Controlled radical polymerization of styrene in miniemulsion mediated by PEO-based trithiocarbonate macromolecular RAFT agents†
Thomas
Boursier
a,
Isabelle
Chaduc
a,
Jutta
Rieger
b,
Franck
D'Agosto
*a,
Muriel
Lansalot
*a and
Bernadette
Charleux
a
aUniversité de Lyon, Univ. Lyon 1, CPE Lyon, CNRS UMR 5265, Laboratoire de Chimie Catalyse Polymères et Procédés (C2P2), Equipe LCPP Bat 308F, 43 Bd du 11 novembre 1918, F-69616, Villeurbanne, France. E-mail: dagosto@lcpp.cpe.fr; lansalot@lcpp.cpe.fr; Fax: +33 4 72 43 17 68; Tel: +33 4 72 43 17 70
bUPMC Univ. Paris 6 and CNRS, Laboratoire de Chimie des Polymères, UMR 7610, 4 place Jussieu, Tour 44-54, 75252, Paris Cedex 05, France
First published on 27th September 2010
Abstract
Poly(ethylene oxide) (PEO) based macroRAFT agents with various chemical structures have been used as both stabilizer and control agent for the polymerization of styrene in miniemulsion conditions. Trithiocarbonate (PEO-DTTC (Z = thiododecyl), PEO-PTTC (Z = thiopropyl)) functional groups were attached to a commercial monomethyl ether PEO (Mn = 2000 g mol−1). PEO-DTTC and PEO-PTTC allowed the formation of stable miniemulsions of styrene in water. Our previous results (A. M. Dos Santos, T. Le Bris, C. Graillat, F. D'Agosto, M. Lansalot, Macromolecules, 2009, 42, 946) showed that PEO-based dithiobenzoate (PEO-DB) led to controlled polymerization but also to broad molar mass distribution (PDI = 1.9) and multipopulated polymer chains. The switch from PEO-DB to PEO-DTTC greatly improved the molar mass distribution (PDI = 1.6). This was ascribed to the ability of PEO-DTTC to be localized at the water/monomer droplets interface. An increase in PEO-DTTC concentration improved the control of the polymerization. However, the concomitant formation of micelles favored secondary nucleation. This was attenuated by the use of PEO-PTTC, less prone to form micelles in water which greatly improved both the quality of control (PDI = 1.3) and the particle size distribution and showed that the particles were constituted of well-defined PEO-b-PS polymer chains. These results could be attributed to a more efficient anchoring of PEO-PTTC at the monomer droplet or particle/water interface showing the crucial role of the macroRAFT structure in these systems.
Introduction
A considerable amount of work has been recently dedicated to the transposition of controlled radical polymerization (CRP) via reversible deactivation from bulk and solution to heterogeneous polymerization media.2–4 This can be attributed to the intrinsic features of the corresponding techniques (mainly emulsion, miniemulsion and dispersion) that offer several advantages in terms of process, cost and environmental impact (low viscosity of the reaction medium and of the resulting latex, high polymerization rate, simplified design and operation of chemical reactors, no need for volatile organic compounds). Among the available CRP techniques, reversible addition-fragmentation chain transfer (RAFT) process5 relies on the use of thiocarbonyl thio compounds of structure Z–C(=S)–SR which are very efficient reversible chain transfer agents acting as control agents. In the initial works dedicated to RAFT in emulsion polymerization, the synthesis was successful only when moderately hydrophobic control agents with low chain transfer constants (dithiocarbonate) were used,6 whereas more reactive chain transfer agents such as dithioesters proved to be unsuccessful to provide both good colloidal stability and well-defined polymers.Significant headway was recently made when strategies employing macromolecular chain transfer agents were put forward, and various RAFT-derived hydrophilic (co)polymers were employed to mediate the polymerization of hydrophobic monomers in aqueous dispersed systems for the synthesis of polymer particles.4 These macromolecular RAFT (or macroRAFT) agents can be considered as reactive precursors of surfactants that lead to the in situ formation of amphiphilic block copolymers. They do not only play a key role in the formation of the particles and in the functionalization of their surface, but may also control the growth of the hydrophobic block that will form the particles thus acting as a reversible transurf. In the end, the particles are ideally constituted of amphiphilic block copolymers with a predefined narrowly distributed molar mass. This approach consists of an elegant way of getting rid of low molar mass surfactants which are known to have detrimental effects such as poor latex stability under freezing conditions or under high shear, or diffusion problems during film-formation. In addition, those macromolecular stabilizers being anchored via a covalent bond are less prone to desorption or migration when compared to conventional low molar mass surfactants.7–9
Good control over both the colloidal stability and the molar masses via the macroRAFT strategy was achieved under starved-feed monomer addition when using polyelectrolyte macroRAFT agents based on poly(acrylic acid) (PAA)10–13 or poly(4-vinyl pyridine).14 Successful examples for a batch emulsion processes were then reported using poly(ethylene oxide) (PEO) or poly(dimethyl acrylamide) macroRAFT agents.15–17 It should be noted that, in all these cases, the macroRAFT carry a trithiocarbonate chain end.
