Preparation of emulsion-templated porous polymers using thiol–ene and thiol–yne chemistry
Elaine
Lovelady
a,
Scott D.
Kimmins
a,
Junjie
Wu
bc and
Neil R.
Cameron
*ab
aDepartment of Chemistry, Durham University, South Road, Durham, DH1 3LE, UK. E-mail: n.r.cameron@durham.ac.uk; Fax: +44 (0)191 3844737; Tel: +44 (0)191 3342008
bBiophysical Sciences Institute, Durham University, South Road, Durham, DH1 3LE, UK
cSchool of Engineering and Computer Sciences, Durham University, South Road, Durham, DH1 3LE, UK
First published on 24th December 2010
Abstract
Highly porous polymeric materials are prepared by thiol–ene and thiol–yne mediated network formation using high internal phase emulsion templates. The efficiency of network formation is between 80 and 90% for all materials while the thiol–yne materials display enhanced strength and toughness due to their higher degree of crosslinking.
Thiol–ene chemistry, in which a thiyl radical adds to an alkene, has been known for many years.1,2 It has been used extensively as a means to prepare linear and network polymers, via the reaction between di- or multifunctional thiols and alkenes (enes).3,4 Recently, this approach to polymer formation has attracted intense interest due to its ease and versatility (it is classed as a form of ‘click’ chemistry), leading to the production of a wide range of functional polymers and macromolecular materials.5–8 A related reaction involves the radical addition of thiols to alkynes (thiol–yne chemistry).9 This differs from the thiol–ene reaction in that each alkyne can react with two thiols leading to networks with higher crosslinking densities than thiol–ene approaches. Similarly to thiol–ene, thiol–yne chemistry has been the subject of much interest recently as a strategy to prepare functional materials.9–16
There are very few previous examples of the use of thiol–ene/yne chemistry to prepare porous materials.17,18 Such materials have many important technological uses, including, inter alia, supports for a variety of catalysts and reagents, chromatography stationary phases, substrates for 3D cell culture and tissue engineering and hydrogen storage media. Amongst the various methods available to prepare porous polymeric materials, emulsion templating is particularly attractive as it allows control over the materials' morphology, porosity, pore diameter and other parameters.19–22 This paper describes the facile preparation of well-defined, highly porous polymeric materials by thiol–ene and thiol–yne photopolymerisation using commercially available starting materials.
Initial work employed a triacrylate (trimethylolpropane triacrylate, 1) and a trithiol (trimethylolpropane tris(3-mercaptopropionate), 3) (Scheme 1) in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio of –SH to C
1 molar ratio of –SH to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C groups to ensure efficient network formation by step growth polymerisation. A high internal phase emulsion (HIPE) consisting of 25 vol% organic phase and 75 vol% aqueous phase was prepared, using the polymeric surfactant Hypermer B246, which is known to be suitable for preparing acrylate-based polyHIPE materials by photopolymerisation.23 The HIPE formed without any noticeable phase separation and was cured successfully by UV-initiated thiol–ene reaction, following transfer to a polymerisation mould. Subsequently, the HIPE composition was changed, in the first instance to incorporate more aqueous phase to increase the porosity, then by replacing triacrylate 1 with octadiyne 2. In the latter case, a trithiol to di-yne molar ratio of 4
C groups to ensure efficient network formation by step growth polymerisation. A high internal phase emulsion (HIPE) consisting of 25 vol% organic phase and 75 vol% aqueous phase was prepared, using the polymeric surfactant Hypermer B246, which is known to be suitable for preparing acrylate-based polyHIPE materials by photopolymerisation.23 The HIPE formed without any noticeable phase separation and was cured successfully by UV-initiated thiol–ene reaction, following transfer to a polymerisation mould. Subsequently, the HIPE composition was changed, in the first instance to incorporate more aqueous phase to increase the porosity, then by replacing triacrylate 1 with octadiyne 2. In the latter case, a trithiol to di-yne molar ratio of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 was employed to maintain the –SH to C
3 was employed to maintain the –SH to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C ratio at 1
C ratio at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (each alkyne group counts as two C
1 (each alkyne group counts as two C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds since it reacts twice). The composition of each HIPE is shown in Table 1.
