To our surprise, the pseudorotaxaneR1 and R2 show no obvious ICD signals, while supramolecular polymerP shows a large wide Cotton peak at about 357 nm (ESI, Fig. S7†), which might be attributed to the molecular conglomeration.
The electrochemical properties of the supramolecular polymer were further studied by cyclic voltammetry experiments. As shown in Fig. 5, the free K containing viologen dication portion undergoes two consecutive electron reduction processes to the neutral form. The first reduction peaks exhibit negative shifts of 89 mV and 11 mV for the second one. This indicates that in the first reduction process it is much more difficult to immerse the methyl-pyridium portion into the cavity, and also that the complex stability of pseudorotaxane decreases gradually upon reduction. As expected, the second redox curve is mostly similar, while the corresponding oxidation peak of the first reduction undergoes a major negative shift of 73 mV, which indicates that p-sulfonatocalix[4]arene can effectively include K+˙, despite the negative influence arising from one-electron reduction.
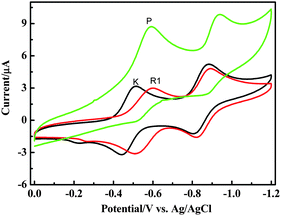 | ||
| Fig. 5 Cyclic voltammetry curves recorded in 2 × 10−4 M DMSO solution, 0.1 M TBAClO4, r. t., scan rate 100 mV s−1) for the first and second reduction of the viologen unit in K, R1 and polymerP. | ||
To focus on the CV curves of polymerP and pseudorotaxane unimer R1, the first reduction and its corresponding re-oxidation peak undergoes a minor shift from the unimer, while the second reduction peak undergoes a major negative shift of 57 mV. We can infer that the second reduction process is more difficult in polymer than in the unimer. When CF3COOH added to constructpolymerP in the solution, all the phenolic hydroxyls of Bis-SC4A were protonated. In this case, the cavity possesses the most compact framework and highest π-electron density, leading to more effective π-stacking interactions and then a more stable complex with K+. The CV curves of polymerP in Fig. S10 (ESI†) show obvious variance when the scan rate changes, especially with a scan rate of 2000 mV s−1, which proves the occurrence of the extensive polymer disassembly when the viologen portion was fully reduced and consequently weakened the complexation stability of Bis-SC4A.8
In summary, a novel supramolecular polymer based on homoditopic calixarene and a viologen dication connector decorated with a dimethylamino portion and fluorophore was constructed. The assembly/disassembly process of the polymer can be reversibly controlled electrochemically and by protonation/deprotonation stimuli via two pathways, with two pseudorotaxanes as unimers. For the deprotonation route, the polymer disassembles to a pseudorotaxeneR1. For another electrochemical route, in the oxidized form, the two components assemble to the linear polymers with lengths of micro-magnitudes, whereas in the reduced form, the polymer disassembles back to another pseudorotaxaneR2. Such a reversible noncovalent system can be potentially applicable to design sophisticated polymeric materials with excellent responsive properties at the nanoscale.
Finally, the National Basic Research 973 Program (2011CB808400) and NSFC/China (20972053 & 20802019) are acknowledged for financial support. X. Ma thanks the support from Fundamental Research Funds for Central Universities (WJ0911001), Ph. D. Programs Foundation of Ministry of Education of China (200802511029) and ‘Chen Guang’ project supported by Shanghai Municipal Education Commission and Shanghai Education Development Foundation. X. Ma and R. Sun contributed equally to the work.
Notes and references
- L. Brunsveld, J. B. Folmer, E. W. Meijer and R. P. Sijbesma, Chem. Rev., 2001, 101, 4071 CrossRef CAS.
- V. Berl, M. Schmutz, M. J. Krische, R. G. Khoury and J. M. Lehn, Chem.–Eur. J., 2002, 8, 1227 CrossRef CAS; P. R. Andres and U. S. Schubert, Adv. Mater., 2004, 16, 1043 CrossRef CAS; T. Park and S. C. Zimmerman, J. Am. Chem. Soc., 2006, 128, 13986 CrossRef CAS.