While this strategy seems to be the way to control both the colloidal and the macromolecular features of polymer colloids obtained by emulsion polymerization, a long way is still to go to fully understand the parameters that impact the nucleation, the kinetics and the resulting achievable morphologies.18 In this context, the transposition of this strategy to miniemulsion polymerization appears particularly appealing. One of the main obstacles to industrial acceptance of this process is the difficulty in scaling up the miniemulsification step. However, readily scalable techniques for manufacturing scale are being investigated.19 In addition, apart from a simplified nucleation step, this process is a powerful synthetic tool for localizing the polymerization in nanoreactors that are originally formed by the monomer droplets.20–22 In addition, the miniemulsion process brings the benefit of the potential encapsulation of hydrophobic species such as inorganic particles or water-sensitive components. Nevertheless, as for emulsion polymerization, stabilization and surfactant desorption issues still need to be addressed in a conventional miniemulsion process. Using macroRAFT agents located at the monomer droplets/water interface as both stabilizer and control agent will be the way to improve the versatility already achieved by this process and allegedly expand the range of possible particle morphologies by controlling the polymerization inside the particles.
To date, only a few studies report the use of macroRAFT agents as both stabilizer and control agent for aqueous miniemulsion polymerization. Pham et al.23 obtained stable poly(n-butyl acrylate) (PBA) or polystyrene (PS) latexes with a good control over molar masses using trithiocarbonate-based amphipathic block copolymers composed of either PAA-b-PBA or PAA-b-PS. Later on, Luo and coworkers described the synthesis of nanocapsules viaminiemulsion polymerization using either dithioester-based amphiphilic copolymers of styrene and maleic anhydride of various hydrophilicity (after hydrolysis/aminolysis treatment)24,25 or an amphiphilic block copolymer of PAA-b-PS synthesized from a trithiocarbonate carrying an alkyl chain (C12H25).26 Taking into account initiator-derived chains and introducing a factor of efficiency for the macroRAFT agent, the molar masses agreed with the theoretical ones and low polydispersity indexes (below 1.6) were observed. To improve the proportion of nanocapsules versus solid particles as well as the homogeneity of the nanocapsules in terms of overall size, shell thickness and shape symmetry, SDS was however used as an additional surfactant or post-added in some cases.25,26 These examples are all related to the use of amphiphilic copolymers.
In our group, we recently studied a PEO-based dithiobenzoate macroRAFT agent as both stabilizer and control agent for styrene miniemulsion polymerization.1 In line with this study, the present paper aims at assessing the use of PEO-based trithiocarbonate macroRAFT agents that have been designed to be localized at the water/monomer droplets interface (Scheme 1). The targets are here to efficiently control the polymerization while still keeping the colloidal stability that makes the process referred to as a miniemulsion polymerization.
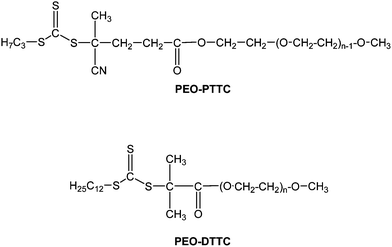 | ||
| Scheme 1 The chemical structures of the two PEO-based macroRAFT agents used in this study. | ||
Experimental section
Materials
Water was deionized before use (Purelab Classic UV, Elga LabWater). Styrene (S, 99%, Aldrich) was distilled under vacuum. Dichloromethane (>99.8%, Aldrich) was dried over molecular sieves. Hexadecane (HD, 99%, Acros Organics), poly(ethylene oxide) monomethyl ether (PEO-OH, Mn = 2000 g mol−1, Fluka), 4-dimethylaminopyridine (DMAP, >99%, Fluka), N,N′-dicyclohexylcarbodiimide (DCC, >99%, Fluka), 4,4′-azobis(4-cyanopentanoic acid) (ACPA, >98%, Fluka) were used as received. 2,2′-Azobis(isobutyronitrile) (AIBN, Fluka, 98%) was purified by re-crystallization from ethanol at 40 °C.Synthesis of PEO-based macroRAFT agents
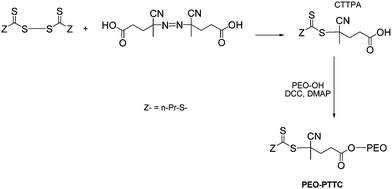 | ||
| Scheme 2 The synthetic route to PEO-based trithiocarbonate PEO-PTTC. | ||
CTTPA: 1H NMR 250 MHz (CDCl3, 273 K), δ: 0.95 (3H, t, CH3-CH2), 1.75 (2H, sext, CH3–CH2–CH2–), 1.85 (3H, s, CH3), 2.20–2.80 (4H, m, HOOC–CH2–CH2–), 3.30 (2H, t, CH3–CH2–CH2–).