C bonds since it reacts twice). The composition of each HIPE is shown in Table 1.
 | ||
| Scheme 1 Monomers used to prepare polyHIPE materials by thiol–ene and thiol–yne polymerisation. | ||
| Aqueous phase content (%) | 1/ml | 2/ml | 3/ml | CHCl3/ml | H2O/ml |
|---|---|---|---|---|---|
| 75 | 3.94 | 0 | 4.81 | 8.75 | 52.5 |
| 80 | 3.15 | 0 | 3.85 | 7 | 56 |
| 75 | 0 | 2.03 | 6.72 | 8.75 | 52.5 |
| 80 | 0 | 1.62 | 5.38 | 7 | 56 |
In all cases, the HIPE formed successfully and no obvious signs of phase separation were observed. However, increasing the aqueous phase content beyond 80 vol% did not result in the formation of a stable HIPE.
The morphology of the resulting polyHIPE materials was investigated by SEM. Micrographs of the thiol–ene and thiol–yne materials are shown in Fig. 1. The interconnected, open-cell foam morphology typical of polyHIPE materials is observed, suggesting that the emulsions were stable up to the gel-point of the system. Void diameters were determined by analysis of SEM images. A histogram showing the frequency of voids within specific diameter ranges for the materials prepared is shown in Fig. 2. All materials gave similar void size distributions with the majority of voids between 10 and 30 μm. Unfortunately, due to the nature of the morphology of the 80% porosity thiol–yne sample (Fig. 1d), it was not possible to determine void diameter by image analysis. Also shown in Fig. 2 are the interconnecting window diameters, as determined by mercury porosimetry. The average void (〈D〉) and window (〈d〉) diameters are presented in Table 2, together with the parameter 〈d〉/〈D〉 which gives an indication of the extent of openness of the voids. The 〈d〉 value for TY-75 appears to be higher than that suggested by SEM (Fig. 1c). This may be due to the relatively small number of windows visible in the SEM image.
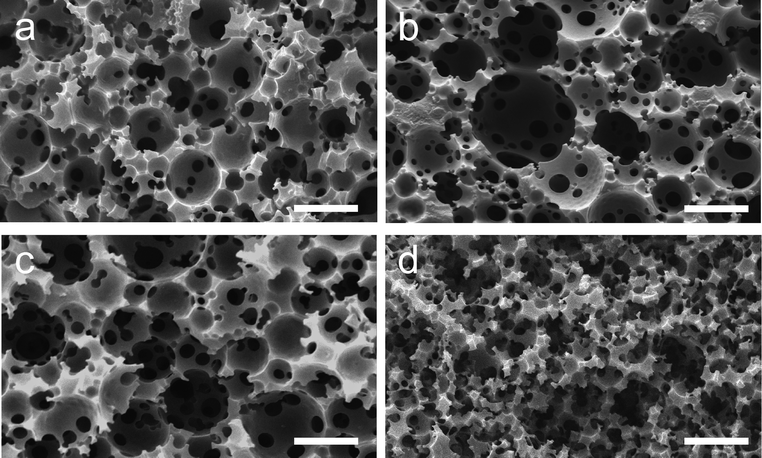 | ||
| Fig. 1 SEM images of thiol–ene and thiol–yne polyHIPE materials: (a) sample TE-75; (b) sample TE-80; (c) sample TY-75; (d) sample TY-80. Scale bar = 20 μm. See Table 2 footnote for sample codes. | ||
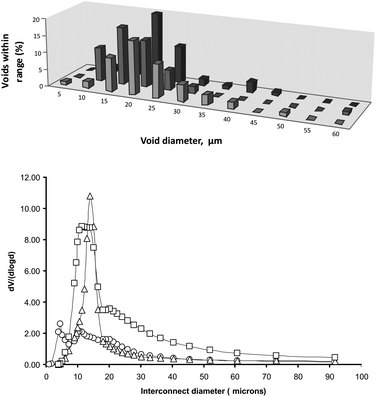 | ||
| Fig. 2 Void and interconnect diameters of thiol–ene and thiol–yne polyHIPEs: (a) void diameter distribution by analysis of SEM images, from front to back TE-75, TE-80 and TY-75; (b) window diameter distribution by mercury porosimetry: circles TE-75, triangles TY-75, and squares TY-80. | ||
| Polymer a | 〈D〉/μm | 〈d〉/μm | 〈d〉/〈D〉 | %Sthb | %Sexpb | T g/°C |
|---|---|---|---|---|---|---|
