Functional polymers for optoelectronic applications by RAFT polymerization
Graeme
Moad
*,
Ming
Chen
,
Matthias
Häussler
,
Almar
Postma
,
Ezio
Rizzardo
and
San H.
Thang
CSIRO Materials Science and Engineering, Bayview Ave, Clayton, VIC 3168, Australia. E-mail: graeme.moad@csiro.au.
First published on 23rd September 2010
Abstract
This review focuses on the approaches to the synthesis of functional polymers for optoelectronic applications that make use of radical polymerization with reversible addition–fragmentation chain transfer (RAFT) polymerization. Optoelectronic applications include hole/electron transport in photovoltaics (OPVs), light emitting diodes (OLEDs and PLEDs), thin-film transistors (TFTs), sensors, light-harvesting and related applications. In this context we consider metallopolymers (polymers that incorporate a metal or possess metal ligating functionality as a pendant group to the backbone, as an end-group or as a connecting group), organic semiconductors (polymers with an organic semiconductor moiety either as a block or as a pendant group), and various surfaces, nanoparticles and quantum dots that are formed by RAFT polymerization or where a RAFT-synthesized polymer forms an integral part of the process or structure.
 Graeme Moad | Graeme Moad obtained his PhD in 1977 from Adelaide University in Organic Free Radical Chemistry. Between 1977 and 1979 he postdoced at Pennsylvania State University. He joined CSIRO in 1979 where he is currently a chief research scientist and Project Team Leader. He is also a project leader in the CRC for Polymers. Dr Moad is author of more than 130 journal papers, co-inventor of more than 32 patent families and coauthor of the book “The Chemistry of Radical Polymerization”. Research interests lie in polymer design and synthesis (radical polymerization, reactive extrusion and polymerization kinetics and mechanism). |
 Ming Chen | Ming Chen received his PhD from The University of Melbourne in 2004 and MSc (2000) and BSc (First Class Honours, 1997) from Tsinghua University, China. He has been working at CSIRO since 2001, first as a PhD student co-supervised by Prof. Ken Ghiggino at The University of Melbourne and Drs Gerry Wilson and San Thang at CSIRO, then as a CSIRO Postdoctoral Fellow under the supervision of Dr Ezio Rizzardo from 2005, and more recently as a research scientist working in the cross-disciplinary area of electroactive materials for organic electronics. |
 Matthias Häussler | Matthias Häussler completed his MSc in chemistry at the Martin-Luther University, Halle-Wittenberg, Germany in 2002 and undertook his PhD in conjugated hyperbranched polymers at the Hong Kong University of Science & Technology in 2006. Afterwards, he joined the electroactive materials group at CSIRO as a postdoctoral fellow and was recently promoted to Research Scientist. |
 Almar Postma | Almar Postma is a graduate from the University of Surrey, UK (1996). After working at CSIRO on RAFT polymerisation he commenced a PhD (2001) at the University of New South Wales under the supervision of Prof. Thomas P. Davis, Dr Graeme Moad and Dr Michael O'Shea in the fields of controlled radical polymerisation and reactive extrusion. He joined CSIRO as a research scientist in 2008 after a postdoc with Prof. Frank Caruso's group (2005) at the University of Melbourne. His research interests lie at the interface of polymer design/synthesis and their applications in nanomedicine and optoelectronics. |
 Ezio Rizzardo | Ezio Rizzardo received his PhD from the University of Sydney for his studies on the photochemistry of organic nitro compounds. He joined CSIRO in 1976 after postdoctoral research on the synthesis of biologically active organic compounds at Rice University, RIMAC, and the Australian National University. His CSIRO research has focussed on developing methods for controlling free radical polymerization. For this he has received a number of awards including the RACI Australian Polymer Medal and the CSIRO Chairman's Gold Medal. Ezio is a CSIRO Fellow and a Fellow of both the Australian Academy of Science and the Royal Society of London. |
 San H. Thang | San H. Thang obtained his PhD from Griffith University in the field of Organic Chemistry. In 1986, he joined CSIRO as a Research Fellow and then moved to ICI Australia in late 1987 to undertake the industrial research on UV-sunscreens and agrochemicals. He re-joined CSIRO in December 1990, currently is a Senior Principal Research Scientist where his research focuses on the interface between biology, organic and polymer chemistry. Dr Thang has over 100 papers in refereed journals and is responsible for several key inventions in the area of controlled/living radical polymerization. Significantly, he is a co-inventor of the RAFT process. |
Introduction
This review focuses on the synthesis of functional polymers for optoelectronic applications that make use of radical polymerization with reversible addition–fragmentation chain transfer (RAFT) polymerization in some part of the overall process. These optoelectronic applications include hole/electron transport in organic photovoltaics (OPVs), in organic and polymer light emitting diodes (OLEDs and PLEDs), in thin-film transistors (TFTs), in sensors, in light-harvesting and related applications. The use of RAFT in this context was most recently reviewed by Favier et al.1Control of radical polymerization with the addition of thiocarbonylthio compounds that serve as reversible addition–fragmentation chain transfer (RAFT) agents was first reported in 1998.2,3 Since that time much research carried out in these laboratories and elsewhere4–11 has demonstrated that polymerization with reversible addition–fragmentation chain transfer is a reversible deactivation radical polymerization (RDRP);12 an extremely versatile process that satisfies most of the established criteria for a living polymerization.13,14 It can be applied to form polymers with a narrow molecular weight distribution. These may be homopolymers or copolymers from most monomers amenable to radical polymerization. There is compatibility with a wide range of functionality in monomers, solvents and initiators. Stars, blocks, microgel and hyperbranched structures, supramolecular assemblies and other complex architectures are accessible and can have high purity. A further significant advantage of RAFT polymerization in the context of optoelectronic applications is that no undesired metal species are introduced during the polymerization process.
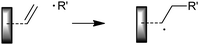
The overall RAFT process can be viewed simply as an insertion of monomer units into the C–S bond of a suitable thiocarbonylthio compound (the RAFT agent, 1) as shown in Scheme 1. A key feature of the process is that the thiocarbonylthio groups, present in the initial RAFT agent (1), are retained in the polymeric product (2). The polymeric products of the process are thus also RAFT agents. These macroRAFT agents (2) are a dormant form of the corresponding propagating radicals and under RAFT polymerization conditions are living polymers. This renders the RAFT process eminently suitable for synthesizing block copolymers and end functional polymers for optoelectronic and other applications.
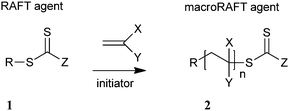 | ||
| Scheme 1 Overall RAFT process. | ||
The review covers three main classes of functional polymers, namely:
• Metallopolymers. The synthesis of polymers which either incorporate a metal complex or possess metal ligating functionality either as a pendant group or as an end-group.
• Organic semiconductors. The synthesis of polymers with an organic semiconductor moiety either as a block or as a pendant group to the backbone. We also consider polymers with attached dyes for use in light-harvesting, photochromic and some imaging applications.