In a second step, the PEO-based propyltrithiocarbonate was obtained by esterification of the carboxylic acid group with PEO-OH. PEO-OH was first dried by three successive azeotropic distillations performed in toluene. A typical esterification reaction was carried out as follows. A solution of PEO-OH (1.400 g, 0.706 mmol), DMAP (16 mg, 0.130 mmol, 0.18 eq), and CTTPA (2 eq) in 10 mL of dichloromethane was prepared under argon atmosphere. After 10 min of stirring at 0 °C, a solution of 303 mg of DCC (1.147 mmol) in 5 mL of dichloromethane was added dropwise. The resulting mixture was stirred for 20 h at room temperature. The obtained suspension was then filtered, and the filtrate added to a large excess of cold hexane to precipitate the polymer. This operation was repeated twice and final precipitated polymer washed with diethyl ether. The recovered yellow polymer was then dried under vacuum for several hours.
End functionalized PEO-PTTC and PEO-DTTC were obtained with functionalities higher than 89% as determined by 1H NMR.
Partitioning of PEO-RAFT between water and styrene
A mixture of styrene, water and PEO-RAFT of the same composition as in Latex 1 recipe (see Table 1) was vigorously stirred at 75 °C for 20 min, and then left at the same temperature until complete separation of styrene and water. The amount of emulsifier in the aqueous phase was determined by gravimetric analysis, and the amount in styrene calculated by mass balance.| Expt | PEO-RAFT | [PEO-RAFT]/mol L−1em | Time/h | Conv (%) | D p b/nm–Poly | Np/mL−1Latex |
|---|---|---|---|---|---|---|
| a All experiments were carried out with 10 wt% of styrene; [AIBN] = 9 × 10−3 (mol L−1em); hexadecane: 5.2 wt%/styrene; T = 75 °C. b Obtained by dynamic light scattering. c Latex 1: 23.5 h – 100% conversion; Latex 2: 22.5 h – 100% conversion; Latex 3: 25 h – 86% conversion. | ||||||
| Latex 1 | PEO-PTTC | 2.13 × 10−3 | 7.0 | 76c | 440–0.25 | 2.3 × 1012 |
| Latex 2 | PEO-DTTC | 2.30 × 10−3 | 6.5 | 53c | 362–0.28 | 4.2 × 1012 |
| Latex 3 | PEO-DTTC | 4.15 × 10−3 | 7.0 | 64c | 371–0.28 | 3.4 × 1012 |
| Latex 4 | PEO-PTTC | 4.22 × 10−3 | 6.0 | 56 | 300–0.06 | 6.6 × 1012 |
| Latex 5 | PEO-PTTC | 8.50 × 10−3 | 7.0 | 92 | 203–0.08 | 2.2 × 1013 |
Miniemulsion polymerization procedure
Batch miniemulsion polymerizations of styrene were performed at 75 °C in a three-necked round bottom flask equipped with a condenser and a nitrogen inlet. Styrene was first mixed with hexadecane and AIBN as inititator. This organic phase was then added to the aqueous phase (water and PEO-RAFT) under vigorous stirring. After 15 min, the resulting mixture was ultrasonicated (750W Vibracell 75042, amplitude 90%) for two minutes. The obtained stable miniemulsion was then transferred to the round-bottom flask and deoxygenated by purging with nitrogen for 30 min at room temperature. The introduction of the round-bottom flask into the preheated oil bath corresponded to time zero of the polymerization. The regular withdrawal of samples allowed us to follow the conversion of monomer as a function of time and the evolution of molar masses and molar mass distributions as a function of monomer conversion. Table 1 shows the experimental conditions for the various miniemulsion polymerizations carried out in this study.Characterization techniques
Monomer consumption was followed by gravimetric analysis of samples withdrawn from the polymerization medium at different times. The size of the monomer droplets or of the particles (hydrodynamic diameter) was measured by dynamic light scattering (Zetasizer HS1000 from Malvern Instruments), and the data were collected using the fully automatic mode of the Zetasizer system. The mean size distribution, Poly, is a dimensionless measure of the distribution broadness. For a monodisperse sample, the Poly value should theoretically be zero. In practice, a Poly value for a “monodisperse” latex lies between 0 and 0.05. The poly value can be considered to have lost its significance for values above 0.15, but below a value of 0.5 useful comparisons between samples can be made.The number of droplets, Nd, or particles, Np (mL−1Latex), is calculated using the diameter obtained from DLS (either Dd or Dp, nm) according to eqn (1), with τ the solids content of the dispersed phase (comprising the monomer and polymer present for a given conversion and expressed in g mL−1Latex), and ρ (g cm−3) the density of the particles (taking into account the conversion).