| a T = thiol, E = ene, Y = yne, 75/80 = nominal porosity. b % Sth = calculated sulfur content, % Sexp = sulfur content by elemental analysis. c Could not be determined as the material was too elastic for mercury porosimetry. d Could not be calculated due to missing parameter. e The morphology did not allow determination of void diameter by image analysis. | ||||||
| TE-75 | 15.4 | 7.2 | 0.46 | 13.80 | 11.80 | 20.4 |
| TE-80 | 20.3 | c | d | 13.80 | 11.63 | 28.8 |
| TY-75 | 19.5 | 13.0 | 0.67 | 20.10 | 16.64 | 26.7 |
| TY-80 | e | 4.2 | d | 20.10 | 17.01 | 34.6 |
Table 2 also gives sulfur elemental analysis data for the polyHIPE materials. The networks are formed by a step growth process, for which an exact balance of thiols and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C groups is required to ensure efficient network formation. As can be seen, the experimental values are not in complete agreement with the theoretical ones, indicating that network formation occurs with between 80 and 85% efficiency. Solid-state 13C NMR spectroscopy was used to investigate further the difference between the experimental and calculated sulfur content. Comparison of the integral due to C
C groups is required to ensure efficient network formation. As can be seen, the experimental values are not in complete agreement with the theoretical ones, indicating that network formation occurs with between 80 and 85% efficiency. Solid-state 13C NMR spectroscopy was used to investigate further the difference between the experimental and calculated sulfur content. Comparison of the integral due to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C groups (∼130 ppm) to that of carbonyls (∼170 ppm) allows an estimation of the extent of reaction. The results indicate that all samples are reacted to 89% completion. These values are in good agreement with those determined from the elemental analysis data in Table 2 and suggest that the network forming reaction is limited to between 80 and 90% completion due to the loss of monomer 3. One possible explanation is that this monomer partitions to a small extent into the aqueous phase of the emulsion.
C groups (∼130 ppm) to that of carbonyls (∼170 ppm) allows an estimation of the extent of reaction. The results indicate that all samples are reacted to 89% completion. These values are in good agreement with those determined from the elemental analysis data in Table 2 and suggest that the network forming reaction is limited to between 80 and 90% completion due to the loss of monomer 3. One possible explanation is that this monomer partitions to a small extent into the aqueous phase of the emulsion.
Glass transition temperature values are also given in Table 2. The thiol–yne materials have a higher Tg than thiol–ene, reflecting the higher crosslink density of the former. A small increase in Tg is observed as porosity increases.
