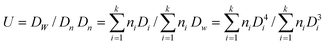Synthesis of monodisperse SiO2/P(DVB-SO3H)/SiO2/P(DVB-SO3H) tetra-layer polyelectrolyte microspheres and the corresponding hollow P(DVB-SO3H) polyelectrolyte microspheres with a shell-in-shell structure
Min
Ji
,
Hongli
Liu
and
Xinlin
Yang
*
Key Laboratory of Functional Polymer Materials, the Ministry of Education, Institute of Polymer Chemistry, Nankai University, Tianjin, 300071, China. E-mail: xlyang88@nankai.edu.cn; Fax: +86-22-23503510; Tel: +86-22-23502023
First published on 12th October 2010
Abstract
The hollow poly(divinylbenzyl sulfonic acid) (P(DVB-SO3H)) polyelectrolyte (PE) microspheres with a shell-in-shell structure were prepared by selective etching of the silica core and third-layer with hydrofluoric acid from the corresponding SiO2/P(DVB-SO3H)/SiO2/P(DVB-SO3H) tetra-layer PE microspheres, which were synthesized by a combination of the controlled sol–gel hydrolysis of tetraethylorthosilicate (TEOS) for the preparation of silica core and third-layer, and the distillation precipitation polymerization of divinylbenzene (DVB) in acetonitrile for the construction of polydivinylbenzene (PDVB) layer with subsequent surface modification of the phenyl group to afford P(DVB-SO3H) PE second and outer shell-layer. The silica/poly(divinylbenzylsulfonate 4-vinylpyridinium) (SiO2/P(DVB-SO3−VPyH+) core-shell PE microspheres were prepared by the neutralization of 4-vinylpyridine (4-VPy) with sulfonic acid groups on the surface of SiO2/P(DVB-SO3H) core-shell microspheres, which were synthesized by the distillation precipitation polymerization of DVB in the presence of 3-(methacryloxy)propyl trimethacrylate (MPS)-modified silica nanoparticles as seeds to afford SiO2/PDVB core-shell microspheres with subsequent sulfonation of the phenyl groups of PDVB shell in concentrated sulfuric acid. SiO2/P(DVB-SO3H)/SiO2/P(DVB-SO3H) tetra-layer PE microspheres were synthesized by sulfonation of SiO2/P(DVB-SO3−VPyH+)/SiO2/PDVB tetra-layer microspheres in concentrated sulfuric acid, which were prepared by distillation precipitation polymerization of DVB with MPS-modified SiO2/P(DVB-SO3−VPyH+)/SiO2 tri-layer microspheres as seeds via coating of silica-layer onto SiO2/P(DVB-SO3−VPyH+) nanoparticles. The morphology and properties of the resultant microspheres, and the corresponding hollow P(DVB-SO3H) microspheres with a shell-in-shell structure were characterized by transmission electron microscopy (TEM), Fourier transform spectroscopy (FT-IR), and X-ray photoelectron spectroscopy (XPS).
Introduction
The development of materials with a novel structure has been a fundamental focus of chemical research, which promotes the advances in both academic and industrial areas. Recently, organic/inorganic hybrid materials have attracted increasing interest from materials scientists due to the combination of the various functional groups from the polymeric components and the advantages of the thermally stable and robust inorganic substrate.1,2 Among them, silica/polymer hybrid/composite particles with various interesting morphologies, including silica core/organic shell,3 organic core/silica shell,4 raspberry-like,5,6 snowman-like,7 daisy-shaped and multipod-like,8 and raisinbun-like,9 have been afforded by different techniques. The synthesis of these silica/polymer hybrid/composite particles can be generally classified as two categories: the self-assembly of the resultant silica and polymer particles via physical or physicochemical interaction, and the direct polymerization of monomers on the surface of silica particles. Bourgeat-Lami et al.10–12 synthesized silica/polymer hybrids with nanospheres as seeds by emulsion polymerization, in which the reactive vinyl groups were introduced by 3-(trimethoxysilyl)propyl methacrylate (MPS)-modification on the surface of silica.The inorganic/polymer hierarchical multi-layer microspheres have attracted considerable attention as materials for drug-delivery systems, diagnostic, coating and catalysis, originating from their novel and excellent properties, such as mechanical, chemical, electrical, rheological, plastic, optical, magnetic, and catalytic. These multi-layer microspheres can be facilely tuned by altering the size, monodispersity, building blocks, composition and morphology of each-layer.13–17 In these materials, the interaction between or among the hierarchical layers with various compositions and thickness has provided unique characteristics comparing to those of the single-composition microspheres. The tetra-layer concentric nanoshell plasmonic structures behaved new resonances for the analysis of the plasma resonant modes and energy controllable hybrid modes via precise tuning the thickness of the designed shell-layer and the total diameter of the whole particles.18 The multi-layer polymer microspheres with quantum dots (QDs) loaded core and alternating layers with low and refractive indices were used as optical resonators via increasing the refractive index contrast between the alternating layers and excluding polymers.19 For these applications, it is essential to tune the controllable thickness of each layer and overcome the in-compatibility between the connecting layers. Therefore, it is highly necessary to develop a facile method for the construction of hierarchical multi-layer microspheres with adjustable dimension, building components and structure.