 | (1) |
1H NMR measurements were carried out on a Bruker DRX 300 spectrometer using deuterated chloroform (CDCl3) as solvent, at room temperature. The chemical shift scale was calibrated relative to tetramethylsilane peak used as reference. Molar masses and molar mass distributions of the dried polymers were determined by size exclusion chromatography (SEC) using a modular system comprising a Jasco PU-2080 Plus pump and an autosampler 717Plus (Waters). Three columns [two PLgel 5 μm Mixed C (300 × 7.5 mm2) and one PLgel 5 μm 500 Å (300 × 7.5 mm2)] thermostated at 40 °C were used with THF as eluent at a flow rate of 1 mL min−1. Waters 410 refractometer was used for detection and molar masses were calculated on the basis of a calibration curve using polystyrene standards.
Results and discussion
In the present study two PEO-based macroRAFT agents namely PEO-PTTC and PEO-DTTC (Scheme 1) were used as both control agent and stabilizer for the miniemulsion polymerization of styrene in water. These PEO-based trithiocarbonates chain transfer agents were carrying tertiary reinitiating R group and either a thiopropyl (PEO-PTTC) or thiododecyl group (PEO-DTTC) as Z group, respectively (Scheme 1). They were compared to a PEO-based dithiobenzoate (PEO-DB) employed in our previous investigations1 to mediate the polymerization of styrene miniemulsions. In this study,1 we showed that stable PS particles sterically stabilized by the PEO segments could be obtained. However, although molar masses increased with conversion, rather broad molar mass distributions were observed. This was ascribed to the presence of different populations of controlled chain length arising from the partitioning of PEO-RAFT between the aqueous phase, the droplets and the water/droplets interface. This also led to a loss of the miniemulsion features with Np/Nd well below the expected value of 1. Complex mechanisms induced by PEO-DB partitioning were indeed corroborated by the presence of holes observed by transmission electron microscopy when PEO-DB concentration was increased, and were likely to arise from the formation of buried PEO-b-PS block copolymers. As the localization of the macroRAFT agent appeared to be of paramount importance for controlling both the polymerization process and the colloidal stability of the system, we envisioned that the use of PEO macroRAFT agent end capped with hydrophobic alkyl chains such as PEO-PTTC and PEO-DTTC would be less prone to partition between water and the organic phase and thus be better candidates to achieve controlled miniemulsion polymerization of styrene.Influence of the structure of the PEO-RAFT agent
In the first series of experiments, the influence of the structure of the macroRAFT agent was evaluated (Latexes 1–2, Table 1). As shown in Fig. 1a, the use of PEO-PTTC or PEO-DTTC did not significantly impact the conversion versus time profile with however a slightly faster polymerization for the miniemulsion polymerization carried out in the presence of PEO-PTTC (ca. 50% conversion in 6.5 h for PEO-DTTCversus 76% in 7 h for PEO-PTTC). It is worth noting here the absence of inhibition period contrary to what is often observed in macroRAFT mediated batch emulsion polymerization.15–17 Whatever the used PEO-RAFT, full conversion could be achieved if the polymerization was allowed to proceed for longer times (i.e. 22 h). The obtained latexes were stable although exhibiting quite broad particle size distribution (Poly close to 0.3, Table 1). In each case, the molar masses increased with conversion in agreement with a controlled