The tensile properties of the thiol–ene and thiol–yne materials were investigated. Dumbbell-shaped samples of gauge length 16 mm and cross-sectional area of 6 mm2 were cut from materials produced by photopolymerisation and subjected to mechanical properties analysis. The TE-75 sample proved to be stronger than the TE-80 material, and could withstand a load of up to 1.10 N. Using the initial gradient of a plot of stress against strain, the initial Young's modulus was calculated to be 0.45 MPa for the TE-75 sample and 0.20 MPa for the TE-80 material. At the outset, both samples have very similar properties but as load is increased, the 80% porosity sample extends to a greater extent for a given load and fractures earlier due to its greater elasticity. Likewise, the TY-75 polyHIPE sample proved to be stronger than the TY-80 sample. The difference in strength between the two was far greater however than in the case of the thiol–ene polyHIPEs; for a 5% increase in porosity, strength decreased by a factor of 12.7. The initial Young's modulus was calculated to be 31.9 MPa for TY-75 and 0.27 MPa for TY-80. Both samples extend further than the thiol–ene polyHIPEs but it is the TY-75 material that can withstand the much higher load (up to 14.5 N). At both porosities, the thiol–yne polyHIPEs are stiffer than their thiol–ene counterparts. This could be due to the higher degree of crosslinking within the former materials. The relatively poor performance of the TY-80 specimen could be due to its internal morphology, which was not as well-defined as the other samples (Fig. 1d). The lack of well-defined voids may mean that the specimen could not endure the constant strain and hence fractured early on in the testing.
In conclusion, novel, highly porous polymeric materials have been prepared by thiol–ene and thiol–yne polymerisation of the continuous phase of high internal phase emulsions. The network forming reactions are highly efficient, leading to materials with well-defined morphologies. The mechanical properties are dependent on the porosity and the type of network forming reaction used (thiol–ene or thiol–yne).
Experimental
Materials and methods
The monomers trimethylolpropane triacrylate (1), trimethylolpropane tris(3-mercaptopropionate) (2), and octadiyne (3) were obtained from Sigma Aldrich and used without further purification. The photoinitiator, a blend of diphenyl(2,4,6-trimethylbenzoyl)phosphine oxide/2-hydroxy-2-methylpropiophenone (Aldrich), was used as supplied. The surfactant Hypermer B246 was obtained from Croda and is a mix of polyhydroxystearic acid and poly(ethylene glycol). Photopolymerisation was conducted using a Light Hammer 6 variable power UV curing system with benchtop conveyor from Fusion UV Systems, Inc. The system employs an H bulb operating at 200 W cm−2. The morphology of the polyHIPEs was investigated using a Philips/FEI XL30 ESEM operating at between 20 and 25 kV. Fractured specimens were sputter-coated with gold and mounted on carbon fibre pads adhered to aluminium stubs. Image J24 was used to calculate the average void size, using a random sample of 50 voids from each SEM image. A statistical correction factor25 was employed to provide accurate void diameters. Mercury intrusion porosimetry analysis was performed using a Micromeritics Autopore IV. Intrusion and extrusion mercury contact angles of 130° were used and intrusion pressures did not exceed 1600 psi. Penetrometers had a stem volume of 1.190 ml and a bulb volume of 4.2 ml. The intrusion volume always comprised between 45% and 80% of the stem volume. The sulfur content was determined using an Ion DX-120 Ion Chromatograph. Solid state 13C NMR spectra were recorded on a Varian VNMRS 400 spectrometer, using direct polarization with proton decoupling during acquisition at a frequency of 100.562 MHz. Samples for tensile testing were carefully cut into dumbbell shapes using a scalpel and a metal template. Specimens were then subjected to tensile testing using an Instron 5565 Materials Testing System. Samples were mounted between rubber-padded grips and tested at room temperature under a constant strain rate (10−3s−1).Preparation of polyHIPE materials
This procedure is based on the work carried out by Pierre et al.23 An oil phase consisting of triacrylate 1 or di-yne 3, trithiol 2, chloroform, photoinitiator (7 wt% relative to monomer content) and the surfactant Hypermer B246 (2.5 wt% of oil phase) was added to a 150 ml beaker. The oil phase was stirred continually at 350 rpm using a D-shaped PTFE paddle connected to an overhead stirrer. An aqueous phase consisting of deionised water was added dropwise until a HIPE had formed. After addition of the aqueous phase, the HIPE was stirred for a further five minutes. The HIPE was then poured into a PTFE 50 × 50 × 5 mm square frame mould, and secured between two glass plates. This mould was then passed under the UV irradiator four times on each side at a speed of 3.5 m min−1. The resulting polyHIPE was washed with acetone and dried overnight in a vacuum oven at 50 °C.Notes and references
- T. Posner, Ber. Dtsch. Chem. Ges., 1905, 38, 646–657 CrossRef.