• Surfaces, nanoparticles and quantum dots. The formation of grafts or brushes on various (electroactive) substrates.
For the most part, we limit our consideration to structures that are formed by RAFT polymerization or where a RAFT-synthesized polymer forms an integral part of the overall process or product.
RAFT agents
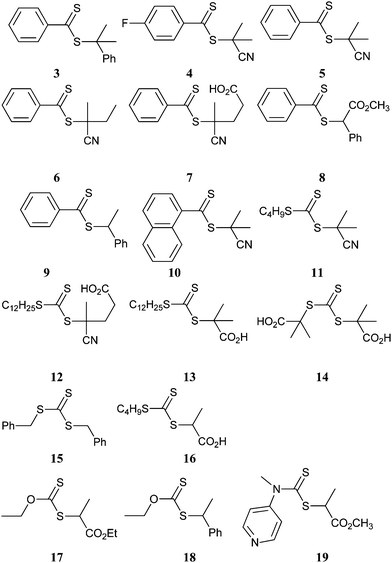
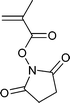
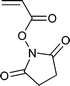
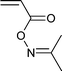
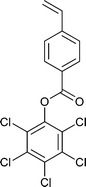
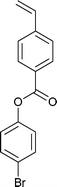
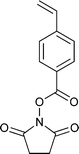
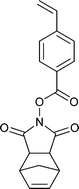
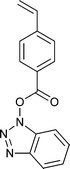
A wide range of thiocarbonylthio RAFT agents (ZC(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S)SR, 1) has now been reported. A broad summary of these and the factors which influence the choice of RAFT agent (1) for a particular polymerization can be found in our previous reviews.4,6–8,15 The effectiveness of a RAFT agent depends on the monomer being polymerized and is determined by the properties of the free radical leaving group ‘R’ and the ‘Z’ group. Some examples of RAFT agents used in the context of this review are 3–19. Other RAFT agents with specific functionality are mentioned in the sections which follow.
S)SR, 1) has now been reported. A broad summary of these and the factors which influence the choice of RAFT agent (1) for a particular polymerization can be found in our previous reviews.4,6–8,15 The effectiveness of a RAFT agent depends on the monomer being polymerized and is determined by the properties of the free radical leaving group ‘R’ and the ‘Z’ group. Some examples of RAFT agents used in the context of this review are 3–19. Other RAFT agents with specific functionality are mentioned in the sections which follow.The ‘Z’ group is chosen to activate or deactivate the thiocarbonyl double bond of the RAFT agent (1) and modify the stability of the intermediate species. RAFT agents such as dithioesters (1, Z = aryl or alkyl) or trithiocarbonates (1, Z = alkylthio) suitable for controlling polymerization of ‘more-activated’ monomers (MAMs) (e.g. MMA, S, MA, AM, and AN) inhibit or retard polymerizations of ‘less activated’ monomers (LAMs, e.g., VAc, NVP, and NVC). Similarly RAFT agents suitable for controlling polymerizations of LAMs such as xanthates (1, Z = alkoxy) and N,N-dialkyl- or N-alkyl-N-aryl-dithiocarbamates (1, Z = N,N-dialkylamino or N-alkyl-N-arylamino) tend to be ineffective with MAMs.
The reduced effectiveness of the xanthate and dithiocarbamate RAFT agents with MAMs relates to their lower reactivity towards radical addition and consequent lower transfer constants.16 The double-bond character of the thiocarbonyl group is reduced by the contribution of zwitterionic canonical forms which localize a positive charge on nitrogen and negative charge on sulfur.16,17 On the other hand, the tendency of dithioesters or trithiocarbonates to inhibit polymerization of LAMs is a consequence of the poor homolytic radical leaving group ability of propagating species with a terminal LAM unit. A consequence of this has been that the direct synthesis of narrow dispersity polyMAM-b-polyLAM is difficult or not possible using conventional RAFT agents.
A new class of stimuli-responsive switchable RAFT agents that can be switched to offer good control over polymerization of both MAMs and LAMs and a route to polyMAM-b-polyLAM have been reported.18,19N-(4-Pyridinyl)-N-methyldithiocarbamates (e.g., 19) behave as other N-aryl-N-alkyldithiocarbamates, and are effective in controlling the polymerization of LAMs but have relatively low transfer constants when used in MAM polymerization. However, in the presence of a strong acid, the protonated form of the RAFT agent provides excellent control over the polymerization of MAMs.18,19
In the present context of optoelectronic polymers, this allows the synthesis of well-defined block copolymers comprising MAMs such as functional styrene and (meth)acrylate derivatives and LAMs such as NVC.
In the present context, these click reactions include the copper-catalyzed azide–alkyne 1,3-dipolar cycloaddition (Scheme 2)25 and the active ester–amine reaction (Scheme 3). It also includes processes that involve either the thiocarbonylthio-group directly (the hetero-Diels–Alder reaction31–39) or the thiol end-group derived from thiocarbonylthio-group (e.g., the thiol–ene reaction40–42 and various thiol-trapping reactions—vide infra).
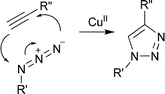 | ||
| Scheme 2 Copper-catalyzed azide–alkyne 1,3-dipolar cycloaddition. | ||
The chemistry of the thiocarbonylthio group is well known from small molecule chemistry70–73 and much of this knowledge is applicable to transforming the thiocarbonylthio groups present in RAFT-synthesized polymers.2 Some common methods used for end-group removal are summarized in Scheme 4. Thiocarbonylthio groups undergo reaction with nucleophiles and ionic reducing agents (e.g.amines, hydroxide and borohydride) to provide thiols. They also react with various oxidizing agents (including NaOCl, H2O2, tBuOOH, peracids and ozone) and are sensitive to UV irradiation. These reactions may leave reactive end-group functionality and thus are not appropriate in all circumstances. Thermolysis64,74–76 and radical-induced reactions (e.g., addition–fragmentation transfer,77 addition–fragmentation coupling78,79 and oxidation80,81) provide another solution and give complete desulfurization. Reviews focussing on end-group transformation/removal include those by Willcock and O'Reilly,82 Moad et al.83,84 and Barner and Perrier.85
![Processes for thiocarbonylthio-group transformation. (R′˙ is a radical, [H] is a hydrogen atom donor)](/image/article/2011/PY/c0py00179a/c0py00179a-s4.gif) | ||
| Scheme 4 Processes for thiocarbonylthio-group transformation. (R′˙ is a radical, [H] is a hydrogen atom donor) | ||
In designing polymer architectures it will normally be preferable to introduce functionality in ‘R’. Any functionality introduced on ‘Z’ will be lost if the thiocarbonylthio group is removed.