polymerization process (Fig. 1b). The discrepancy observed between the theoretical (without taking into account the initiator derived chains) and experimental molar mass values may be related to the PS calibration and/or the high amount of initiator used compared to PEO-RAFT ([PEO-trithiocarbonate]/[AIBN] = 1/4). This ratio was originally fixed by previous experiments performed with PEO-DB that showed that AIBN concentrations which were too low (i.e. [PEO-DB]/[AIBN] = 4) did not lead to an efficient nucleation.1 A better fit of experimental molar mass values could be obtained by taking into account initiator derived chains (Fig. 1b) using the following equation: | (2) |
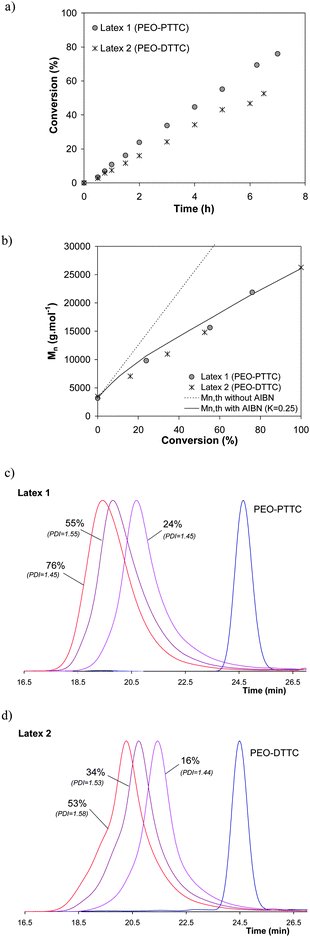 | ||
| Fig. 1 Influence of the structure of poly(ethylene oxide)-based macromolecular agents for reversible addition-fragmentation chain transfer (PEO-RAFT) for the PEO-RAFT mediated miniemulsion polymerization of styrene, initiated by 2,2′-azobis(isobutyronitrile) (AIBN) at 75 °C (Latex 1: PEO-PTTC; Latex 2: PEO-DTTC). Evolution of (a) monomer conversion vs. time, (b) number average molar masses (Mn) with conversion, (c,d) SEC chromatograms with conversion for Latex 1 and Latex 2. | ||
These results are in favor of a localization of the PEO-PTTC and PEO-DTTC at the water/monomer droplet interface or/and in the monomer droplet rather than a strong partitioning of the PEO-RAFT between the different phases as previously observed for PEO-DB.1 To further investigate this point, we determined under our miniemulsion conditions the partitioning of PEO-DTTC and PEO-PTTC between water and styrene at 75 °C and found that only 23 wt% of PEO-DTTC and 13 wt% of PEO-PPTC remained in the aqueous phase whereas 46 wt% of PEO-DB was located in water.1 This corroborates the quality of the control and the PDI observed when using the three PEO-RAFT. In addition, the observed difference between PEO-DTTC and PEO-PTTC may be rationalized by the possible formation of micelles in water. A critical aggregation concentration (CAC) of 0.5 mM was determined by Rieger et al.15 for PEO-DTTC. The presence of a thiopropyl Z group in PEO-PTTC, which is less hydrophobic than the thiododecyl Z group in PEO-DTTC, may lead to a higher CAC value for PEO-PTTC (not determined) and thus may disfavor the presence of PEO-PTTC in water either solubilized or in the form of micelles. It is worth mentioning that, while micelles would certainly make the secondary nucleation more severe, reasonably high concentrations of macroRAFT agent in the aqueous phase, even at sub-CAC concentrations, could contribute to homogeneous nucleation. Additionally, although the initial concentrations of the macroRAFT agents were the same for each experiment, the interpretation of the resulting data must take into account the fact that the starting styrene miniemulsions did not exhibit the same monomer droplet size (data not shown) although they were prepared under the same experimental conditions.
Even if very interesting results were obtained in terms of control of the molar masses, the system did not strictly speaking follow the trends of a miniemulsion polymerization (particle size not constant vs. conversion and Np/Nd varying with conversion). Therefore, in an attempt to obtain colloidal features of a miniemulsion polymerization while maintaining a good control over the molar masses, higher initial concentrations of either PEO-DTTC or PEO-PTTC were used in the next series of experiments.
Influence of PEO-RAFT concentration