- M. S. Kharasch, A. T. Read and F. R. Mayo, Chem. Ind., 1938, 752 Search PubMed.
- C. R. Morgan, F. Magnotta and A. D. Ketley, J. Polym. Sci., Part A: Polym. Chem., 1977, 15, 627–645 Search PubMed.
- C. E. Hoyle, T. Y. Lee and T. Roper, J. Polym. Sci., Part A: Polym. Chem., 2004, 42, 5301–5338 CrossRef CAS.
- C. E. Hoyle, A. B. Lowe and C. N. Bowman, Chem. Soc. Rev., 2010, 39, 1355–1387 RSC.
- M. J. Kade, D. J. Burke and C. J. Hawker, J. Polym. Sci., Part A: Polym. Chem., 2010, 48, 743–750 CrossRef CAS.
- A. B. Lowe, Polym. Chem., 2010, 1, 17–36 RSC.
- A. B. Lowe and M. A. Harvison, Aust. J. Chem., 2010, 63, 1251–1266 CrossRef CAS.
- J. W. Chan, H. Zhou, C. E. Hoyle and A. B. Lowe, Chem. Mater., 2009, 21, 1579–1585 CrossRef CAS.
- R. Hoogenboom, Angew. Chem., Int. Ed., 2010, 49, 3415–3417 CAS.
- A. B. Lowe, C. E. Hoyle and C. N. Bowman, J. Mater. Chem., 2010, 20, 4745–4750 RSC.
- J. W. Chan, J. Shin, C. E. Hoyle, C. N. Bowman and A. B. Lowe, Macromolecules, 2010, 43, 4937–4942 CrossRef CAS.
- J. A. Carioscia, H. Lu, J. W. Stanbury and C. N. Bowman, Dent. Mater., 2005, 21, 1137–1143 CrossRef CAS.
- J. A. Carioscia, L. Schneidewind, C. O'Brien, R. Ely, C. Feeser, N. Cramer and C. N. Bowman, J. Polym. Sci., Part A: Polym. Chem., 2007, 45, 5686–5696 CrossRef CAS.
- J. A. Carioscia, J. W. Stansbury and C. N. Bowman, Polymer, 2007, 48, 1526–1532 CrossRef CAS.
- B. D. Fairbanks, T. F. Scott, C. J. Kloxin, K. S. Anseth and C. N. Bowman, Macromolecules, 2009, 42, 211–217 CrossRef CAS.
- R. A. Prasath, M. T. Gokmen, P. Espeel and F. E. Du Prez, Polym. Chem., 2010, 1, 685–692 RSC.
- X. Q. Gong, W. J. Wen and P. Sheng, Langmuir, 2009, 25, 7072–7077 CrossRef CAS.
- D. Barby and Z. Haq, EP Pat., 60138, 1982.
- N. R. Cameron, Polymer, 2005, 46, 1439–1449 CrossRef CAS.
- N. R. Cameron and D. C. Sherrington, Adv. Polym. Sci., 1996, 126, 163–214 CAS.
- H. Zhang and A. I. Cooper, Soft Matter, 2005, 1, 107–113 RSC.
- S. J. Pierre, J. C. Thies, A. Dureault, N. R. Cameron, J. C. M. van Hest, N. Carette, T. Michon and R. Weberskirch, Adv. Mater., 2006, 18, 1822–1826 CrossRef CAS.
- http://rsbweb.nih.gov/ij/ .
- A. Barbetta and N. R. Cameron, Macromolecules, 2004, 37, 3188–3201 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2011 |