- V. Balzani, M. Venturi and A. Credi, Molecular Devices and Machines- Concepts and Perspectives for the Nanoworld, Wiley-VCH, Weinheim, Germany, 2008 Search PubMed; E. J. F. Klotz, T. D. W. Claridge and H. L. Anderson, J. Am. Chem. Soc., 2006, 128, 15374 Search PubMed; H. Tian and Q. C. Wang, Chem. Soc. Rev., 2006, 35, 361 CrossRef CAS; E. R. Kay, D. A. Leigh and F. Zerbetto, Angew. Chem., Int. Ed., 2007, 46, 72 RSC; X. Ma and H. Tian, Chem. Soc. Rev., 2010, 39, 70 CrossRef CAS.
- Y. Ho Ko, K. Kim, J. K. Kang, H. Chun, J. W. Lee, S. Sakamoto, K. Yamaguchi, J. C. Fettinger and K. Kim, J. Am. Chem. Soc., 2004, 126, 1932 CrossRef CAS; E. N. Guidry, J. Li, J. F. Stoddart and R. H. Grubbs, J. Am. Chem. Soc., 2007, 129, 8944 CrossRef CAS.
- P. B. Wan, Y. G. Jiang, Y. P. Wang, Z. Q. Wang and X. Zhang, Chem. Commun., 2008, 5710 RSC; D. H. Qu, Q. C. Wang and H. Tian, Angew. Chem., Int. Ed., 2005, 44, 5296 CrossRef CAS.
- G. Latini, L.-J. Parrott, S. Brovelli, M. J. Frampton, H. L. Anderson and F. Cacialli, Adv. Funct. Mater., 2008, 18, 2419 CrossRef CAS; A.-M. L. Fuller, D. A. Leigh and P. J. Lusby, Angew. Chem., Int. Ed., 2007, 46, 5015 CrossRef CAS.
- L. L. Zhu, X. Li, F. Y. Ji, X. Ma, Q. C. Wang and H. Tian, Langmuir, 2009, 25, 3482 CrossRef CAS.
- R. M. Yebeutchou, F. Tancini, N. Demitri, S. Geremia, R. Mendichi and E. Dalcanale, Angew. Chem., Int. Ed., 2008, 47, 4504 CrossRef CAS; D. S. Guo, S. Chen, H. Q. Zhang and Y. Liu, Chem. Commun., 2010, 46, 2620 RSC.
- D. S. Guo, L. H. Wang and Y. Liu, J. Org. Chem., 2007, 72, 7775 CrossRef CAS.
- J. W. Steed, C. P. Johnson, C. L. Barnes, R. K. Juneja, J. L. Atwood, S. Reilly, R. L. Hollis, P. H. Smith and D. L. Clark, J. Am. Chem. Soc., 1995, 117, 11426 CrossRef CAS.
- Q. C. Wang, D. H. Qu, J. Ren, K. C. Chen and H. Tian, Angew. Chem., Int. Ed., 2004, 43, 2661 CrossRef CAS; L. L. Zhu, X. Ma, F. Y. Ji, Q. C. Wang and H. Tian, Chem.–Eur. J., 2007, 13, 9216 CrossRef CAS.
- K. Kano and Y. Ishida, Chem.–Asian J., 2008, 3, 678 CrossRef CAS; D. S. Guo, K. Chen, H. Q. Zhang and Y. Liu, Chem.–Asian J., 2009, 4, 436 CrossRef CAS.
- X. Ma, D. H. Qu, F. Y. Ji, Q. C. Wang, L. L. Zhu, Y. Xu and H. Tian, Chem. Commun., 2007, 1409 RSC; X. Ma, Q. C. Wang and H. Tian, Tetrahedron Lett., 2007, 48, 7112 CrossRef CAS; X. Ma, Q. C. Wang, D. H. Qu, Y. Xu, F. Y. Ji and H. Tian, Adv. Funct. Mater., 2007, 17, 829 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis, 1H NMR spectra and MSspectra of the compounds. Instructions for instruments. DLS of the polymerP. ICD spectra of R1, R2, P and P-DH. CV curves of K, R1 and P. See DOI: 10.1039/c0py00419g |
| This journal is © The Royal Society of Chemistry 2011 |