The first considered trithiocarbonate–based PEO-RAFT was PEO-DTTC which carries a thiododecyl group as Z group. Using a twofold initial concentration under otherwise identical experimental conditions (Latex 4), slightly higher conversions were obtained (Fig. 2a). The final particle size and Np were not strongly impacted by the initial PEO-RAFT concentration (Table 1). The evolution of particle size and Np/Ndversus conversion clearly shows in both cases the occurrence of secondary nucleation (Fig. 2b and 2c). The higher the initial PEO-DTTC concentration was, the more important this phenomenon. These results can be interpreted by considering the critical agregation concentration value of PEO-DTTC (0.5 mM).15 Given the initial PEO-RAFT concentrations (Latex 3: 5 × CAC, Latex 4: 9 × CAC), the aqueous phase is likely to contain PEO-RAFT micelles, allowing a secondary nucleation to occur, giving rise to the formation of small particles originally stabilized by PEO-DTTC and then by PEO-b-PS-DTTC upon conversion of styrene. Consequently a higher particle number than expected was observed. In both cases, the molar masses increased with conversion and agreed well with the theoretical values (Fig. 2d). As expected in a controlled radical polymerization, using a twofold concentration of PEO-DTTC led to lower molar masses and accordingly induced a narrowing of the molar mass distribution as illustrated in Fig. 2e (PDI decreased from 1.6 to 1.5).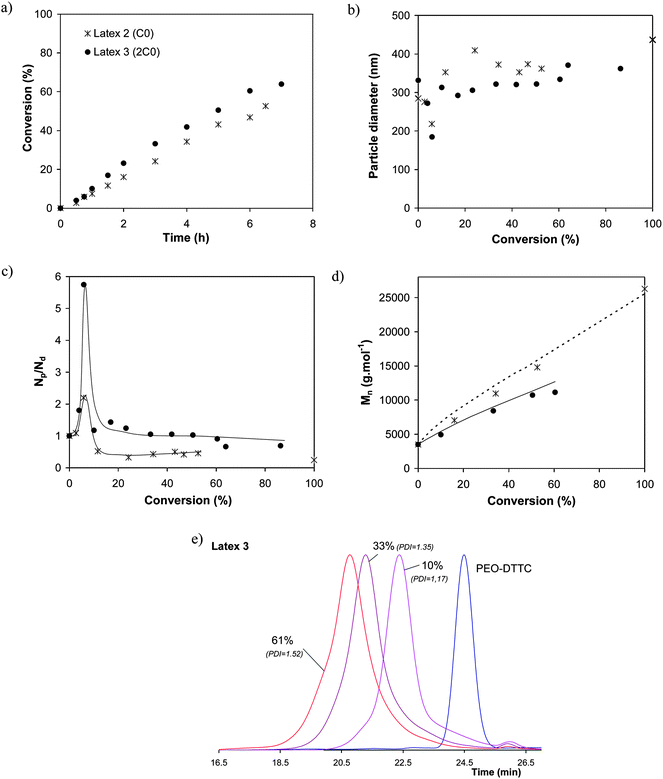 | ||
| Fig. 2 Influence of PEO-DTTC concentration (2.3 × 10−3 mol L−1em (Latex 2); 4.15 × 10−3 mol L−1em (Latex 3)) for the PEO-RAFT mediated miniemulsion polymerization of styrene at 75 °C (see Table 1 for detailed experimental conditions). Evolution of (a) monomer conversion vs. time, (b) particle diameter vs. conversion, (c) ratio of the number of particles (Np) to the number of initial droplets (Nd) vs. conversion, (d) number average molar mass (Mn) vs. conversion (solid and dotted lines correspond to theoretical molar mass values calculated with eqn (2) and K = 0.25) and (e) SEC chromatogramsvs. conversion for Latex 3. | ||
In a second series of experiments the concentration of PEO-PTTC, which is comparable to PEO-DTTC but possesses a shorter hydrophobic alkyl chain, was varied. When increasing the PEO-PTTC concentration (Latex 1, 4 and 5 in Table 1), although the starting monomer droplet size was similar for the three experiments, higher conversion were observed particularly for Latex 5 (4C0) (Fig. 3a). Consistently, the final particle size decreased and the final number of particles Np increased when going from Latex 1 to Latex 5, showing that the number of polymerization loci was increased (Table 1). With the lowest concentration of PEO-PTTC (Latex 1, C0), some coalescence was observed as the particle size increased and the ratio of Np/Nd decreased with conversion (Fig. 3b and 3c). This clearly indicates that no secondary nucleation occurred and that PEO-PTTC was thus mainly located at the surface of or in the monomer droplets ensuring a good control of the polymerization. The concentration was however too low to avoid coalescence (also displayed by the higher Poly values). Unfortunately, increasing the concentration of PEO-PTTC (Latexes 4 and 5) led to occurrence of secondary nucleation due to the possible presence of micelles of PEO-PTTC in water (Fig. 3c). Consequently, these systems do not strictly speaking display the expected colloidal features of a miniemulsion. Nevertheless, it is worth noting that the particle size distribution was improved in these two experiments with Poly value lower than 0.1 (Latexes 4 and 5, Table 1).