Hollow structures, especially those with complex components and double-shelled or multi-shelled structures, have attracted concern associated with their unique properties and potential applications.20–23 Different from the single-layered hollow spheres, the concentric multi-layered hollow spheres containing various composition and complex structures exhibit unique performance. Double-walled polymethacrylic acid/poly(N-isopropyl acrylamide) (PMAA/PNIPAAm) concentric hollow polymeric microspheres with unique, thermal and pH dual-stimuli responsive properties as the controlled drug-release carriers.20 The polymeric capsules possessing shell-in-shell structures showed the higher permeability and remarkable better mechanical stability than single-layer capsules.21,22 Eccentric sphere-in-sphere titanium (TiO2) hollow spheres23 and SiO2/TiO2 microspheres24 can scatter UV-light more efficiently inside the cavity so as to greatly enhance the photocatalytic activity. The preparation of the hollow microspheres with a shell-in-shell structure can be generally classified into two categories: direct polymerization both on the interior and exterior shell-surfaces of the hollow inorganic or polymer templates with channels or mesoporous shell25,26 and layer-by-layer (LbL) technique.21
Polyelectrolyte (PE) polymer has been used to modify the surface properties of various materials and found wide applications, including contact lenses, modification of the surfaces for titanium implants, wound dressings for serious burns, separation membranes for gases and dissolved species.27–30
In our previous work, we reported the synthesis of poly(ethyleneglycol dimethacrylate-co-methacrylic acid@poly(ethyleneglycol dimethacrylate-co-4-vinyl pyridinium benzylchloride)/silica/polymer (P(EGDMA-co-MAA)@P(EGDMA-co-VPyBzCl)/SiO2) tetra-later microspheres and the subsequent development of hollow polymer microspheres with movable PE core and functional groups on the shell-layer via ethching of the sandwiched silica layer in hydrofluoric acid (HF).31 Herein, we describe the facile synthesis of monodisperse hollow poly(divinybenzyl sulfonic acid) (P(DVB-SO3H)) PE microspheres with a shell-in-shell structure via the selective etching of silica species from the corresponding (SiO2/P(DVB-SO3H)/SiO2/P(DVB-SO3H) tetra-layer PE microspheres in HF, which were prepared by the combination of the sol–gel hydrolysis of TEOS for the formation of silica components and the distillation precipitation polymerization of DVB in acetonitrile for coating of PDVB layers with subsequent surface modification for the formation of PE second and outer shell-layers.
Experimental
Chemicals
Ammonia (25 wt%, aqueous solution) was obtained from Tianjin Dongzheng Fine Chemical Reagent Factory, China. Tetraethylorthosilicate (Si(OEt)4, TEOS) and 3-(methacryloxy)propyl trimethoxysilane (MPS) were supplied by Aldrich, in which was later purified by vacuum distillation. Divinylbenzene (DVB, 80% DVB isomers) was provided as technical grade by Shengli Chemical Technical Factory, Shandong, China, which was washed with 5 wt% aqueous sodium hydroxide (NaOH) and water, then dried over anhydrous magnesium sulfate prior to utilization. Benzoyl peroxide (BPO) was available from Chemical Factory of Nankai University and recrystallized from methanol. 4-Vinylpyridine (4-VPy) was obtained from Alfa Aesar and purified by vacuum distillation. Concentrated sulfuric acid (96 wt%) was provided by Tianjin Yingda Reagent II Co.). Acetonitrile (analytical grade, Tianjin Chemical Reagent II Co.) was dried over calcium hydride and purified by distillation before use. Hydrofluoric acid (HF, containing 40 wt% of HF) was available from Tianjin Chemical Reagent Institute. All the other reagents were used as received without any further purification.Synthesis of monodisperse SiO2/P(DVB-SO3−VPyH+) core-shell PE microspheres
Two-semibatch mode distillation precipitation polymerization was used for the synthesis of monodisperse silica/polydivinylbenzene (SiO2/PDVB) core-shell microspheres with smooth surface. The first-semibatch distillation precipitation polymerization of DVB for the synthesis of SiO2/PDVB core-shell microspheres was very similar to the procedure reported in our previous work,32 in which the mass ratio of inorganic MPS-modified silica seeds to DVB monomers was 1/2 (0.40 g seeds and 0.80 mL of DVB) together with 0.016 g of BPO initiator (2 wt% relative to the DVB monomers) in 160 mL of acetonitrile for the polymerization. For the second-semibatch polymerization, 1.60 mL of DVB monomer together with 0.40 g of SiO2/PDVB hybrids from the first-semibatch polymerization as seeds and 0.032 g of BPO initiator were well-dispersed in 160 mL of acetonitrile suspension in a 250 mL two-necked round-flask. The flask attaching with a fractionating column, Liebig condenser, and receiver was submerged in a heating mantle. The reaction mixture was heated from ambient temperature to the boiling state within 10 min and the reaction system was kept under the refluxing state for a further 20 min. The polymerization was ended after 80 mL of acetonitrile was distilled off the reaction system within 80 min. After the second-semibatch polymerization, the resultant SiO2/PDVB core-shell microspheres were purified by repeating centrifugation, decantation, and resuspension in acetone with ultrasonic irradiation for three times. The SiO2/PDVB hybrids were dried in a vacuum oven at 50 °C until a constant weight was reached.The resultant 0.40 g SiO2/PDVB core-shell microspheres were sulfonated by 5.0 mL of concentrated sulfuric acid (96 wt%) at 40 °C for 4.5 h to get SiO2/poly(divinylbenzyl sulfonic acid) (SiO2/P(DVB-SO3H)) core-shell PE microspheres as following. After the modification, the anionic SiO2/P(DVB-SO3H) PE microspheres were separated by vacuum filtration over a G-5 sintered glass filter and with subsequent washing with de-ionized water until the filtrate reached the pH of 7. The anionic SiO2/P(DVB-SO3H) core-shell PE hybrids were dried in a vacuum oven at 50 °C until a constant weight was reached.
Monodisperse pyridinium silica/poly(divinylbenzyl pyridinium sulfonate) (SiO2/(P(DVB-SO3−VPyH+)) PE microspheres were prepared as follows: 0.40 g of SiO2/P(DVB-SO3H) PE microspheres were dispersed in 50 mL of 4-VPy solution in acetonitrile (CH3CN, containing about 0.40 mL, 3.6 mmol 4-VPy). The neutralization was carried out at room temperature for 24 h with mild stirring. After the neutralization, the resultant pyridinium SiO2/P(DVB-SO3−VPyH+) PE microspheres were separated by vacuum filtration over a G-5 sintered glass filter and successively washed with ethanol three times. The pyridinium SiO2/PE core-shell microspheres were separated by vacuum filtration over a G-5 sintered glass filter and successively washed with ethanol for three times. The pyridinium SiO2/P(DVB-SO3−VPyH+) core-shell PE microspheres were dried in a vacuum oven at 50 °C until a constant weight was reached.