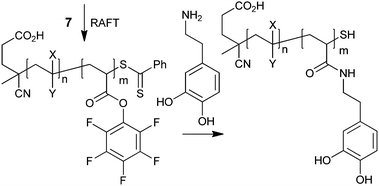
Specific end-group functionality may be introduced through addition–fragmentation coupling,16,86,87thiol end-group modification by the thiol–ene reaction,88–93 the thiol–isocyanate reaction,93disulfide formation through reaction with functional methanethiosulfonates or pyridyl disulfide derivatives, and other processes.55,91,94,95 These reactions have been much used in forming biopolymer conjugates and several examples in the optoelectronic field will be found in the later sections of this review.
A recent paper by Koo et al.96 examined the use of radical catalyzed thiol–ene processes for polymer conjugation. The reaction was found to be problematic because of the incidence of side reactions and difficulties in achieving high conversions unless one reagent was in large excess. The authors concluded that the radical catalyzed thiol–ene reaction should not be considered a “click” reaction when used for polymer–polymer conjugation.96
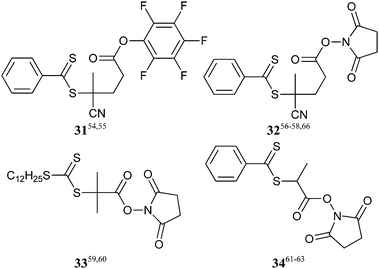
An example that demonstrates the versatility of end-group transformation is shown in Scheme 5.95 The chain ends of PDEGMA formed with RAFT agent 31 are sequentially and quantitatively transformed by the active ester–amine and the thiol–methanethiosulfonate “click” reactions.
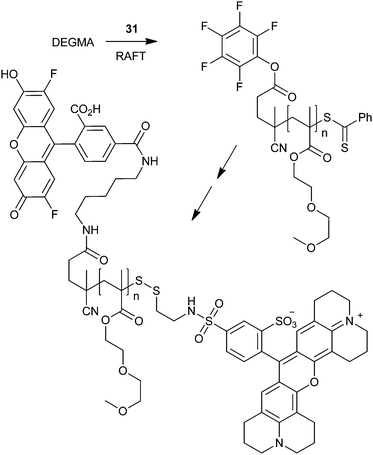 | ||
| Scheme 5 Use of the active ester–amine and the thiol–methanethiosulfonate “click” reactions for selective end-group transformation.95 | ||
RAFT agents and macro-RAFT agents with electron withdrawing ‘Z’ (e.g., Z = pyridyl, phosphonate and phenylsulfonyl) have been shown to undergo hetero-Diels–Alder reactions with suitable dienes (Scheme 6).31–39 The process has been developed as a route to block copolymers,32,37 star polymers31,32,35 and modified surfaces.36,38
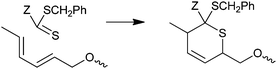 | ||
| Scheme 6 Hetero-Diels–Alder reaction. | ||
Monomers for RAFT polymerization
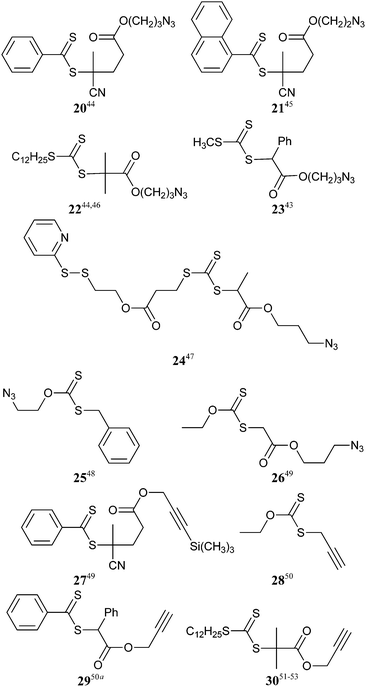
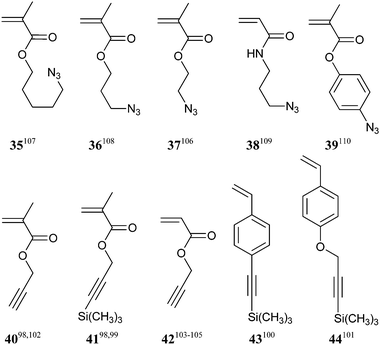
With appropriate choice of RAFT agent, RAFT polymerization is applicable to most monomers amenable to radical polymerization. Monomers used include all of the usual classes (e.g., methacrylates, acrylates, methacrylamides, acrylamides, acrylonitrile, styrene derivatives and vinyl monomers) and a range of monomers with reactive functionality, for example, active ester, alkyne, ammonium, azide, betaine, boronic acid, carboxy, halo, hydroxyl, pyridyl disulfide, tertiary amino and thiirane. A comprehensive survey of monomers that have been used in RAFT polymerizations can be found in our recent reviews.4,8Azide functional polymers have also been prepared from RAFT-synthesized polymers containing 3-chloropropyl acrylate units which are converted to 3-azidopropyl acrylate units post-polymerization by reaction with sodium azide.104,105
Most work has focused on copper-catalyzed azide–alkyne 1,3-dipolar cycloaddition. The copper catalyst is required to achieve acceptable reaction rates and conversions. However, good results can be achieved with copper-free reactions with strained alkynes.106
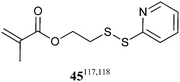
The thiol–ene reaction40 and disulfide coupling are other “click” processes for functionalization post-RAFT polymerization.41,119 Both processes require as substrate a polymer with thiol functionality. However, monomers with thiol functionality are not compatible with RAFT polymerization. The monomer (45), which contains protected thiol functionality, has been used in conjunction with RAFT polymerization to make biopolymer conjugates.117,118 Monomers with ‘ene’ functionality amenable to RAFT (co)polymerization have been described.120–122
Metallopolymers
Metallopolymers may contain main group metals, transition metals, lanthanides or actinides. A range of possible structural types exist depending on how the metal centres are incorporated and the linkages between them. The metal centres can be either in the main chain or in a side group structure. They can be linear, branched or dendritic. The metal centres can be incorporated through stable covalent bonds or through non-covalent coordination bonds in metallosupramolecular polymers.142In this section we consider RAFT synthesized polymers which incorporate a metal complex or which incorporate metal ligating functionality either as an end-group or connecting group, through use of a functional RAFT agent, or as a side or pendant group, through polymerization of a functional monomer.