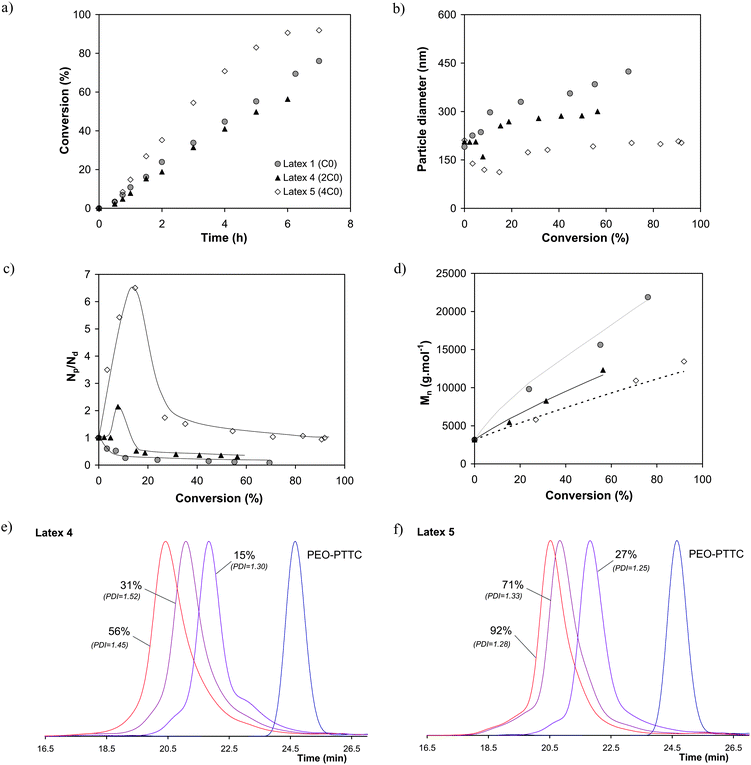 | ||
| Fig. 3 Influence of PEO-PTTC concentration (2.13 × 10−3 mol L−1em (Latex 1); 4.22 × 10−3 mol L−1em (Latex 4) and 8.50 × 10−3 mol L−1em (Latex 5)) for the PEO-RAFT mediated miniemulsion polymerization of styrene at 75 °C (see Table 1 for detailed experimental conditions). Evolution of (a) monomer conversion vs. time, (b) particle diameter vs. conversion, (c) ratio of the number of particles (Np) to the number of initial droplets (Nd) vs. conversion, (d) number average molar mass (Mn) vs. conversion (solid and dotted lines correspond to theoretical molar mass values calculated with eqn (2) and K = 0.25), (e,f) SEC chromatogramsvs. conversion for Latex 5 and 6 (see Fig. 1c for Latex 1). | ||
Considering the same initial PEO-RAFT concentrations, the smaller monomer droplet size obtained for PEO-PTTC (around 190 nm) compared to PEO-DTTC (around 300 nm) indicates that, as foreseen, the chemical structure of PEO-PTTC ensures a more efficient anchoring at the monomer droplet/water interface. As mentioned above in the text, an improved control on the molar mass was observed when switching from PEO-DTTC to PEO-PTTC. As shown in Fig. 3d, an increase of the molar masses with conversion in good agreement with theoretical molar mass values evolution was observed in all the cases and the final PDI was lower than in the case of PEO-DTTC (Fig. 3e and 3f). Upon increasing PEO-PTTC concentration, a small shoulder on the high molar mass side of the SEC trace appeared (Fig. 3e and 3f), that may be the result of termination reaction by coupling favoured in the case of shorter targeted chains. However, the control was further improved: the final PDI was 1.45 for Latexes 1 and 4, and decreased to 1.3 for Latex 5, showing that the final particles were composed of well-defined PEO-b-PS block copolymer chains
Conclusions
MacroRAFT agents based on PEO and carrying trithiocarbonate chain ends were synthesized and used as both control agent and stabilizer in miniemulsion polymerization of styrene. The nature of the chain ends (thiopropyl or thiododecyl) in the trithiocarbonate functionalized PEO (PEO-PTTC and PEO-DTTC, respectively) helped to localize the PEO chains at the water/monomer droplets interface. As a result, much narrower molar mass distributions (PDI = 1.6) were obtained compared to the polymerization mediated by a dithiobenzoate functionalized PEO (PEO-DB, PDI = 1.9). Increasing the concentration of PEO-DTTC improved the control and stable latex (ca. 360 nm) composed of well defined polymer chains (PDI = 1.5) was formed. However, the propensity of PEO-DTTC to form micelles in water impeded the system to obey to a strict miniemulsion system as secondary nucleation occurred. Although not suppressed, this phenomenon was attenuated by the use of PEO-PTTC which provided stable latexes with improved particle size distribution (Poly lower than 0.1) together with a better control over the molar masses (PDI = 1.3) indicating that the particles were constituted of well-defined PEO-b-PS polymer chains. These results were attributed to a more efficient anchoring of PEO-PTTC at the monomer droplet or particle/water interface showing the crucial role of the macroRAFT agent structure in these systems.Acknowledgements
Ana Cenacchi Pereira (LCPP Team) is acknowledged for her help in the PEO-PTTC synthesis.References
- A. M. Dos Santos, T. Le Bris, C. Graillat, F. D'Agosto and M. Lansalot, Macromolecules, 2009, 42, 946 CrossRef.
- P. B. Zetterlund, Y. Kagawa and M. Okubo, Chem. Rev., 2008, 108, 3747 CrossRef CAS.
- M. F. Cunningham, Prog. Polym. Sci., 2008, 33, 365 CrossRef CAS.
- B. Charleux, F. D'Agosto and G. Delaittre, Adv. Polym. Sci., 2010 DOI:10.1007/12_2010_64 , in press.
