Thiol–isocyanate “click” reactions: rapid development of functional polymeric surfaces†
Ryan M.
Hensarling
a,
Santosh B.
Rahane
a,
Arthur P.
LeBlanc
a,
Bradley J.
Sparks
a,
Evan M.
White
b,
Jason
Locklin
b and
Derek L.
Patton
*a
aSchool of Polymers and High Performance Materials, University of Southern Mississippi, 118 College Drive #5050, Hattiesburg, MS, USA. E-mail: derek.patton@usm.edu; Fax: +1 601-266-5880; Tel: +1 601-266-4792
bDept. of Chemistry, Faculty of Engineering, and the Center for Nanoscale Science and Engineering, University of Georgia, Athens, GA 30602, USA
First published on 18th October 2010
Abstract
Functional, micropatterned and multicomponent polymer brush surfaces can be rapidly fabricated via base-catalyzed thiol–isocyanate “click” reactions.
Applications for advanced functional polymeric surfaces that possess precisely engineered properties are expanding rapidly. Such demands necessitate the development of fabrication methods for soft material surfaces with precise control over functionality, architecture, reactivity and domain size for an array of applications ranging from biosensors to microelectronics.1 Among several surface functionalization strategies recently developed, those involving robust and efficient click reactions are particularly attractive for orthogonal and site-selective immobilization of functional moities.2 Several outstanding examples involving conventional Cu-catalyzed azide–alkyne3–5 and photoactivated Cu-free azide–alkyne cycloadditions6 illustrate the power of click strategies for tailor-made surfaces. Additionally, we and others have demonstrated thiol-click reactions, including thiol–ene7–10 and thiol–yne,11 as rapid, robust, and efficient immobilization strategies toward patterned, multicomponent surfaces. Our current efforts focus on expanding the “toolbox” of modular reactions that allow immobilization of functionally complex molecules onto solid substrates by exploiting efficient linking strategies. Herein, we report thiol–isocyanate (thiol–NCO) chemistry as a modular approach toward surface engineering by demonstrating the rapid generation of a library of functional, patterned, and multicomponent polymer brush surfaces using a single substrate precursor.
The base-catalyzed reaction of thiols with isocyanates yielding thiourethanes has been known for over 50 years,12,13 but has only recently been recognized for its potential as a click reaction.14,15 Despite impressive potential, these reactions have been scarcely exploited for polymer synthesis16,17 and postmodification.18,19 For functional surfaces, isocyanate chemistry has only been explored in a few instances that focused on reactions with amines (urethane linkages) for immobilizing functional moieties on self-assembled monolayers for biosurfaces,20,21 photoswitchable wettability,22 organometallic surfaces,23 and self-cleaning/anti-fog surfaces.24 To our knowledge, thiol–isocyanate chemistry has yet to be explored as a modular approach to surface engineering. Considering rapid kinetics, quantitative conversions, and vast libraries of commercially available and/or naturally occurring thiols, we envisioned the fabrication of highly functional surfaces using base-catalyzed thiol–NCO reactions to modify densely tethered NCO-containing polymer brush surfaces. As we will show, this approach works equally well with thiols or amines. This platform is analogous, but orthogonal to our recently reported radical-mediated thiol–yne click approach.11 Additionally, the NCO group is inert to radical polymerization conditions eliminating any need and synthetic effort to protect the “clickable” moiety during surface-initiated photopolymerization (SIP).
As shown in Fig. 1a, silicon substrates were first functionalized with a chlorosilane derivative of commercially available 2-hydroxy-4′-(2-hydroxyethoxy)-2-methylpropiophenone (Irgacure 2959) photoinitiator.11,25 These substrates were subsequently inserted into a microchannel reactor containing 2-isocyanatoethyl methacrylate (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 v/v in dry THF) and irradiated with UVλmax=365nm light (∼140 mW cm−2, 20 min, ∼28 nm brush thickness). For the fabrication of a 15 mm × 65 mm substrate only 1 mL of monomer solution was required to fill the microchannel reactor, drastically reducing the cost of this approach. After extensive washing in THF and toluene, the brush surfaces were analyzed by grazing-angle attenuated total reflection FTIR (GATR-FTIR), ellipsometry and water contact angle measurements. Polymer brush formation was confirmed by the presence of the asymmetric stretching vibration of the isocyanate group (2275 cm−1) and carbonyl stretching vibration for esters (1729 cm−1) (Fig. 3).26 The resulting NCO-containing polymer brushes served as a “universal” reactive precursor for subsequent thiol–NCO click reactions eliminating the synthetic effort associated with the use of multiple functional monomers. Despite the known sensitivity of NCO functional groups, no special handling of the substrates was required prior to thiol modification. NCO-modified surfaces could be stored up to two weeks in nitrogen-flushed, septum-sealed test tubes with no observable loss in functionality or degradation in reactivity.