Polymers with organometallic functionality or with metal ligating functionality as end-groups or as connecting groups
Polymers with organometallic functionality or metal ligating functionality can be formed by making use of an appropriately designed RAFT agent which includes the desired functionality as part of ‘Z’ or ‘R’. Examples of such RAFT agents are shown in Table 2 (organometallic RAFT agents) or Table 4 (RAFT agents containing metal ligating functionality).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S)–R) with organometallic functionality in ‘R’
S)–R) with organometallic functionality in ‘R’
| RAFT agenta | Polymers b |
|---|---|
| a References provide a synthesis of the RAFT agent. b In the case of block copolymers the first mentioned block was prepared first. | |
|
115 143 |
| 58 143 | |
|
115,143–145115-b-NIPAM144,145 |
| 59 143 | |
|
St/StB,146 St/StB-b-MMA,146EGDMA/MMA,147DVB/St147 |
| 60 146,147 | |
|
StB,146StB-b-St146 |
| 61 146 | |
|
St148 |
| 62 148 |
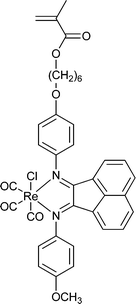
A few polymers have been synthesized directly from organometallic RAFT agents (Tables 2 and 3). However, this strategy is not always possible because of the intrinsic properties of the organometallic species and its compatibility with radical polymerization and, in some cases, the thiocarbonylthio functionality of the RAFT agent. Thus, a second route to metallopolymers makes use of RAFT agents containing metal ligating functionality (Table 4). Such polymers have been used as precursors to metallo-supramolecular polymers, a sub-class of main chain supramolecular polymers which have metal–ligand bonds within the main chain of a copolymer located at the junction between polymer blocks.149,150 A wide range of block or multiblock copolymers can be achieved. The metal ligating functionality can also be introduced into RAFT-synthesized polymers by end-group modification.151
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S)R) with organometallic functionality in ‘Z’
S)R) with organometallic functionality in ‘Z’
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S)R) containing metal ligating functionality in ‘R’
S)R) containing metal ligating functionality in ‘R’
| RAFT agenta | Polymers b | Metalc | RAFT agenta | Polymers b | Metalc |
|---|---|---|---|---|---|
| a References provide a synthesis of the RAFT agent. b In the case of block copolymers the first mentioned block was prepared first. c Metal species incorporated into the polymer post-polymerization. | |||||
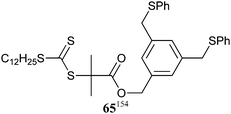
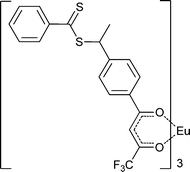
|
MA,154MA-b-tBA154 | Pd | |||
| 65 154 | |||||
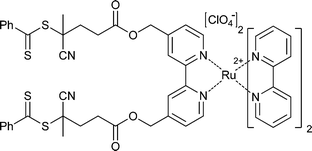
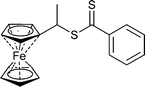
|
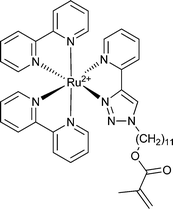
St,155,156NIPAM155,157 | Ru |
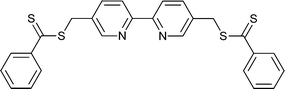
|
St,158NIPAM159 | Ru |
| 66 155 | 67 158 | ||||
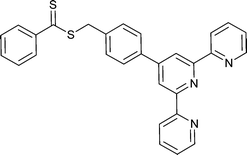
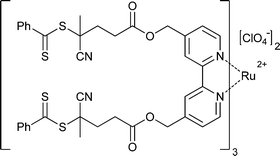
|
St,156,160 | Ru |
|
St,161 BA,161St-b-BA,161BA-b-St161 | Ru |
| 68 160 | 69 161 | ||||
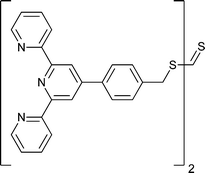
|
t BA,162 St162 |
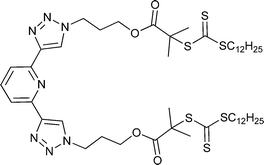
|
MMA,162tBA,162 St,162St-b-tBA162 | RuII, EuIII, FeII | |
| 70 162 | 71 162 |
Polymers with thiocarbonylthio or derived thiol functionality have been shown to bind certain metals and particles. Polymer brushes on surfaces can be formed by making use of this property. Such systems are covered in the section Surfaces, Nanoparticles and Quantum Dots.
Polymers with metal species or metal ligating functionality as pendants
The synthesis and properties of polymers with pendant or side-chain organometallic groups have been reviewed.163Polymers synthesized by direct (co)polymerization of monomers containing organometallic groups as substituents are shown in Table 5. Only a few monomers (Table 6) have been subjected to RAFT polymerization directly. The more common approach to this form of metallopolymers is to polymerize monomers containing metal ligating functionality (Table 7) and introduce a metal species post-polymerization. The derived polymers formed are indicated in Table 8.| Polymer a | RAFT agent | Metalsb | Ref. |
|---|---|---|---|
| a In the case of block copolymers the first mentioned block was prepared first. b Metal species incorporated into the polymer post-polymerization. c From deprotection of PSt-b-P83. | |||
| P76-b-PSt | 5 | AlIII | 168 |
| P77 | 5 | SmIII | 169 |
| PNVC-b-P78 | 18 | (ZnII) | 165 |
| P(MMA-co-79) | 5 | EuIII | 170 |
| P(PEGMA)-b-P80 | 3 | FeII | 171 |
| P(MMA-co-80) | 6 | IrIII | 172 |
| P(St-co-81) | 10 | CuII, EuIII | 173 |
| PMMA-b-P(MMA-co-82) | 5 | CuII, CoII | 174,175 |
| PSt-b-PSOH c | 3 | RuII | 176 |
Some of the results reported in Tables 5 and 8 deserve further comment. O-Alkyl xanthate RAFT agents generally do not offer good control over the polymerization of methacrylates (MMA).177 Furthermore the PNVC propagating radical is anticipated to be a poor leaving group with respect to either P73˙ or P78˙. However, the polymers PNVC-b-P73 and PNVC-b-P78 were synthesized with xanthate RAFT agent 18 and with PNVC-b-P78, good control (a low dispersity polymer) was reported.165
Organic semiconductors
The fully conjugated polymers that have seen use in the organic semiconductors cannot themselves be made by the RAFT process or other RDRP methods. Nonetheless, RAFT polymerization can be used in the synthesis of polymers or blocks that form one or more of the active components of optoelectronic devices. The RDRP methods can be used to form materials which comprise segments of these polymers either as blocks or grafts. They are also used to form polymers which contain electroactive molecules as pendant units.Two significant benefits of RAFT polymerization are the ability to form polymers with narrow molecular weight distributions and to construct block copolymers and other designed architectures. A particular advantage of narrow molecular weight distributions is the possibility of eliminating the low molecular weight “impurities” which can act as hole or electron traps in organic semiconductors while, at the same time, targeting the modest molecular weights that offer advantages in solubility, processing and film forming characteristics.