- C. Barner-Kowollik, Handbook of RAFT Polymerization. Wiley-VCH: Weinheim, 2008 Search PubMed.
- M. J. Monteiro and J. de Barbeyrac, Macromolecules, 2001, 34, 4416 CrossRef CAS.
- A. Guyot and K. Tauer, Adv. Polym. Sci., 1994, 111, 43–65 CAS.
- A. Guyot, K. Tauer, J. M. Asua, S. Van Es, C. Gauthier, A. C. Hellgren, D. C. Sherrington, A. Montoya-Goni, M. Sjoberg, O. Sindt, F. Vidal, M. Unzue, H. Schoonbrood, E. Shipper and P. Lacroix-Desmazes, Acta Polym., 1999, 50, 57 CrossRef CAS.
- A. Guyot and K. Tauer, Polymerizable and Polymeric Surfactants, in Reactions and Synthesis in Surfactants Systems, ed. J. Texter, Marcel Dekker, New York, 2001, vol. 100, XXVIII, pp. 547 Search PubMed.
- C. J. Ferguson, R. J. Hughes, D. Nguyen, B. T. T. Pham, R. G. Gilbert, A. K. Serelis, C. H. Such and B. S. Hawkett, Macromolecules, 2005, 38, 2191 CrossRef CAS.
- C. J. Ferguson, R. J. Hughes, B. T. T. Pham, B. S. Hawkett, R. G. Gilbert, A. K. Serelis and C. H. Such, Macromolecules, 2002, 35, 9243 CrossRef CAS.
- E. Sprong, J. S. K. Leswin, D. J. Lamb, C. J. Ferguson, B. S. Hawkett, B. T. T. Pham, D. Nguyen, C. H. Such, A. K. Serelis and R. G. Gilbert, Macromol. Symp., 2006, 231, 84 CAS.
- D. E. Ganeva, E. Sprong, H. de Bruyn, G. G. Warr, C. H. Such and B. S. Hawkett, Macromolecules, 2007, 40, 6181 CrossRef CAS.
- J. Božović-Vukić, H. T. Manon, J. Meuldijk, C. Koning and B. Klumperman, Macromolecules, 2007, 40, 7132 CrossRef CAS.
- J. Rieger, F. Stoffelbach, C. Bui, D. Alaimo, C. Jérôme and B. Charleux, Macromolecules, 2008, 41, 4065 CrossRef CAS.
- J. Rieger, G. Osterwinter, C. Bui, F. Stoffelbach and B. Charleux, Macromolecules, 2009, 42, 5518 CrossRef CAS.
- J. Rieger, W. Zhang, F. Stoffelbach and B. Charleux, Macromolecules, 2010, 43, 6302 CrossRef CAS.
- S. Boissé, J. Rieger, K. Belal, A. Di-Cicco, P. Beaunier, M.-H. Li and B. Charleux, Chem. Commun., 2010, 46, 1950 RSC.
- U. El-Jaby, M. Cunningham and T. F. L. McKenna, Ind. Eng. Chem. Res., 2009, 48, 10147 CrossRef CAS.
- M. Antonietti and K. Landfester, Prog. Polym. Sci., 2002, 27, 689 CrossRef CAS.
- J. M. Asua, Prog. Polym. Sci., 2002, 27, 1283 CrossRef CAS.
- F. J. Schork, Y. Luo, W. Smulders, J. P. Russum, A. Butté and K. Fontenot, Adv. Polym. Sci., 2005, 175, 129 CAS.
- B. T. T. Pham, D. Nguyen, C. J. Ferguson, B. S. Hawkett, A. K. Serelis and C. H. Such, Macromolecules, 2003, 36, 8907 CrossRef CAS.
- Y. Luo and H. Gu, Macromol. Rapid Commun., 2006, 27, 21 CrossRef CAS.
- Y. Luo and H. Gu, Polymer, 2007, 48, 3262 CrossRef CAS.
- F. Lu, Y. Luo and B. Li, Macromol. Rapid Commun., 2007, 28, 868 CrossRef CAS.
- G. Bouhadir, N. Legrand, B. Quiclet-Sire and S. Z. Zard, Tetrahedron Lett., 1999, 40, 277 CrossRef CAS.
- S. H. Thang, B. Y. K. Chong, R. T. A. Mayadunne, G. Moad and E. Rizzardo, Tetrahedron Lett., 1999, 40, 2435 CrossRef CAS.
Footnote |
| † Footnote: This paper is part of a Polymer Chemistry issue highlighting the work of emerging investigators in the polymer chemistry field. Guest Editors: Rachel O'Reilly and Andrew Dove. |
| This journal is © The Royal Society of Chemistry 2011 |
