Propylenedioxythiophene (ProDOT)–phenylene copolymers allow a yellow-to-transmissive electrochrome†
Chad M.
Amb
,
Justin A.
Kerszulis
,
Emily J.
Thompson
,
Aubrey L.
Dyer
and
John R.
Reynolds
*
The George and Josephine Butler Polymer Research Laboratory, Department of Chemistry, Center for Macromolecular Science and Engineering, University of Florida, Gainesville, FL 32611, USA. E-mail: reynolds@chem.ufl.edu
First published on 1st February 2011
Abstract
In order to create multicolour displays using electrochromic (EC) technologies, the modulation of the intensity of three primary subtractive colours (red, yellow, blue, or cyan, magenta, yellow) could be used to express any colour in the spectrum. Herein we report the first cathodically colouring yellow-to-transmissive switching EC polymer, which completes a full colour palette and promises to fulfil the requirements necessary to create multicolour EC displays.
Non-emissive display technologies utilizing electrochromic (EC) materials are at the forefront of materials research today. EC devices have the ability to be viewed in a wide variety of lighting conditions, and large area devices can be fabricated using convenient and inexpensive printing techniques. Importantly, EC displays utilizing materials that can exhibit three primary colours could be employed to create full colour displays where the expression of any colour can be achieved through the control of the intensity of each of the primary colours. There has been much discussion in the literature on the additive primary colours red, green, and blue;1–4 however, for displays which are truly non-emissive, the primary subtractive colours must be employed to allow any colour to be produced.5 Kobayashi et al. have elegantly demonstrated this principle with molecular electrochromic species, but unfortunately the potential at which colouration occurs is quite high (−2 V), and switching occurs in tens of seconds rather than subsecond speeds necessary for dynamic, yet non-video rate applications.6
Cathodically colouring EC polymers have demonstrated attractive properties for display type applications (i.e. fast switching speed, high contrast), but the lack of cathodically colouring yellow ECPs has limited production of multicolour display prototypes utilizing these materials. Polymers with high band gaps between 2.3 and 2.7 eV that display the colour yellow have not been realized as cathodically colouring (ECPs), as the high band gap required to achieve the yellow colour has not allowed shifting of the visible absorption bands into the NIR on oxidation, prohibiting a transmissive state.7 A few anodically colouring yellow polymers have been produced,8,9 but in practice these polymers will be difficult to incorporate into display devices as application of a narrow potential window is required to achieve the yellow colour without displaying brownish hues, and long term stability to more than 500 switches has not been reported. In the closest example of a cathodically colouring yellow-to-transmissive EC polymer, our group reported a conjugated oligomer containing pendant acrylate moieties that was photopatterned using UV mediated polymerisation.10 The material was found to switch from a yellow neutral state to a dark blue cation-radical state, and finally a lighter blue dicationic state, but the strong residual blue colour could not be overcome. In order to address these problems, here we demonstrate a simple approach which yields a solution processable yellow-to-transmissive switching EC polymer.
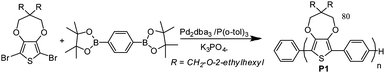
Dioxythiophenes (DOTs) have long been a monomer of choice in cathodically colouring ECPs;11,12 however, homopolymers5,13–15 and donor–acceptor polymers3,4,16–19 incorporating these moieties have resulted in materials with band gaps that are too low to display the desired yellow colour. We hypothesized that the alternation of p-phenylene moieties, with their high aromaticity and subtle steric repulsion from ortho-C–H units, would shift the band gap of these polymers higher while still allowing a sufficient amount of electrochemical doping to facilitate shifting of absorption from the visible well into the NIR. To this end, a Suzuki polycondensation was employed to give the desired alternating ProDOT–phenylene polymer, as shown in Scheme 1. The polymer was endcapped after the reaction using a phenylboronic acid ester, followed by bromobenzene, and was isolated in high yield (83%) with a number average molecular weight (Mn) of 25 kDa (see the ESI† for GPC and NMR data).
Thin films of the polymer were spray-cast onto indium tin oxide (ITO) coated glass slides from a ∼2 mg mL−1toluene solution and electrochemically cycled from 0.00 V to 1.08 V vs. Fc/Fc+ in a 0.2 M LiBTI (lithium bistrifluoromethanesulfonimide)/PC (propylene carbonate) solution until a stable and reproducible voltammogram was obtained (see the ESI† for electrochemical data), then subjected to spectroelectrochemical analysis. Fig. 1 shows the spectroelectrochemical response in terms of percent transmittance as a function of applied potential. As seen in the bold black spectrum, a sharp absorption band with a λmax at 455 nm is present in the neutral state, and the onset of absorption reveals a band gap of around 2.38 eV. Upon oxidation, the intensity of absorption at 455 nm is significantly reduced, while a polaronic transition arises around 650 nm. The intensity of the polaronic transition depletes at higher oxidation levels, giving way to a strong bipolaronic absorption extending beyond 1600 nm. Importantly, when fully oxidized the polymer is greater than 75% transmissive across the entire visible range (400–750 nm).