Preparation of MPS-modified SiO2/P(DVB-SO3−VPyH+)/SiO2 tri-layer composite microspheres
Monodisperse SiO2/P(DVB-SO3−VPyH+)/SiO2 tri-layer composite microspheres were synthesized by coating an outer-layer of silica onto pyridinium SiO2/P(DVB-SO3−VPyH+) core-shell PE microspheres via a modified Stöber sol–gel process: 0.20 g of SiO2/P(DVB-SO3−VPyH+) template particles, 1.0 mL of TEOS and 4.80 mL of ammonium hydroxide were consecutively added into a water–ethanol (40/320 mL) mixture with vigorous stirring. The sol–gel procedure was allowed to proceed for 12 h at room temperature. The resultant MPS-modified SiO2/P(DVB-SO3−VPyH+)/SiO2 tri-layer composite microspheres were afforded by introducing 2.0 mL of MPS into the above mentioned SiO2/P(DVB-SO3−VPyH+)/SiO2 tri-layer composite microspheres water–ethanol suspension with strong stirring and the reaction mixture was stirred further for 48 h at room temperature for complete modification. The resultant MPS-modified SiO2/P(DVB-SO3−VPyH+)/SiO2 tri-layer composite microspheres were purified by centrifugation, decantation and resuspension in ethanol three times to remove the excessive MPS and ammonia. The final SiO2/P(DVB-SO3−VPyH+)/SiO2 tri-layer composite microspheres were dried in a vacuum oven at 50 °C until a constant weight was reached.Synthesis of SiO2/P(DVB-SO3H)/SiO2/P(DVB-SO3H) tetra-layer PE microspheres
The monodisperse SiO2/P(DVB-SO3−VPyH+)/SiO2/PDVB tetra-layer microspheres were prepared by the two-semibatch mode distillation precipitation of DVB in acetonitrile with MPS-modified SiO2/P(DVB-SO3−VPyH+)/SiO2 tri-layer composites as templates. The procedure for this polymerization was very similar to that for the synthesis of monodisperse SiO2/PDVB core-shell microspheres by the second-semibatch polymerization as described, in which the mass ratio of SiO2/P(DVB-SO3−VPyH+)/SiO2 tri-layer composites to DVB monomers was designed as 1/2 in the first-semibatch as well as 1/4 in the second-semibatch polymerization and the BPO initiator was kept at 2 wt%.The sulfonation of SiO2/P(DVB-SO3−VPyH+)/SiO2/PDVB microspheres for the synthesis of SiO2/P(DVB-SO3H)/SiO2/P(DVB-SO3H) tetra-layer PE microspheres was very similar to the procedure for the modification of SiO2/PDVB core-shell microspheres as described above with concentrated sulfuric acid, in which 0.40 g of SiO2/P(DVB-SO3−VPyH+)/SiO2/PDVB microspheres were sulfonated in 5.0 mL of 96 wt% concentrated sulfuric acid.
Synthesis of monodisperse hollow P(DVB-SO3H) PE microspheres with a shell-in-shell structure
The resultant 0.25 g of SiO2/P(DVB-SO3H)/SiO2/P(DVB-SO3H) tetra-layer PE microspheres were immersed in 5.0 mL of 40 wt% HF aqueous solution with low frequency oscillating at room temperature for 6 h. The excessive HF and the formed SiF4 gas were expelled out of the reaction system by vacuum filtration over a G-5 sintered glass filter. The hollow P(DVB-SO3H) PE microspheres with shell-in-shell structure were purified by several centrifugation/washing cycles in de-ionized water until the pH of the suspension was 7. The resultant hollow P(DVB-SO3H) PE microspheres with a shell-in-shell structure were then dried in a vacuum oven at 50 °C until a constant weight was reached.All the syntheses of these microspheres were confirmed by several duplicate and triplicate experiments.
Characterization
The morphology, size and size distribution of silica core, SiO2/PDVB core-shell microspheres, SiO2/P(DVB-SO3H) PE microspheres, SiO2/P(DVB-SO3−VPyH+) PE microspheres, SiO2/P(DVB-SO3−VPyH+)/SiO2 tri-layer composite, SiO2/P(DVB-SO3−VPyH+)/SiO2/PDVB tetra-layer microspheres, and SiO2/P(DVB-SO3H)/SiO2/P(DVB-SO3H) tetra-layer PE microspheres, hollow P(DVB-SO3H) PE microspheres with shell-in-shell structure were determined by transmission electron microscopy (TEM, Tecnai G2 20S-TWIN). All the size and size distribution reflect the averages about 100 particles each, which are calculated according to the following formula:where U is the polydispersity index, Dn is the number-average diameter, Dw is the weight-average diameter, and Di is the particle diameters of the determined microparticles. The shell-thickness is calculated as half of difference between the number average diameter of core microspheres and that of core-shell microspheres.
Fourier-transform infrared spectra (FT-IR) were scanned over the range of 400–4000 cm−1 with potassium bromide pellet on a Bio-Rad FTS 135 FT-IR spectrometer.
X-Ray photoelectron spectroscopy (XPS) analysis was carried out with a PHI 5300 XPS surface analysis system (Physical Electronics, Edn Prairie, MN, US) using a Mg-Kα X-ray source operating at 250 W and 13 kV (hγ = 101253.6 eV). The electron binding energy of C1s (284.6 eV) is used as the internal standard.