6 v/v in dry THF) and irradiated with UVλmax=365nm light (∼140 mW cm−2, 20 min, ∼28 nm brush thickness). For the fabrication of a 15 mm × 65 mm substrate only 1 mL of monomer solution was required to fill the microchannel reactor, drastically reducing the cost of this approach. After extensive washing in THF and toluene, the brush surfaces were analyzed by grazing-angle attenuated total reflection FTIR (GATR-FTIR), ellipsometry and water contact angle measurements. Polymer brush formation was confirmed by the presence of the asymmetric stretching vibration of the isocyanate group (2275 cm−1) and carbonyl stretching vibration for esters (1729 cm−1) (Fig. 3).26 The resulting NCO-containing polymer brushes served as a “universal” reactive precursor for subsequent thiol–NCO click reactions eliminating the synthetic effort associated with the use of multiple functional monomers. Despite the known sensitivity of NCO functional groups, no special handling of the substrates was required prior to thiol modification. NCO-modified surfaces could be stored up to two weeks in nitrogen-flushed, septum-sealed test tubes with no observable loss in functionality or degradation in reactivity.
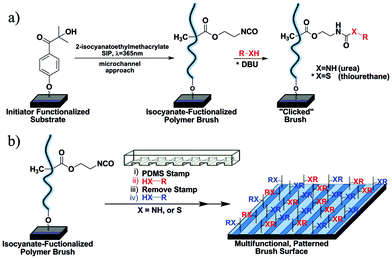 | ||
| Fig. 1 (a) Schematic procedure for surface-initiated photopolymerization of 2-isocyanatoethyl methacrylate and subsequent X-isocyanate functionalization (X = thiol or amine). (b) Schematic procedure for patterning NCO-containing polymer brush surfaces with sequential X-isocyanate reactions. | ||
The nucleophilic addition of primary thiols or amines to isocyanates generates a thiourethane or urea linkage, respectively (Fig. 1a). Amine–NCO reactions are self-catalyzed while thiol–NCO reactions require the addition of a base catalyst—the identity of which is known to have a pronounced effect on the reaction kinetics.17Tertiary amine catalysts facilitate rapid reactions via generation of (1) a more electron deficient carbonyl carbon within the isocyanate moiety and (2) a strongly nucleophilic thiolate ion.15,17 For thiol–NCO reactions herein, 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) (0.2 mol% with respect to thiol) was used as catalyst. To explore the efficacy of the isocyanate click reactions on surfaces, we selected a library of commercially available thiols and amines for functionalization (Fig. 2): 3-mercaptopropionic acid (MPA) (pH responsive), 1-dodecanethiol (DDT) (hydrophobic), 1-thioglycerol (hydrophilic), N-acetyl-L-cysteine (model peptide attachment), benzyl mercaptan, 1-adamantanethiol, thiocholesterol (biomembrane attachment), 3-mercaptopropyl polyhedral oligomeric silsequioxane (POSS), furfuryl mercaptan, hexyl amine, and benzyl amine. These reactions were carried out under ambient air, temperature, and humidity conditions to afford functional polymeric brushes. Subsequently, the substrates were washed extensively with multiple solvents to eliminate any physisorbed material prior to characterization. GATR-FTIR was used to follow the functionalization of the brushes with the various thiols and amines. For the entire series of functional brushes, quantitative conversion of the tethered isocyanates was observed within minutes as indicated by the disappearance of the peak associated with the isocyanate group (2275 cm−1) (Fig. 3) and appearance of peaks indicative of the incorporated thiols and amines (see Table S1† for additional peak assignments). Triethylamine also carried the thiol–NCO reaction to quantitative conversion, albeit in several hours rather than minutes as observed with DBU. Static water contact angles revealed expected changes in wettability related to the functional moieties incorporated into the polymer brushes (Fig. S1†). An increase in thickness of the polymer brushes was observed after functionalization with the various thiols and amines due to an increase in the molar mass of the monomer repeat unit, resulting in an increase in the molecular weight of the brush. Additionally, to broaden the utility of this approach, fluorescent brushes were easily obtained by absorbing acridine orange (fluorescent dye) onto deprotonated MPA functionalized polymer brushes (Fig. S3†). The fluorescent dye adheres to the functionalized polymer brush through an ionic interaction and demonstrates the potential use of orthogonal covalent/non-covalent interactions for fabrication of functional polymer surfaces.