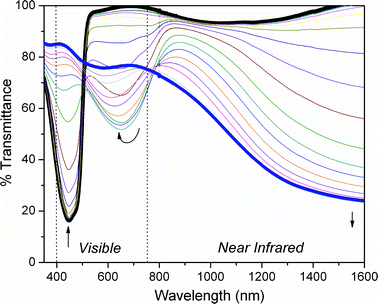 | ||
| Fig. 1 Spectroelectrochemical analysis P1. A thin film was spray-cast from a ∼2 mg mL−1 solution in toluene, and electrochemical oxidation was carried out in 0.2 M lithium bistrifluoromethanesulfonimide (LiBTI)/propylene carbonate (PC) solution, using a Ag/Ag+ reference electrode (10 mM AgNO3/0.1 M Bu4NPF6 in MeCN, calibrated to the Fc/Fc+ redox couple, EFc/Fc+ − EAg/Ag+ = 80 mV) and a Pt wire counter electrode. The applied potential was increased in 50 mV steps from 180 mV to 1080 mV vs. Fc/Fc+. | ||
In order to evaluate the colours of the polymer films as the human eye perceives them, the L*a*b* values (CIE 1976 L*a*b* Color Space) for three polymer films of differing optical density were measured as a function of electrochemical doping level, and the a*b* values for each step (50 mV steps, 180 to 1080 mV vs.Fc/Fc+) are shown in Fig. 2, and photographs of each film in the neutral and fully oxidized states are inset. The extremely high b* values in the neutral state indicate that the material is a highly saturated yellow as viewed by the human eye, as is also evident in the photographs. Surprisingly, the films also displayed slightly negative a* values indicative of a green hue, which is likely due to yellow-green emission from the polymer films when irradiated with white light (see the ESI†). At intermediate doping levels, the polaronic transition results in negative b* values, indicative of a blue colour state. At around 1040 mV, only slightly negative b* (−5 to −10) values remain and the a* values are near zero (0 to +2), as the films are faintly sky blue coloured and highly transmissive, also shown in the photographs.
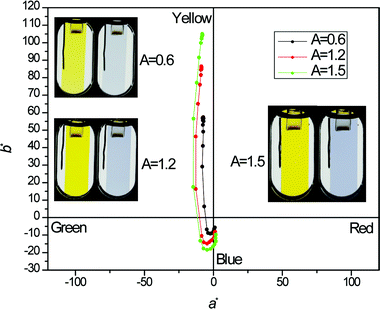 | ||
| Fig. 2 Colourimetric a*b* (CIE 1976 L*a*b* Color Space) colour coordinates of thin films of P1 taken as a function of level of electrochemical oxidation (180 to 1080 mV vs. Fc/Fc+, 50 mV steps), with inset photographs of the three films of differing optical density (A) studied in their neutral (180 mV) and fully oxidized (1080 mV) states. The neutral-state optical density of each film at 455 nm is listed next to each photoset. | ||
Another important parameter that governs an ECPs ability to be utilized in display-type devices (such as e-readers and signage) is the speed at which the polymer can be switched from one colour state to another, as rapidly switching materials are required. Fig. 3 shows the change in % transmittance at the λmax of a spray-cast film of P1, as a function of time, as potential square waves were applied. As can be seen in Fig. 3, this film exhibits an excellent 73% contrast at 455 nm, and is also able to retain a high level of that contrast at switching rates as high as 1 s per potential step, and requires 0.8 s to achieve 95% of a full switch. Even at a switching rate of 0.5 s per potential, the polymer still retains 63% contrast.
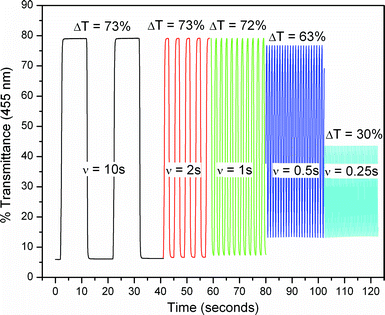 | ||
| Fig. 3 Potential square wave absorptometry of P1 (measured at 455 nm, 180 to 1080 mV vs. Fc/Fc+ in 0.2 M Li BTI/PC solution, Pt wire CE). The time each potential was held is indicated by ν and the transmittance contrast by ΔT. | ||
As mentioned previously, the necessity for a cathodically colouring yellow-to-transmissive electrochrome is paramount in completing the colour palette for non-emissive displays using the subtractive colour mixing theories of CMY and RYB. To demonstrate subtractive primary colour mixing, we have spray-cast overlaying films of yellow (from this work), red,5 and blue19 ECPs through circular masks sequentially as shown in Fig. 4a and b.