Results and discussion
Scheme 1 illustrated the synthesis of SiO2/P(DVB-SO3H)/SiO2/P(DVB-SO3H) tetra-layer PE microspheres mainly through a four-stage reaction and the further development of hollow P(DVB-SO3H) PE microspheres with shell-in-shell structure via the selective etching components in aqueous HF solution from the tetra-layer PE microspheres. The SiO2/P(DVB-SO3H)/SiO2/P(DVB-SO3H) tetra-layer PE microspheres were prepared by the combination of the controlled hydrolysis of TEOS in ethanol–water (4/1) mixture to afford silica core and third-layer, and the distillation precipitation polymerization of DVB in acetonitrile with MPS-modified silica particles as seeds for coating of PDVB layer together with the subsequent surface modification of the PDVB shell-layers for the formation of PE layers.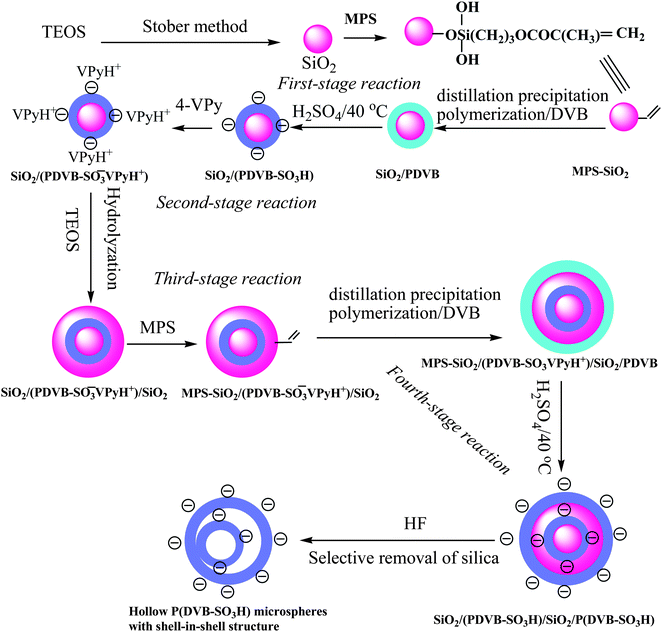 | ||
| Scheme 1 Synthesis of SiO2/P(DVB-SO3H)/SiO2/P(DVB-SO3H) tetra-layer polyelectrolyte microspheres and the corresponding hollow P(DVB-SO3H) polyelectrolyte microspheres with a shell-in-shell structure. | ||
Synthesis of monodisperse SiO2/P(DVB-SO3H) core-shell microspheres
The MPS-modified silica nanospheres were prepared by the controlled-hydrolysis of TEOS via the Stöber method to get silica nanoparticles with subsequent surface MPS modification in presence of ammonium hydroxide as catalyst in water–ethanol solvent according to the procedures in the literature.10,11 The TEM micrographs of silica and MPS-modified silica microspheres in Fig. 1A and 1B indicated that these nanoparticles had a spherical shape and smooth surface with average diameters of 224 and 228 nm, and a monodispersity index (U) of 1.022 and 1.008, respectively, as summarized in Table 1 (Entries A and B). The MPS-modification of the silica nanospheres was confirmed by the FT-IR spectrum in Fig. 2a with presence of the peaks at 1704 and 1638 cm−1 corresponding to the stretching vibration of carbonyl groups and vinyl groups of the MPS component. The residual vinyl groups on the PDVB polymer microspheres were essential for the growth of polymer microspheres for distillation precipitation polymerization of DVB,33 during which the newly formed oligomers and monomers were captured by the reactive vinyl groups. The incorporated vinyl groups on the surface of MPS-modified SiO2 nanospheres would permit the growth of the polymer shell onto the silica seeds by radical capture of the newly formed oligomers and monomers during the next-stage distillation precipitation polymerization of DVB to result in SiO2/PDVB core-shell hybrid microspheres.32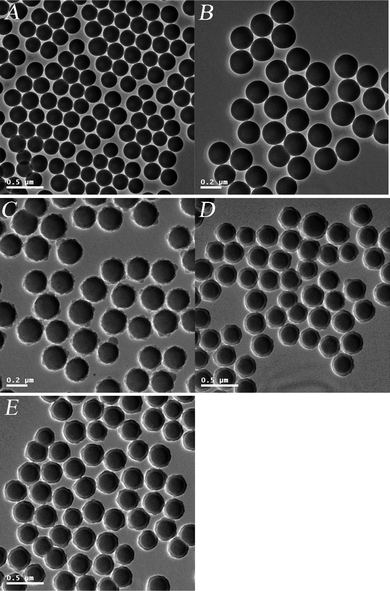 | ||
| Fig. 1 TEM micrographs: (A) SiO2 nanospheres; (B) MPS-modified SiO2 nanospheres; (C) SiO2/PDVB core-shell microspheres after the first semibatch polymerization; (D) SiO2/PDVB core-shell microspheres after the second semibatch polymerization; (E) SiO2/P(DVB-SO3H) core-shell PE microspheres. | ||
| Entry | A | B | C | D | E | F | G | H | I | J | K |
|---|---|---|---|---|---|---|---|---|---|---|---|
| D n (nm) | 224 | 226 | 249 | 301 | 300 | 300 | 393 | 431 | 511 | 510 | 525 |
| D w (nm) | 229 | 228 | 251 | 304 | 303 | 302 | 395 | 435 | 515 | 514 | 528 |
| U | 1.022 | 1.008 | 1.009 | 1.010 | 1.010 | 1.007 | 1.005 | 1.009 | 1.008 | 1.007 | 1.006 |
| Thickness of outer layer/nm | — | 1.0 | 11.5 | 37.5 | — | — | 46.5 | 19.0 | 59 | — | — |
 | ||
| Fig. 2 FT-IR spectra: (a) MPS-modified SiO2 nanospheres; (b) SiO2/PDVB core-shell microspheres; (c) SiO2/P(DVB-SO3H) core-shell PE microspheres; (d) SiO2/P(DVB-SO3−VPyH+) core-shell PE microspheres; (e) hollow P(DVB-SO3H) microspheres with a shell-in-shell structure. | ||
To obtain the SiO2/PDVB core-shell nanoparticles with monodispersion and a smooth surface, two-semibatch mode distillation precipitation polymerization was used for the synthesis of these core-shell hybrid microspheres. Fig. 1C shows the TEM micrograph of SiO2/PDVB microspheres after the first semibatch polymerization of DVB in the presence of MPS-modified SiO2 nanoparticles as seeds. The result shown in Fig. 1C indicates that these core-shell hybrid particles had slight rough surface, which may be due to the rigid of PDVB network much thin-layer of PDVB after the first-semibatch polymerization. The average diameter of the resultant SiO2/PDVB core-shell nanospheres was 249 nm with narrow-dispersity index (U) of 1.009 as shown in Table 1 (entry C), which implied that the PDVB shell-thickness was 11.5 nm after the first-semibatch polymerization. The TEM micrograph in Fig. 1D demonstrates that the SiO2/PDVB microspheres had a smooth surface and spherical shape having a typical core-shell structure with a deeper contrast of inorganic silica core and a lighter contrast PDVB shell-layer after the second-semibatch polymerization. The size of these SiO2/PDVB core-shell microspheres was 301 nm with a monodispersity index (U) of 1.010 (entry D, Table 1), which implied that the thickness of PDVB shell-layer was significantly increased from 11.5 to 37.5 nm during the second-semibatch polymerization. In such a two-semibatch distillation precipitation polymerization, monodisperse SiO2/PDVB core-shell microspheres with PDVB shell-thickness of ranging from 11.5 to 37.5 nm and smooth surface were synthesized, which was proven further by the FT-IR spectrum of SiO2/PDVB particles in Fig. 2b with presence of a new peak at 710 cm−1 corresponding to the typical absorption of the phenyl group of PDVB component as comparing to that of MPS-modified silica core in Fig. 2a.