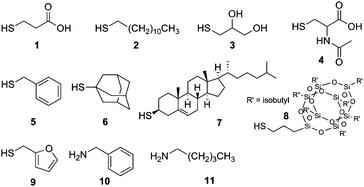 | ||
| Fig. 2 Commercially available thiols/amines used for X-isocyanate click reactions: mercaptopropionic acid (1), 1-dodecanethiol (2), thioglycerol (3), N-acetyl-L-cysteine (4), benzyl mercaptan (5), 1-adamantanethiol (6), thiocholesterol (7), 3-mercaptopropyl polyhedral oligomeric silsequioxane (POSS) (8), furfuryl mercaptan (9), benzyl amine (10), and hexyl amine (11). | ||
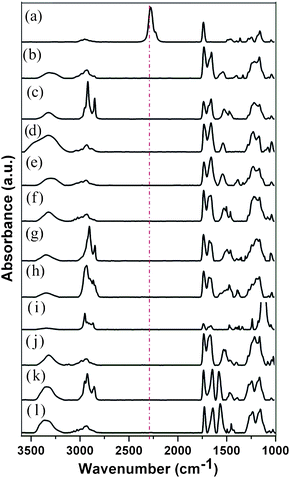 | ||
Fig. 3
GATR-FTIR
spectra of brushes on SiOx substrates (key peaks are identified): (a) poly(2-isocyanatoethyl methacrylate) brush (2275 cm−1, NCO) (red) reacted with (b) 3-mercaptopropionic acid (3320–3000 cm−1, COO–H), (c) 1-dodecanethiol (2954, 2924, 2852 cm−1, C–H), (d) 1-thioglycerol (3573–3125 cm−1, OH), (e) N-acetyl-L-cysteine (3450–3162 cm−1, CO–NH), (f) benzyl mercaptan (3084, 3058, 3025 cm−1, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H; 1517, 1493, 1451 cm−1, C C–H; 1517, 1493, 1451 cm−1, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), (g) 1-adamantanethiol (2906, 2850 cm−1, C–H), (h) thiocholesterol (2936, 2903, 2865, 2850 cm−1, C–H), (i) 3-mercaptopropyl polyhedral oligomeric silsequioxane (1109 cm−1, Si–O), (j) furfuryl mercaptan (1204, 1156 cm−1, C–O (cyclic), 1068 cm−1, C–O–C (5-membered rings), (k) hexyl amine (2954, 2930, 2856 cm−1, C–H), and (l) benzyl amine (3085, 3061, 3025 cm−1, C), (g) 1-adamantanethiol (2906, 2850 cm−1, C–H), (h) thiocholesterol (2936, 2903, 2865, 2850 cm−1, C–H), (i) 3-mercaptopropyl polyhedral oligomeric silsequioxane (1109 cm−1, Si–O), (j) furfuryl mercaptan (1204, 1156 cm−1, C–O (cyclic), 1068 cm−1, C–O–C (5-membered rings), (k) hexyl amine (2954, 2930, 2856 cm−1, C–H), and (l) benzyl amine (3085, 3061, 3025 cm−1, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H; 1565, 1493, 1451 cm−1, C C–H; 1565, 1493, 1451 cm−1, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C). C). | ||
With the development of thiol–NCO click reactions as a platform for surface engineering in mind, the modularity and versatility of our approach was demonstrated by conducting sequential/area-selective thiol–NCO brush modifications using an elastomeric microcapillary patterning process.27 The process is schematically shown in Fig. 1b. A line-patterned PDMS stamp (linewidth: 15.0 µm) was used to create defined, micropatterned polymeric surfaces. The stamp was placed in direct contact with the brush surface and a solution of 3-mercapto-1-propanesulfonic acid (300![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mol/mol thiol
1 mol/mol thiol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DBU) in methanol was wicked in subsequently reacting for 12 min yielding a micropatterned sulfonate/NCO surface. After removing the stamp and washing with methanol and toluene, the unexposed and unreacted isocyanate groups were then subjected to a second thiol–NCO click reaction with DDT (500
DBU) in methanol was wicked in subsequently reacting for 12 min yielding a micropatterned sulfonate/NCO surface. After removing the stamp and washing with methanol and toluene, the unexposed and unreacted isocyanate groups were then subjected to a second thiol–NCO click reaction with DDT (500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mol/mol thiol