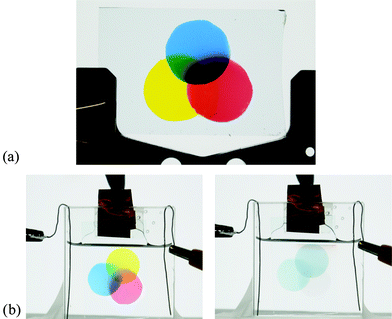 | ||
| Fig. 4 (a) Thin films of blue, red, and yellow polymers spray-cast onto ITO slides through circular shadow masks. (b) Thinner films are immersed in a tetrabutylammonium hexafluorophosphate (TBAPF6)/PC solution and held at −0.25 V (left) and 1.2 V (right) vs. Ag wire. | ||
As is seen in Fig. 4a and b, for the neutralized state of all three polymers where the films overlap, a subtractive mixture of the primary colours is created. For example, where yellow and red overlap, the colour is orange, where red and blue overlap, there is purple, and where blue and yellow overlap, there is green. In the center where all of the colours overlap, a black colour appears as is especially apparent using the thicker films shown in Fig. 4a. When a sufficiently positive potential is applied across the electrode, all three polymers are oxidized to their highly transmissive states shown in the right photograph in Fig. 4b. This is the first demonstration of the utilization of the subtractive primaries (yellow, red, and blue) of electrochromic polymers in colour mixing to form additional colours that all switch to highly transmissive, as needed for full-colour electrochromic displays.
Conclusions
Yellow-to-transmissive electrochromic materials are necessary for the development of multicolour electrochromic displays which use the subtractive primaries (red, blue, yellow or cyan, magenta, yellow) to display any desired colour. We report a new alternating propylenedioxythiophene–phenylene copolymer which is the first polymer that is yellow in its neutral state and displays high transmissivity over the entire visible region in its oxidized state. The polymer achieves over 70% transmittance contrast at the absorption maximum, and attains 95% of a full switch in less than 1 second. We also demonstrate the importance of this polymer as subtractive colour mixing is used to show how the expression of three subtractive primary colours could be used to create displays that can exhibit essentially any colour in the spectrum. This new material promises to allow development of full multicolour EC devices.Acknowledgements
The authors would like to thank the AFOSR (FA9550-09-1-0320) and BASF for funding.Notes and references
- G. Sonmez, C. K. F. Shen, Y. Rubin and F. Wudl, Angew. Chem., Int. Ed., 2004, 43, 1498–1502 CrossRef CAS.
- G. Sonmez, H. B. Sonmez, C. K. F. Shen, R. W. Jost, Y. Rubin and F. Wudl, Macromolecules, 2005, 38, 669–675 CrossRef CAS.
- G. E. Gunbas, A. Durmus and L. Toppare, Adv. Funct. Mater., 2008, 18, 2026–2030 CrossRef CAS.
- M. Icli, M. Pamuk, F. Algi, A. M. OÌnal and A. Cihaner, Chem. Mater., 2010, 22, 4034–4044 CrossRef CAS.
- A. L. Dyer, M. R. Craig, J. E. Babiarz, K. Kiyak and J. R. Reynolds, Macromolecules, 2010, 43, 4460–4467 CrossRef CAS.
- N. Kobayashi, S. Miura, M. Nishimura and H. Urano, Sol. Energy Mater. Sol. Cells, 2008, 92, 136–139 CrossRef CAS.
- T. Dey, M. A. Invernale, Z. Buyukmumcu and G. A. Sotzing, Polym. Prepr., 2010, 51, 576 CAS.
- G.-S. Liou and H.-Y. Lin, Macromolecules, 2008, 42, 125–134.
- H.-M. Wang and S.-H. Hsiao, Polym. Chem., 2010, 1, 1013–1023 RSC.
- C. B. Nielsen, A. Angerhofer, K. A. Abboud and J. R. Reynolds, J. Am. Chem. Soc., 2008, 130, 9734–9746 CrossRef CAS.
- A. L. Dyer and J. R. Reynolds, in Handbook of Conducting Polymers, ed. T. A. Skotheim and J. R. Reynolds, CRC Press, Boca Raton, FL, 2007, vol. 1, ch. 20 Search PubMed.
- P. M. Beaujuge and J. R. Reynolds, Chem. Rev., 2010, 110, 268–320 CrossRef CAS.
- B. D. Reeves, C. R. G. Grenier, A. A. Argun, A. Cirpan, T. D. McCarley and J. R. Reynolds, Macromolecules, 2004, 37, 7559–7569 CrossRef CAS.
- H. W. Heuer, R. Wehrmann and S. Kirchmeyer, Adv. Funct. Mater., 2002, 12, 89–94 CrossRef CAS.
- E. Poverenov, M. Li, A. Bitler and M. Bendikov, Chem. Mater., 2010, 22, 4019–4025 CrossRef CAS.
- A. Balan, G. Gunbas, A. Durmus and L. Toppare, Chem. Mater., 2008, 20, 7510–7513 CrossRef CAS.
- P. M. Beaujuge, S. Ellinger and J. R. Reynolds, Nat. Mater., 2008, 7, 795–799 CrossRef CAS.
- P. M. Beaujuge, S. Ellinger and J. R. Reynolds, Adv. Mater., 2008, 20, 2772–2776 CrossRef CAS.
- C. M. Amb, P. M. Beaujuge and J. R. Reynolds, Adv. Mater., 2010, 22, 724–728 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details and electrochemical analysis of P1. See DOI: 10.1039/c0py00405g |
| This journal is © The Royal Society of Chemistry 2011 |