The anionic SiO2/P(EVB-SO3H) core-shell PE microspheres were prepared by the sulfonation of the phenyl group of PDVB shell-layer with concentrated sulfuric acid. The TEM micrograph of SiO2/P(DVB-SO3H) PE microspheres in Fig. 1E indicated that these PE nanoparticles maintained their spherical shape and smooth surface with a deeper contrast of silica core and a lighter contrast of P(DVB-SO3H) PE shell-layer. The average diameter of SiO2/P(DVB-SO3H) core-shell PE microspheres was kept at 300 nm with a monodispersity index (U) of 1.010 as summarized in Table 1 (entry E), which was almost the same as those (Dn = 301 nm, U = 1.010) of SiO2/PDVB core-shell microspheres (entry D). All these results implied that the core-shell microspheres were physically stable and remained monodisperse during the sulfonation process for the synthesis of SiO2/P(DVB-SO3H) core-shell PE microspheres.
The successful sulfonation of SiO2/PDVB core-shell microspheres was confirmed further by the XPS spectra as shown in Fig. 3, in which the electronic binding energy of C1s (284.6 eV) was used as the internal standard. The spectrum in Fig. 3a of SiO2/PDVB core-shell microspheres had the strong peaks at 531.9 and 282.9 eV ascribing to the binding energy of O1s and C1s, respectively. For the XPS spectrum of SiO2/P(DVB-SO3H) core-shell PE microspheres with sulfonic acid surfaces in Fig. 3b, the strong peaks at 530.2 and 282.7 eV together with a weak peak at 163.4 eV were clearly observed, which were ascribed to the binding energy of O1s, C1s, and S2p, respectively. The atom concentration on the surface of the SiO2/P(DVB-SO3H) core-shell PE microspheres in Fig. 3b indicated that the S concentration on the surface of core-shell PE microspheres was calculated as high as 1.22% comparing to the S value of 0.0 for the SiO2/PDVB core-shell microspheres before sulfonation as shown in Fig. 3a. These results proved the successful synthesis of SiO2/P(DVB-SO3H) core-shell PE microspheres by the distillation precipitation polymerization of DVB in acetonitrile in the presence of MPS-modified SiO2 nanoparticles as seeds and the further sulfonation modification of the phenyl group on the PDVB shell-layer of the resultant SiO2/PDVB core-shell microspheres in concentrated sulfuric acid.
 | ||
| Fig. 3 XPS-spectra: (A) SiO2/PDVB core-shell microspheres; (B) SiO2/P(DVB-SO3H) core-shell PE microspheres; (C) SiO2/P(DVB-SO3−VPyH+) core-shell PE microspheres. | ||
Synthesis of SiO2/P(DVB-SO3−VPyH+)/SiO2 tri-layer composite microspheres
The Stöber method has been adopted to prepare polymer/silica core-shell particles by coating the surfaces of the monodisperse polystyrene (PSt) beads with uniform silica shells in the pH range of 10–12 to balance the nucleation and growth of silica sols.34 Monodisperse polymer/SiO2 core-shell microspheres were synthesized by coating of silica shell-layer onto the polymer core via a sol–gel process with the aid of hydrogen-bonding interaction35 and efficient electrostatic interaction31,36 between the surface of polymer core and the newly formed silica species.It is difficult to directly perform the uniform hydrolysis of TEOS on the strong negative surface of SiO2/P(DVB-SO3H) core-shell PE microspheres for the preparation of SiO2/PE/SiO2 tri-layer microspheres with smooth surface and controllable morphology due to the strong electrostatic repulsion between the anionic sulfonic acid groups on the surface of SiO2/P(DVB-SO3H) templates and the newly formed negative silica species during the sol–gel hydrolysis of TEOS. To overcome this problem, the sulfonic acid group was further modified with 4-VPy to form pyridinium species on the surface of SiO2/P(DVB-SO3−VPyH+) core-shell microspheres, which would have a suitable electrostatic interaction between the SiO2/PE core-shell particles and the newly formed silica-coating layer. The TEM micrograph of SiO2/P(DVB-SO3−VPyH+) microspheres in Fig. 4A indicated that the pyridinium nanoparticles had a spherical shape and smooth surface together with a core-shell structure with a deeper inorganic silica core and a lighter contrast of P(DVB-SO3−VPyH+) core. The mean diameter of pyridinium SiO2/P(DVB-SO3−VPyH+) core-shell PE microspheres was kept at 300 nm with monodispersity index (U) of 1.007 as summarized in Table 1 (entry F). These results implied that all the core-shell microspheres were physically stable and remained monodispersion during the pyridinium process. The successful pyridinium modification via the neutralization of the strong sulfonic acid group on the surface of SiO2/P(DVB-SO3H) with weak basic pyridyl group of 4-VPy for the formation of SiO2/P(DVB-SO3−VPyH+) core-shell PE microspheres was confirmed by the FT-IR spectrum in Fig. 2d with presence of the new peaks at 1638 and 1441 cm−1 corresponding to the stretching vibration of vinyl and pyridinium groups of 4-VPyH+ species after modification. Furthermore, the XPS spectrum of the pyridinium SiO2/P(DVB-SO3−VPyH+) core-shell PE microspheres in Fig. 3c has strong peaks at 531.9 and 282.9 eV, ascribed to the binding energy of O1s and C1s together with an obvious peak at 400.1 eV assigning to the binding energy of N1s from 4-VPyH+ component of the P(DVB-SO3−VPyH+) shell-layer and the atom concentration on the surface for S and N were determined as 1.29 and 2.34%, respectively. The presence of a tiny amount N (1.61%) on the P(DVB-SO3−H+) core-shell microspheres at 400.1 eV in XPS spectrum (Fig. 3B) may be originated from the binding energy of chemisorbed N2 molecules, which was very similar to the cases of the chemisorbed N2 on the surfaces of the Fe3O4/TiO2 hollow spheres in the literature.37 Compared to the XPS data of the spectrum in Fig. 3B for SiO2/P(DVB-SO3H) core-shell PE microspheres, the molar ratio between the increased N (0.73%) and S (1.29%) surface atom concentration was 1.29 (very near 1.0), which implied that the pyridinium neutralization with sulfonic acid was quantitatively carried out. All these results proved the successful synthesis of SiO2/P(DVB-SO3−VPyH+) core-shell PE microspheres via the sulfonation of the phenyl group together with further pyridinium modification, which provided the possibility for the synthesis of SiO2/P(DVB-SO3−VPyH+)/SiO2 tri-layer composite microspheres.