Block copolymers have attracted interest because of their ability to self-assemble to give nanophase separation into periodic domains. The dimensions of these domains can be in the range of 5–50 nm which encompasses that required for many semiconductor applications.178–182Block copolymers may also be added as a minor component and control the morphology of a blend by acting as a compatibilizer or structure director (vide infra).182–184
General reviews on organic semiconductors include that by Pron et al.185 Reviews on the use of block copolymers in organic electronics include those by Segalman et al.,186 Kim et al.,179 Scherf et al.187 and Darling.182
Block copolymers comprising fully conjugated polymer segments
Macro-RAFT agents based on organic semiconductor or analogous oligomeric species have been prepared by end-group modification of the organic semiconductors. RAFT polymerizations making use of these are summarized in Tables 9 and 10. The block copolymers formed are a sub-class of rod–coil polymers. Several relevant reviews have appeared on block copolymers for organic optoelectronics186 and on the self-assembly of rod–coil polymers.188| Macro-RAFT agenta | Monomerb |
|---|---|
| a References are to the synthesis of the macro-RAFT agent. b Monomers polymerized. In the case of block copolymer the first mentioned monomer was polymerized first. | |
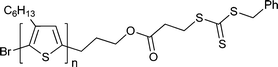
|
St193 |
| 84 poly(3-hexylthiophene) macro-RAFT agent193 | |
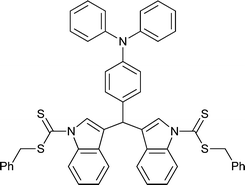
|
St194 |
| 85 194 | |
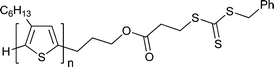
|
Refer Scheme 12195 |
| 86 poly(3-hexylthiophene) macro-RAFT agent195 | |
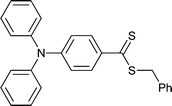
|
St,196 MA,196St-b-MA196 |
| 87 196 |
| Macro-RAFT agenta | Monomerb | Macro-RAFT agenta | Monomerb |
|---|---|---|---|
| a References are to the synthesis of the macro-RAFT agent. b Monomers polymerized. In the case of block copolymer the first mentioned monomer was polymerized first. c Use in polymerization not reported. | |||
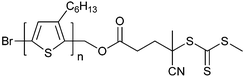
|
St189 |
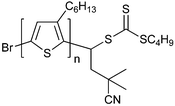
|
St189 |
| 88 poly(3-hexylthiophene) macro-RAFT agent189 | 89 poly(£—hexylthiophene) macro-RAFT agent189 | ||
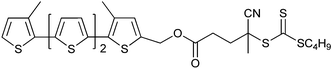
|
MMA,189 St,189 AA,189 MA189 |
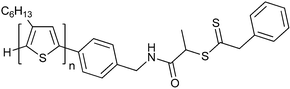
|
122 184 |
| 90 189 | 91 Poly(3-hexylthiophene) macro-RAFT agent184 | ||
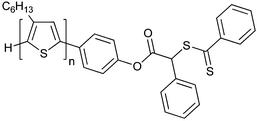
|
—c |
|
NIPAM 198 |
| 92 Poly(3-hexylthiophene) macro-RAFT agent147 | 93 198 | ||
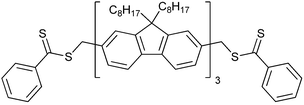
|
114 68 |
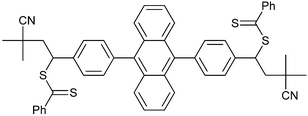
|
114 68 |
| 94 68 | 95 68 | ||
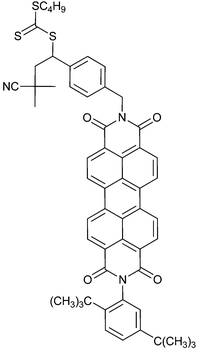
|
110 189 |
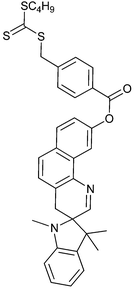
|
St,199 BA,199St-b-BA,199BA-b-St199 |
| 96 Perylene diimide macro-RAFT agent189 | 97 Photochromic dye macro-RAFT agent199 | ||
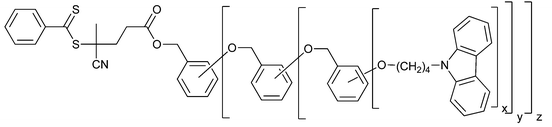
|
St,200 MMA200 | ||
| 98 (Ar-3,5-substitution)200 x = 2, y = z = 0 1st generation dendron RAFT agent; x = y = 2, z = 0 2nd generation dendron RAFT agent; x = y = z = 2 3rd generation dendron RAFT agent | |||
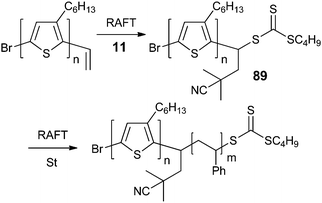
In designing macro-RAFT agents, it is important to note that for ‘Z’-connected RAFT agents (e.g., 84–87, Table 9) the block will be cleaved on end-group removal or polymer degradation. For ‘R’-connected RAFT agents (e.g., 88–98, Table 10), the block linkage is a carbon–carbon bond so the structure should remain intact during processing.189 The macro-RAFT agent 89 was preferred as a precursor to poly(3-hexylthiophene) block copolymers for also having no potentially hydrolysable ester linkages as part of the block juncture.
A method of synthesizing macro-RAFT agents suitable for forming ‘R’-connected block copolymers involves the insertion of a single monomer unit into a RAFT agent structure to form a new macro-RAFT agent as illustrated in Scheme 7.68,189,190 The chain length dependence of propagation is such that, as long as the transfer constant of the RAFT agent is high, there will be substantial conversion to the single monomer “chain” before oligomerization to provide a two unit or longer chain.191,192 RAFT agents 89 and 94–96 (Table 10) were prepared using this methodology.
 | ||
| Scheme 7 Macro-RAFT agent synthesis from macromonomer.189 | ||
The active ester–amine “click” reaction has also been used to synthesize macro-RAFT agents (Scheme 8).61,63 The reaction of amines with the active ester in 34 is substantially more rapid than aminolysis of the dithiobenzoate group such to the extent that the side reaction can be completely excluded.