1 mol/mol thiol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DBU, 12 min) in THF followed by washing with THF and toluene affording the micropatterned, multicomponent surface. Similar patterns could be obtained using sequential combinations of functional thiols and amines. Fig. 4a shows the optical condensation image for the sulfonate/DDT patterned surface. As shown, the hydrophilic sulfonated domains preferentially nucleate condensation of water allowing visualization of the patterned surface.11,28 To complement these results, atomic force microscopy (AFM) imaging was used (Fig. 4b) showing height differences created by the incorporation of the two different functional molecules.
DBU, 12 min) in THF followed by washing with THF and toluene affording the micropatterned, multicomponent surface. Similar patterns could be obtained using sequential combinations of functional thiols and amines. Fig. 4a shows the optical condensation image for the sulfonate/DDT patterned surface. As shown, the hydrophilic sulfonated domains preferentially nucleate condensation of water allowing visualization of the patterned surface.11,28 To complement these results, atomic force microscopy (AFM) imaging was used (Fig. 4b) showing height differences created by the incorporation of the two different functional molecules.
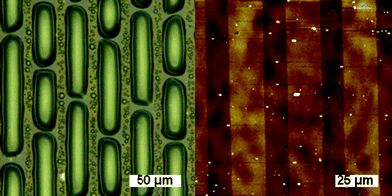 | ||
| Fig. 4 (a) Condensation image of sequential thiol–NCO micropatterned brushes (sulfonate/DDT) showing water droplets selectively nucleating on the hydrophilic sulfonated areas. (b) AFM image of sequential thiol–NCO micropatterned brushes (sulfonate/DDT), 100 × 100 µm, Z-scale = 50.0 nm. | ||
Conclusions
In summary, we have demonstrated thiol–NCO click chemistry as a modular platform for rapid and robust fabrication of highly functional, multicomponent surfaces. Although demonstrated here on polymer brush modified planar substrates, this approach is certainly extendable to a broad range of surfaces, including three-dimensional particle substrates. As a functional handle for post-polymerization modification, we anticipate thiol–NCO click reactions to have a significant impact in many areas of polymer/materials chemistry.Acknowledgements
Support for this research was provided by startup funds from USM and National Science Foundation (Grant #0917730). RMH acknowledges fellowship support from the US Dept. of Education GAANN program (#P200A090066). We thank Baobin Kang in the USM biology department for help with fluorescent microscopy.References
- R. Barbey, L. Lavanant, D. Paripovic, N. Schüwer, C. Sugnaux, S. Tugulu and H.-A. Klok, Chem. Rev., 2009, 109, 5437–5527 CrossRef CAS.
- R. K. Iha, K. L. Wooley, A. M. Nystrom, D. J. Burke, M. J. Kade and C. J. Hawker, Chem. Rev., 2009, 109, 5620–5686 CrossRef CAS.
- S. Im, K. Bong, B.-S. Kim, S. Baxamusa, P. Hammond, P. Doyle and K. Gleason, J. Am. Chem. Soc., 2008, 130, 14424–14425 CrossRef CAS.
- A. Gonzalez-Campo, S.-H. Hsu, L. Puig, J. Huskens, D. N. Reinhoudt and A. H. Velders, J. Am. Chem. Soc., 2010, 132, 11434–11436 CrossRef CAS.