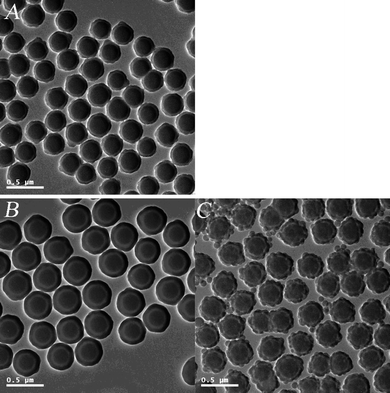 | ||
| Fig. 4 TEM micrographs: (A) SiO2/P(DVB-SO3−VPyH+) core-shell PE microspheres; (B) SiO2/P(DVB-SO3−VPyH+)/SiO2 tri-layer microspheres; (C) SiO2/P(DVB-SO3H)/SiO2 tri-layer microspheres. | ||
In the present work, pyridinium SiO2/P(DVB-SO3−VPyH+) core-shell microspheres were uniformly coated with silica shell-layer via the sol–gel hydrolysis of TEOS with ammonium hydroxide as catalyst, which was shown by the typical TEM micrograph of the resultant SiO2/P(DVB-SO3−VPyH+)/SiO2 composite microspheres having smooth surface and spherical shape in absence of any secondary-silica particles in Fig. 4B. The TEM micrograph in Fig. 4B demonstrated that the SiO2/P(DVB-SO3−VPyH+)/SiO2 composite microspheres had a typical tri-layer structure with deeper contrast silica core and outer shell-layer together with a sandwiched lighter contrast P(DVB-SO3−VPyH+) PE layer. Here, the electrostatic interaction between the pyridinium surface of the SiO2/P(DVB-SO3−VPyH+) templates and the negative charged silica species played an essential role during the hydrolysis of TEOS for the synthesis of SiO2/P(DVB-SO3−VPyH+)/SiO2 tri-layer composite microspheres, which was different from the coating of silica layer onto a positive charged surface of pyridinium benzylchloride to form a uniform P(EGDMA-co-VPyBzCl)/SiO2 core-shell composite in our previous work.32 The essential role of the pyridinium on the surface of SiO2/P(DVB-SO3−VPyH+) PE template was confirmed further by the formation of SiO2/P(DVB-SO3H)/SiO2 tri-layer composite microspheres with rough surfaces together with presence of plenty secondary-silica nanoparticles as shown by the TEM micrograph in Fig. 4C, as there was a strong electrostatic repulsion between the sulfonic acid groups on the surface of SiO2/P(DVB-SO3H) and newly formed negative silica species during the sol–gel hydrolysis of TEOS as discussed above. The particle size of SiO2/P(DVB-SO3−VPyH+)/SiO2 tri-layer composite microspheres was significantly increased from 300 nm of SiO2/P(DVB-SO3−VPyH+) template to 393 nm with a monodispersity index (U) of 1.005 as summarized in Table 1 (entry G). In other words, the silica-layer was uniformly formed with thickness of 46.5 nm by coating of the pyridinium SiO2/P(DVB-SO3−VPyH+) core-shell PE template via the third-stage sol–gel hydrolysis of TEOS as illustrated in Scheme 1.
Synthesis of monodisperse SiO2/P(DVB-SO3H)/SiO2/P(DVB-SO3H) tetra-layer PE microspheres
SiO2/P(DVB-SO3−VPyH+)/SiO2 tri-layer composite microspheres was further modified by MPS for the synthesis of monodisperse SiO2/P(DVB-SO3−VPyH+)/SiO2/PDVB tetra-layer hybrid microspheres. An important concern for the present work was to synthesize SiO2/P(DVB-SO3H)/SiO2/P(DVB-SO3H) tetra-layer PE microspheres with well-defined shape and adjustable polymeric PE shell-thickness, for which a two-semibatch mode distillation precipitation polymerization of DVB in acetonitrile with presence of MPS-modified SiO2/P(DVB-SO3−VPyH+)/SiO2 tri-layer composite microspheres as templates was used. Fig. 5A shows the resultant SiO2/P(DVB-SO3−VPyH+)/SiO2/PDVB tetra-layer microspheres after the first-semibatch polymerization, which have spherical shape with slight rough surface. The diameter of the SiO2/P(DVB-SO3−VPyH+)/SiO2/PDVB tetra-layer microspheres after the first-semibatch polymerization was 431 nm with a monodispersity index (U) of 1.009 as shown in Table 1 (entry H) and the PDVB outer shell-thickness was calculated as 19.0 nm. Fig. 5B shows a TEM micrograph of the final SiO2/P(DVB-SO3−VPyH+)/SiO2/PDVB particles after the second-semibatch polymerization, in which a typical tetra-layer structure was clearly observed with presence of deeper contrast of silica core and sandwiched third-layer, and lighter contrast of sandwiched second-P(DVB-SO3−VPyH+) and PDVB outer shell-layer. The results in Fig. 5A and 5B indicated that the resultant SiO2/P(DVB-SO3−VPyH+)/SiO2/PDVB particles had spherical shape and smooth surface in absence of any secondary-initiated small nanoparticles. The size of the SiO2/P(DVB-SO3−VPyH+)/SiO2/PDVB tetra-layer microspheres was significantly increased from 431 nm of the tetra-layer seeds after the first-semibatch polymerization (entry H) to 511 nm after the second-semibatch polymerization (entry I in Table 1), which meant that 40 nm of PDVB shell-layer was coated onto the tetra-layer seeds during the second-semibatch polymerization. The total thickness of the outer PDVB shell-layer was 59.0 nm after these two-semibatch polymerizations, keeping the final SiO2/P(DVB-SO3−VPyH+)/SiO2/PDVB tetra-layer microspheres monodisperse (U = 1.008). Therefore, the thickness of the outer PDVB shell-layer was facilely controlled by a multi-semibatch polymerization mode in the range of 19 and 59.0 nm in the present work.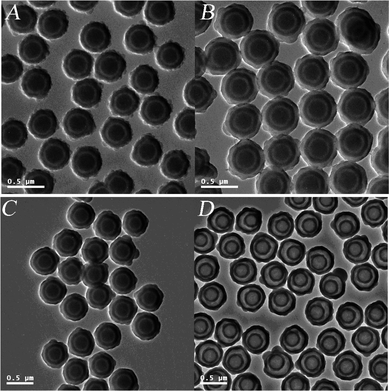 | ||
| Fig. 5 TEM micrographs: (A) SiO2/P(DVB-SO3−VPyH+)/SiO2/PDVB tetra-layer microspheres after the first-semibatch polymerization; (B) SiO2/P(DVB-SO3−VPyH+)/SiO2/PDVB tetra-layer microspheres after the second-semibatch polymerization; (C) SiO2/P(DVB-SO3H)/SiO2/P(DVB-SO3H) tetra-layer polyelectrolyte microspheres; (D) hollow P(DVB-SO3H) microspheres with a shell-in-shell structure. | ||
The SiO2/P(DVB-SO3H)/SiO2/P(DVB-SO3H) tetra-layer PE microspheres were prepared by the further sulfonation of the resultant SiO2/P(DVB-SO3−VPyH+)/SiO2/PDVB tetra-layer microspheres in concentrated sulfuric acid (96%) at 40 °C for 6 h, in which the sandwiched pyridinium P(DVB-SO3−VPyH+) second-layer was transferred to P(DVB-SO3H) in a strong acid environment during the sulfonation process. The TEM micrograph of SiO2/P(DVB-SO3H)/SiO2/P(DVB-SO3H) microspheres in Fig. 5C indicates that the final particles maintained their spherical shape with a smooth surface after the sulfonation modification of the phenyl groups on the outer PDVB shell-layer, in which a typical tetra-layer structure was clearly observed with presence of deeper contrast of silica core and sandwiched third-layer, and slighter contrast of sandwiched second and outer P(DVB-SO3H) layer. The average diameter of the resultant SiO2/P(DVB-SO3H)/SiO2/P(DVB-SO3H) tetra-layer PE microspheres was maintained at 510 nm with a monodispersity index (U) of 1.007 as summarized in Table 1 (entry J).