Polymers and block copolymers with pendant functionality
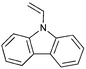
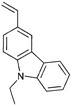
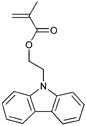
A variety of polymers with pendant functionality for potential use in applications such as thin-film transistors (TFTs), polymer light-emitting diodes (PLEDs) and organic photovoltaics (OPVs) have been synthesized by RAFT polymerization and are shown in Table 11. The monomers used in these polymerizations are listed in Table 12.18,68,137,143–145,165,184,189,201–216| Polymer a | RAFT agent | Application | Ref. |
|---|---|---|---|
| a In the case of block copolymers the first mentioned block was prepared first. b Poly(methylsilsesquioxane) macro-RAFT agent. | |||
| P103-b-P49 | 3 | Photovoltaics | 137 |
| P120 | 3 | F− sensor | 213 |
| P118 | 4 | — | 211 |
| PNVC | 19 | — | 18 |
| PNVP-b-PNVC | 17 | — | 218 |
| P(NVC-co-NIPAM)-b-PDMAEA | 16 | — | 219 |
| PNVC | 18 | — | 165,202 |
| PNVC-b-P78 | 18 | Photovoltaics | 165 |
| PNVC-b-P73 | 18 | Photovoltaics | 165 |
| PMA-b-PNVC | 19 | — | 18 |
| P92-b-P122 | 92 | Photovoltaics | 184 |
| P106 | 11 | — | 206 |
| P107 | 11 | — | 206 |
| P108 | 11 | — | 206 |
| P109 | 11 | — | 206 |
| P104 | 11 | — | 206 |
| P104 | PSSQ b | — | 220 |
| P104-b-P108 | 11 | Photovoltaics | 206 |
| P104-b-P109 | 11 | Photovoltaics | 206 |
| P108-b-P104 | 11 | Photovoltaics | 206 |
| PLA-b-P105 | 13 | Photovoltaics | 207 |
| a References are to the use of the monomer in RAFT polymerization. | ||||||
|---|---|---|---|---|---|---|
|
|
|
|
|
|
|
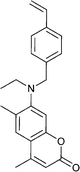
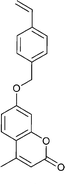
| 99 NVC 18,165,201,202 | 100 203,204 | 101 205 | 102 137 | 103 137 | 104 206 | 105 207 |
|
|
|
|
|
|
|
| 106 206 | 107 206 | 108 206 | 109 206 | 110 189 | 111 205 | 112 208 |
|
|
|
|
|
|
|
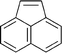
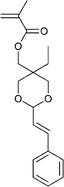
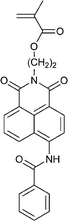
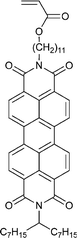
| 113 68 | 114 68 | 115 143–147 | 116 209 | 117 210 | 118 211 | 119 212 |
|
|
|
|
|
|
|
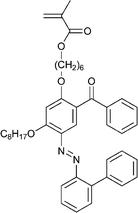
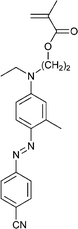
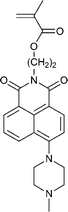
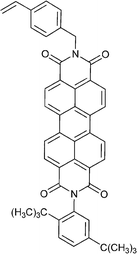
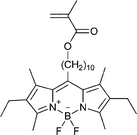
| 120 213 | 121 214 | 122 184 | 123 189 | 124 215 | 125 216 | |
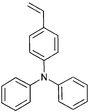
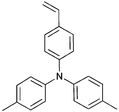
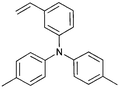
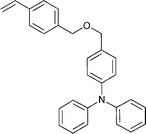
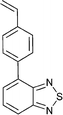
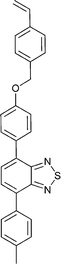
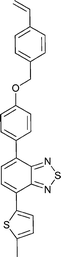
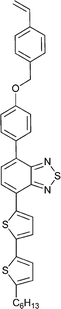
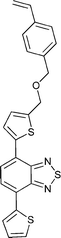
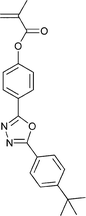
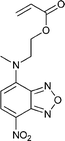
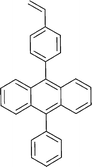
Monomers used in the construction of blocks for hole transport (donors) include the triarylamine and carbazole derivatives 99–10518,165,201–207 and the arylene diimides 120–123.184,189,214 Those used in construction of electron transport (acceptor) blocks include 111,205112208 and the benzothiadiazoles 106–110.109,206
One issue in these polymerizations is the solubility of the monomer and/or the polymers formed. Another potential issue is the intrinsic reactivity of the donor/acceptor functionality towards radicals.
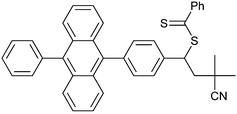
Ring-opening RAFT polymerization (Scheme 9) provided a route to a rod-polymer with chain acene (anthracene) functionality.217
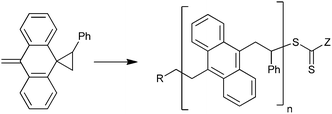 | ||
| Scheme 9 Example of RAFT ring-opening polymerization (R = PhCH2, Z = Ph or N-pyrrole). | ||
Block copolymers by non-RAFT radical polymerization
While RAFT polymerization is attracting much interest, other forms of RDRP such as NMP24 and ATRP20–22 have seen more substantial use. NMP has been mainly applied in synthesizing polymers based on styrenic monomers and, to a lesser extent, acrylates. Examples include P102-b-P122221 and related polymers.222–224ATRP is generally considered a more versatile method and, in the present context, has been widely applied in synthesizing polymers based on methacrylates.Many polymers containing poly(3-hexylthiophene), polyfluorene and other segments based on fully conjugated polymer blocks or pendants have been synthesized using NMP193,221–223,225,226 and ATRP.227–234
Advantages of RAFT polymerization over the “competing technologies” of ATRP and NMP are the absence of metal ions in the polymerization process (required for ATRP), a generally more convenient polymerization process and compatibility with a wider range of monomer types and polymerization conditions.4–11 Advantages seen for ATRP and NMP are the absence of sulfur compounds from the polymerization medium and the polymer product and that no additional initiator is required for polymerization.21,22
Polymers with pendant functionality introduced post-RAFT polymerization
RAFT polymerization allows the synthesis of precursor polymers that allow the semiconductor pendant groups to be introduced in a subsequent polymer modification step. For example, RAFT-synthesized PCMS was used as a scaffold for various pendant groups introduced using Williamson ether synthesis (Scheme 10).123–125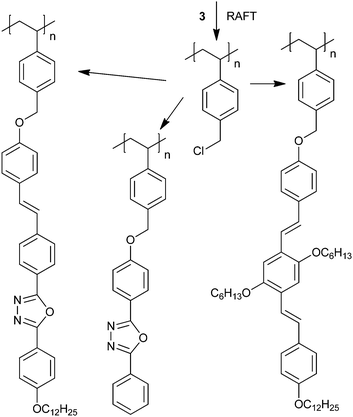 | ||
| Scheme 10 Synthesis of pendant polymers using Williamson ether synthesis.123 | ||
A polymer with pendant terthiophene groups was synthesized by Suzuki coupling as shown in Scheme 11.235 A crosslinked (insoluble) polymer presumed to have pendant polythiophene was also produced using the same methodology.235
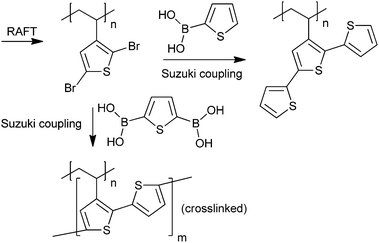 | ||
| Scheme 11 Synthesis of polymer with pendant terthiophene or polythiophene.235 | ||
The donor–acceptor rod–coil block copolymer 126 was produced using macro-RAFT agent 86. The pendant fullerene groups were introduced to provide the copolymer 127 as shown in Scheme 12.195 Care must be taken in using this process since excess hydrazine could potentially cleave the polymer at the trithiocarbonate block linkage.