- S. A. Krovi, D. Smith and S. T. Nguyen, Chem. Commun., 2010, 46, 5277–5279 RSC.
- S. V. Orski, A. A. Poloukhtine, S. Arumugam, L. Mao, V. V. Popik and J. Locklin, J. Am. Chem. Soc., 2010, 132, 11024–11026 CrossRef CAS.
- M. Campos, J. Paulusse and H. Zuilhof, Chem. Commun., 2010, 46, 5512–5514 RSC.
- D. Weinrich, P. Lin, P. Jonkheijm, U. Nguyen, H. Schroder, C. Niemeyer, K. Alexandrov, R. Goody and H. Waldmann, Angew. Chem., Int. Ed., 2010, 49, 1252–1257 CrossRef CAS.
- V. Khire, Y. Yi, N. Clark and C. Bowman, Adv. Mater., 2008, 20, 3308–3313 CrossRef CAS.
- V. Khire, T. Lee and C. Bowman, Macromolecules, 2008, 41, 7440–7447 CrossRef CAS.
- R. M. Hensarling, V. A. Doughty, J. W. Chan and D. L. Patton, J. Am. Chem. Soc., 2009, 131, 14673–14675 CrossRef CAS.
- R. G. Arnold, J. A. Nelson and J. J. Verbanc, Chem. Rev., 1957, 57, 47–76 CrossRef CAS.
- E. Dyer, J. F. Glenn and E. G. Lendrat, J. Org. Chem., 1961, 26, 2919–2925 CrossRef CAS.
- H. C. Kolb, M. G. Finn and B. K. Sharpless, Angew. Chem., Int. Ed., 2001, 40, 2004–2021 CrossRef CAS.
- C. E. Hoyle, A. B. Lowe and C. N. Bowman, Chem. Soc. Rev., 2010, 39, 1355–1387 RSC.
- J. Shin, H. Matsushima, J. W. Chan and C. E. Hoyle, Macromolecules, 2009, 42, 3294–3301 CrossRef CAS.
- J. Shin, H. Matsushima, C. M. Comer, C. N. Bowman and C. E. Hoyle, Chem. Mater., 2010, 22, 2616–2625 CrossRef CAS.
- H. Li, B. Yu, H. Matsushima, C. E. Hoyle and A. B. Lowe, Macromolecules, 2009, 42, 6537–6542 CrossRef CAS.
- J. D. Flores, J. Shin, C. E. Hoyle and C. L. McCormick, Polym. Chem., 2010, 1, 213–220 RSC.
- M. Vigano, R. Suriano, M. Levi, S. Turri, M. Chiari and F. Damin, Surf. Sci., 2007, 601, 1365–1370 CrossRef CAS.
- A. Perzyna, C. d. Zotto, J.-O. Durand, M. Granier, M. Smietana, O. MeInyk, I. G. Stara, I. stary, B. Kepetarova and D. Saman, Eur. J. Org. Chem., 2007, 4032–4037 CrossRef CAS.
- N. Delorme, J. F. Bardeau, A. Bulou and F. Poncin-Epaillard, Langmuir, 2005, 21, 12278–12282 CrossRef CAS.
- A. R. McDonald, H. P. Dijkstra, B. M. J. M. Suijkerbuijk, G. P. M. van Klink and G. van Koten, Organometallics, 2009, 28, 4689–4699 CAS.
- J. A. Howarter and J. P. Youngblood, Adv. Mater., 2007, 19, 3838–3843 CrossRef CAS.
- C. Schuh, S. Santer, O. Prucker and J. Ruhe, Adv. Mater., 2009, 21, 4706–4710 CAS.
- G. Socrates, Infrared and Raman Characteristics Group Frequencies, John Wiley & Sons Ltd., Chichester, 2001 Search PubMed.
- T. Kaufmann and B. Ravoo, Polym. Chem., 2010, 1, 371–387 RSC.
- A. A. Brown, O. Azzaroni, L. M. Fidalgo and W. T. S. Huck, Soft Matter, 2009, 5, 2738–2745 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Synthetic procedures, FTIR assignments, and water contact measurements. See DOI: 10.1039/c0py00292e |
| This journal is © The Royal Society of Chemistry 2011 |