Preparation of hollow P(DVB-SO3H) PE microspheres with a shell-in-shell structure
The silica sections of the SiO2/P(DVB-SO3H)/SiO2/P(DVB-SO3H) tetra-layer PE microspheres can be selectively removed by etching the silica species in hydrofluoric acid (HF) to afford the corresponding hollow P(DVB-SO3H) PE microspheres with a shell-in-shell structure. The driving force for such a selective removal originated from the formation of SiF4 gas, which was given off from the tetra-layer PE microspheres during the etching process. The typical TEM micrograph of the hollow P(DVB-SO3H) PE microspheres with a shell-in-shell structure was illustrated in Fig. 5D, which was developed by the selective removal of silica core and the sandwiched third-layer from the corresponding SiO2/P(DVB-SO3H)/SiO2/P(DVB-SO3H) tetra-layer PE microspheres as shown in Fig. 5C. The TEM micrograph in Fig. 5D of the final hollow P(DVB-SO3H) PE microspheres had a convincing shell-in-shell structure with presence of two circular-rings, an obvious cavity inside the core and a space between the two-circulated shells, and the whole microspheres as well-sectioned and non-segmented spheres. The average diameter of the final hollow P(DVB-SO3H) PE microspheres with shell-in-shell structure was 525 nm with a monodispersity index (U) of 1.006 (entry K in Table 1), which was slightly larger than the size of the former SiO2/P(DVB-SO3H)/SiO2/P(DVB-SO3H) tetra-layer PE microspheres (511 nm, entry J in Table 1). All these results demonstrated that the P(DVB-SO3H) with shell-thicknesses of 49 nm and 59 nm for the inner-shell and outer-shell were thick and strong enough to support the hollow structure without collapse of the whole structure in the present work. Furthermore, the disappearance of the strong wide peak at 1105 cm−1 assigned to the typical stretching vibration of Si–O–Si bond of the silica components in the FT-IR spectrum of Fig. 3e for the final hollow P(DVB-SO3H) with a shell-in-shell structure implied that both the silica sections in the core and sandwiched third-layer were successively removed from the resultant SiO2/P(DVB-SO3H)/SiO2/P(DVB-SO3H) tetra-layer PE microspheres via the etching process. Furthermore, a new peak appears at 1190 cm−1 in Fig. 2e corresponding to the typical asymmetric vibration of S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond of P(DVB-SO3H) shells, which was once hidden by the wide strong peak at 1105 cm−1 of the stretching vibration of Si–O–Si bond as shown in Fig. 2c and 2d.
O bond of P(DVB-SO3H) shells, which was once hidden by the wide strong peak at 1105 cm−1 of the stretching vibration of Si–O–Si bond as shown in Fig. 2c and 2d.
All these results in the present work demonstrated the successful synthesis of SiO2/P(DVB-SO3H)/SiO2/P(DVB-SO3H) tetra-layer negative PE microspheres mainly via a four-stage reaction and the further development of hollow P(DVB-SO3H) microspheres with a shell-in-shell structure, which was different from the synthesis of hollow polymer microspheres containing movable positive PE cores our previous work.31 The hollow PE microspheres with shell-in-shell structure may enable them as potential materials for drug protectrion and delivery system, bio-separation and bio-analysis. The study on the scope of this technique, including the extension of the synthesis of hollow structure particles and the application of these functional hollow microspheres with shell-in-shell structure, such as the enhanced water retention property to confer polymer membranes for high proton conductivity at low humidity, is in progress.
Conclusions
Monodisperse hollow anionic P(DVB-SO3H) PE microspheres with a shell-in-shell structure were synthesized by a facile route mainly with a four-stage reaction and the subsequent removal of the silica sections from the corresponding SiO2/P(DVB-SO3H)/SiO2/P(DVB-SO3H) tetra-layer PE microspheres in HF aqueous solution. The tetra-layer PE microspheres were prepared by the combination of the controlled hydrolysis of TEOS in water–ethanol mixed solvent via a modified Stöber process for the synthesis of silica species and distillation precipitation polymerization of DVB with subsequent modification of the phenyl group of PDVB shell-layer for the formation of PE layers. The pyridinium sulfonate groups on the surface of SiO2/P(DVB-SO3−VPyH+) core-shell PE microspheres were essential to afford monodisperse SiO2/P(DVB-SO3−VPyH+)/SiO2 tri-layer composite microspheres via decrease the electrostatic repulsion between the surface of SiO2/PE surface and the newly formed silica species. The PDVB shell-layers were successively encapsulated onto the MPS-modified silica templates with reactive vinyl groups via the capture newly formed oligomers and monomers from the solution during the second and fourth-stage distillation precipitation polymerization. Further, the thickness of the functional polymer shell can be facilely and well-controlled by a multi-semibatch polymerization affording the thick and strong polymer-shell for the further modification and construction of the resultant hollow P(DVB-SO3H) PE microspheres with a shell-in-shell structure.Acknowledgements
This work was supported by the National Science Foundation of China with project No. of 20874049.References
- Y. D. Zhang, S. H. Lee, M. Yoonessi, K. W. Liang and C. U. Pittman, Polymer, 2006, 47, 2988.