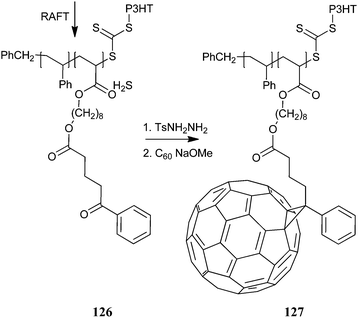 | ||
| Scheme 12 Synthesis of block copolymer from poly(3-hexylthiophene) macro-RAFT agent195. | ||
The star-microgel with active ester groups was prepared by the ‘arm-first’ methodology which was then functionalized with tetra-aniline using the active ester–amine reaction (Scheme 13).60
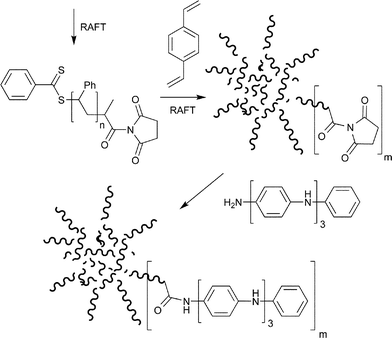 | ||
| Scheme 13 Star-microgel with active ester groups prepared using ‘arm-first’ methodology.60 | ||
Applications of RAFT-synthesized P3HT block copolymers
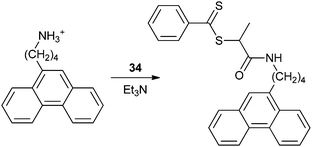
P3HT is one of the most studied organic semiconductors and acts as a p-type material in OFETs and as an electron donor in OPVs.236 Several examples of P3HT blocks by RAFT copolymerization have appeared. The synthesis of P3HT blocks by RAFT polymerization requires synthesis of a P3HT macro-RAFT agent. ‘Z’-connected P3HT block copolymers have been prepared using macro-RAFT agents 84193 or 86195 (Table 9). ‘R’-connected P3HT blocks have been prepared using macro-RAFT agents 88,18989,18991184 or 92197 (Table 10).Metal-free rod–coil P3HT-b-PSt diblock copolymers were prepared from macro-RAFT agent 84.193 Thin films of the block copolymers, prepared by drop-casting from toluene solutions followed by evaporation of the solvent, displayed a nanofibrillar morphology with remarkable long range order, e.g., Fig. 1.193 The width of the fibers corresponded to the weight-average contour length of the polymer chain. The conductivities of the films decreased with increasing insulating polystyrene content but were nonetheless relatively high (4–17 S cm−1).
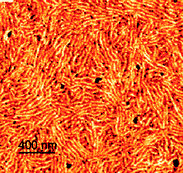 | ||
| Fig. 1 Tapping mode atomic force microscopy phase image (scan size 2 µm × 2 µm) of poly(3-hexylthiophene)-b-polystyrene film (reprinted with permission from the American Chemical Society).193 | ||
Addition of small amounts of a P3HT block copolymer can beneficially influence the morphology of the active layer of OPV devices by acting as a surfactant or compatibilizer.184,195 Introducing an electron acceptor such as C60 into a RAFT-made non-conducting block of 126 provided the donor–acceptor block copolymer 127 (Scheme 12).195 Small amounts (5%) of the block copolymer 127 were introduced into a blend of P3HT and PCBM to provide a substantial improvement in device performance (up to 35%) relative to similar bulk heterojunction solar cells fabricated without the modifier. A similar finding was obtained for a P3HT block copolymer with perylene diimide pendants, another well known electron acceptor (formed by polymerization of monomer 122 with macro-RAFT agent 91).184 A nearly 50% improvement in efficiency was obtained for bulk heterojunction solar cell with the diblock copolymer compatibilizer.
Surfaces, nanoparticles and quantum dots
General reviews on polymer encapsulation of metallic and semiconductor nanoparticles have been published.237 Four approaches have been employed.• The “grafting from” process which embraces surface initiated polymerization.
• The “grafting through” process in which monomer functionality is attached to a substrate to form a macromonomer.
• The “grafting to” process in which preformed polymer is attached to the surface in what can be considered a ligand exchange process.
• In situ particle formation in which the nanoparticle is prepared in the presence of a polymeric surfactant.
Much of the literature on forming polymer brushes by RAFT polymerization relates to “grafting from” silica particles, polymer surfaces and other substrates. A discussion of these processes is beyond the scope of this review. However, many of the methods used can be applied in the present context and the reader is referred to the reviews that have been published.238–242
Two basic approaches are used in “grafting from” nanoparticles by RAFT polymerization. The first involves surface modification to attach RAFT agent functionality and RAFT polymerization as a subsequent step. The second involves forming radicals on the surface (e.g., by irradiation or from attached initiator functionality) so as to have surface-initiated polymerization in the presence of a ‘free’ RAFT agent which becomes attached to the surface as a consequence of RAFT polymerization. The mechanism is then the same as that shown in Scheme 15.
We can also distinguish “away from” processes where the ‘R’ is bound to the substrate (Scheme 14) and “attached to” processes where ‘Z’ is bound to the substrate (Scheme 15). The advantage of the “away from” strategy (Scheme 14) is that propagating radicals are never directly attached to the surface. Radical–radical termination involves reaction of “free” propagating radicals in solution to produce a by-product that can be washed away. All of the thiocarbonylthio functionality remains directly attached to the surface. It might be envisaged that steric factors associated with attack of the propagating radical on the surface-bound RAFT functionality could become an issue particularly at high conversions. A potential disadvantage of the “away from” strategy is that any reaction which cleaves the thiocarbonylthio groups (e.g., hydrolysis and thermolysis) also results in the loss of the graft. With the “attached to” strategy (Scheme 15) most propagating species remain attached to the surface and the thiocarbonylthio functionality is maintained at the chain ends.
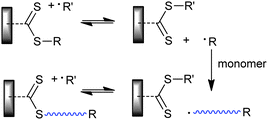 | ||
| Scheme 14 “Grafting from” with ‘Z’ connected RAFT agent. | ||
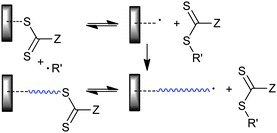 | ||
| Scheme 15 “Grafting from” with ‘R’ connected RAFT agent. | ||
In “grafting through” RAFT polymerization is carried out in the presence of a surface with monomer functionality which is incorporated by copolymerization (Scheme 16). The mechanism is then same as shown in Scheme 15.
 | ||
| Scheme 16 First step in “grafting through”. | ||
To achieve good control over the molecular weight and dispersity of the polymer arms, polymer brush formation by “grafting from” processes should always be conducted in the presence of additional “free”, i.e., unbound, RAFT agent. Similarly, for procedures making use of bound initiator functionality and in “grafting through” processes the RAFT agent should be in excess of the amount of bound initiator or bound monomer respectively. This is necessary because the effective concentration of RAFT agent seen by propagating species is substantially lower than the actual concentration of bound RAFT agent. The concentration of “free” RAFT agent is chosen to give the desired arm length.