- B. Strachotova, A. Strachota, M. Uchman, M. Slouf, J. Brus and J. Plestil, et al. , Polymer, 2007, 48, 1471 CrossRef CAS.
- K. Zhang, H. T. Chen, X. Chen, Z. M. Chen, Z. C. Cui and B. Yang, Macromol. Mater. Eng., 2003, 288, 380 CrossRef CAS.
- I. Tissot, C. Novat, F. Lefebvre and E. Bourgeat-Lami, Macromolecules, 2001, 34, 5737 CrossRef CAS.
- S. Reculusa, C. Poncet-Legrand, S. Ravainane, C. Minogotaud, E. Duguet and E. Bourgeat-Lami, Chem. Mater., 2002, 14, 2354 CrossRef CAS.
- J. Y. Wang and X. L. Yang, Colloid Polym. Sci., 2008, 286, 283 CrossRef CAS.
- A. Derro, S. Reculus, E. Bourgeat-Lami and S. Ravaine, Colloids Surf., A, 2006, 284–285, 78 CrossRef.
- S. Barthlet, C. Mingotaud, E. Bourgeat-Lami, E. Duguet and R. Ravaine, Nano Lett., 2004, 4, 1677 CrossRef CAS.
- C. Barthlet, A. J. Hickey, D. B. Cartins and S. P. Armes, Adv. Mater., 1999, 11, 408 CrossRef CAS.
- E. Bourgeat-Lami and J. Lang, J. Colloid Interface Sci., 1998, 197, 293 CrossRef CAS.
- E. Bourgeat-Lami and J. Lang, J. Colloid Interface Sci., 1999, 210, 281 CrossRef CAS.
- F. Corcos, E. Bourgeat-Lami, C. Novat and J. Lang, Colloid Polym. Sci., 1999, 210, 281.
- F. Caruso, Adv. Mater., 2001, 13, 11 CrossRef CAS.
- J. N. Cha, M. H. Bartl, M. S. Wong, A. Poptitsch, T. J. Deming and G. D. Stucky, Nano Lett., 2003, 3, 907 CrossRef CAS.
- Y. Chan, J. P. Zimmer, M. Stroh, J. S. Streckel, R. K. Jain and M. G. Bawendi, Adv. Mater., 2004, 16, 2092 CrossRef CAS.
- Y. Deng, D. Qi, C. Deng, X. Zhang and D. Zhao, J. Am. Chem. Soc., 2008, 130, 28 CrossRef CAS.
- M. Roca and A. J. Haes, J. Am. Chem. Soc., 2008, 130, 14273 CrossRef CAS.
- C. Radloff and N. J. Holas, Nano Lett., 2004, 4, 1303.
- A. Petukhova, A. S. Paton, Z. X. Wei, A. Gourevich, S. V. Nair, H. E. Ruda, A. Shik and E. Kumacheva, Adv. Funct. Mater., 2008, 18, 1961 CrossRef CAS.
- G. L. Li, G. L. Lei, C. H. Wang, K. G. Neoh, E. T. Kang and X. L. Yang, Macromolecules, 2008, 41, 9487 CrossRef CAS.
- Z. F. Dai, L. Dähne, H. Möhwald and B. Tiersch, Angew. Chem., Int. Ed., 2002, 41, 4019 CrossRef CAS.
- Z. F. Dai, H. Möhwald, B. Tiersch and L. Dähne, Langmuir, 2002, 18, 9553.
- H. Li, Z. Bian, J. Zhu, D. Zhang, G. Li, Y. Huo and Y. Lu, J. Am. Chem. Soc., 2007, 129, 8406 CrossRef.
- H. Zhang, X. Zhang and X. L. Yang, J. Colloid Interface Sci., 2010, 348, 431 CrossRef CAS.
- W. Wei, C. L. Zhang, S. J. Ding, X. Z. Qu, J. G. Liu and Z. Z. Yang, Colloid Polym. Sci., 2008, 286, 881 CrossRef CAS.
- X. W. Lou, D. Deng, J. Y. Lee and L. A. Archer, Chem. Mater., 2008, 20, 6562 CrossRef CAS.
- M. Schönhoff, J. Phys.: Condens. Matter, 2003, 15, R1781 CrossRef.
- P. Bertrand, A. Jonas, A. Laschewsky and R. Legras, Macromol. Rapid Commun., 2000, 21, 319 CrossRef CAS.
- G. Decher, Science, 1997, 277, 1232 CrossRef CAS.
- W. Fabianowski, M. Roszko and W. Brodzinska, Thin Solid Films, 1998, 327–329, 143.
- M. Ji, B. Liu, X. L. Yang and J. Y. Wang, Polymer, 2009, 50, 5970 CrossRef CAS.
- G. Y. Liu, H. Zhang, X. L. Yang and Y. M. Wang, Polymer, 2007, 48, 5896 CrossRef CAS.
- F. Bai, X. L. Yang and W. Q. Huang, Macromolecules, 2004, 37, 9746 CrossRef CAS.
- Y. Lu, J. McLellan and Y. N. Xia, Langmuir, 2004, 20, 3464 CrossRef CAS.
- H. F. Ji, S. P. Wang and X. L. Yang, Polymer, 2009, 50, 133 CrossRef CAS.
- J. J. Yuan, O. O. Mykhaylk, A. J. Ryan and S. P. Armes, J. Am. Chem. Soc., 2007, 129, 1717 CrossRef CAS.
- S. H. Xuan, W. Q. Jiang, X. L. Gong, Y. Hu and Z. Y. Chen, J. Phys. Chem. C, 2009, 113, 553 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2011 |