The “grafting-to” approach involves separate RAFT synthesis of polymers with an end-group or block structure that can bond to a surface.
The thiocarbonylthio functionality of RAFT agents effectively binds to some metal surfaces and quantum dots and this property has been utilized both in “grafting to” processes and in attaching RAFT agent to surfaces for use in “grafting from” processes.
This section is subdivided according to the type of substrate.
Gold and other transition metal surfaces and particles
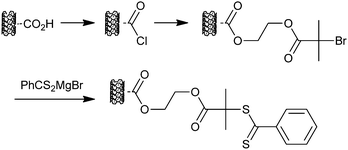
McCormick et al.243 were the first to report the potential of RAFT polymerization as a convenient source of polymers with thiol end-groups and explore the use of RAFT-synthesized polymers in forming gold nanoparticles. The dithiobenzoate end-groups were reduced with NaBH4 in the presence of HAuCl4 with the formation of gold nanoparticles. The approach was applied to a range of water soluble polymer compositions (PAMPS, PVBTAC, PDMAM and PMAEDAPS-b-PDMA) and in the formation of silver, platinum and rhodium colloids. Other examples of the use of thiols derived from RAFT-synthesized polymers used to prepare gold nanoparticlesin situ include PNIPAM,244–247 PMAA-b-PNIPAM,248 PMA249PAA-b-PAN250 and PNIPAM with Au/Pd mixed metal nanoparticles.251 Shan et al.245,246 showed that it was more effective to use PNIPAM with dithiobenzoate ends directly in this type of process than to first form a PNIPAM polymer with thiol ends in a separate process.There are also examples involving the use of RAFT-derived polymeric thiols (glycopolymers,252P(PEGA)-b-NIPAM253 and PNIPAM254) with pre-formed gold nanoparticles. Dithioester or trithiocarbonate groups can, however, be used directly as anchoring groups on gold surfaces in a “grafting-to” approach. For example, the RAFT agents (benzyl dithiobenzoate and dibenzyl trithiocarbonate) and derived RAFT-synthesized polystyrenes were shown to bind to form monolayers on gold surfaces without prior transformation of these thiocarbonylthio groups to thiols.255 This strategy has been used in forming grafts on preformed gold nanoparticles, for example: (nanorods with PDMAEMA, PAA or PSt),256 (POEGA-b-P(St-co-MMA), PHPMA-b-P(St-co-MMA))257 (PAA, PDHPAM, PAEAM)258 or (PAEMAM, PAA, PDMAEA, PNIPAM, PDEGA, POEGA, P(DEGA-co-OEGA)).259Polymers with pyrrolecarbodithioate end-groups (PDEGMA-co-tBA, PDEGMA-co-tBA-b-PGMA, PGMA, PSt) have also been used.260
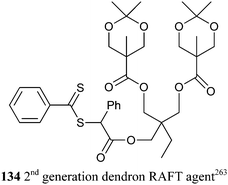
RAFT-synthesized PS-b-P2VP was converted to the thiol-terminated polymeric ligand by aminolysis and used in forming gold nanoparticles.261 However, the grafting density of polymeric ligands which contain secondary thiol groups was not sufficient to prevent the pyridine groups also interacting with the gold surface. End-group modification by addition–fragmentation coupling provided polymeric ligands with primary thiol end which in turn gave a higher grafting density.262 RAFT-synthesized dendritic-linear block copolymers based on the 2nd generation dendron RAFT agent 134 were functionalized with (±)-thioctic acid anhydride to provide highly efficient dispersants containing multiple disulfide linkages for gold nanoparticles.263
Alkyne end-functional PNIPAM prepared with RAFT agent 30 was “clicked” to azide end-functional Au nanoparticles.53Dithiobenzoate end-groups were converted to methanethiosulfonate end-groups to provide better surface coverage, particularly for methacrylate polymers.91
A “grafting-from” approach has also been applied in forming PNIPAM coated gold nanoparticles.264Carboxy-dithiobenzoate 7 was coupled to hydroxy-functional gold nanoparticles (formed with 11-mercaptoundecan-1-ol) using dicyclohexylcarbodiimide (DCC). The dithiobenzoate-functional nanoparticles so formed were then used to mediate the polymerization of NIPAM.
Iron oxide nanoparticles
Polymer stabilized magnetic iron oxide nanoparticles have been synthesized mainly for use in diagnostics and imaging applications.171,265–271 Processes involving “grafting from”, “grafting to” and in situ particle formation have been reported.“Grafting from” processes:
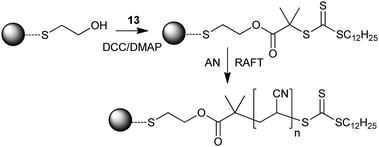
• Oleic acid-stabilized Fe3O4 nanoparticles were converted to nanoparticles with surface trithiocarbonate groups by treatment with 13 in a ligand exchange process.265 These particles were then used in mediating RAFT copolymerization of NIPAM and acrolein.
• RAFT polymerization of AA or St was initiated from ozone treated iron oxide nanoparticles.266
“Grafting to” processes:
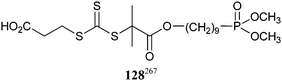
• Stabilized iron oxide nanoparticles were formed in the presence of PEGMA-b-P80 synthesized with cumyl dithiobenzoate (3).171 A variety of polymers were synthesized using trithiocarbonate 128.267 These were converted to the desired heterotelechelic polymers capable of both stabilizing iron oxide nanoparticles and binding biopolymers by transforming the di(methyl)phosphonate group into a phosphonic acid group and the trithiocarbonate into ethylpyridyl disulfide group. PAA-b-PNIPAM-b-P(PEGA) synthesized with 13 was used.268
• The surfactant on oleic acid stabilized nanoparticles was exchanged with carboxy end-functional PNIPAM or biotin end-functional PNIPAM.269 The PNIPAM was formed by RAFT polymerization with trithiocarbonate 14.
In situ particle formation:
• PNIPAM was synthesized using RAFT agent 13 to have a hydrophobic dodecyl group at one end and a carboxyl group at the other end.270 The PNIPAM chains form micelles in tetraglyme solvent with dodecyl groups at the core. The micelles were loaded with Fe(CO)5 to form γ-Fe2O3 containing magnetic iron nanoparticles. Particle size was defined by the size of the precursor micelle.
Quantum dots
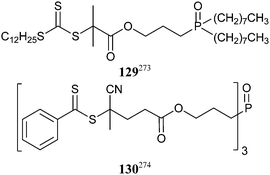
Simple RAFT agents, e.g., the sodium salt of 13, have been used as “surfactants” in solubilising quantum dots in aqueous solution.272The tri-n-octylphosphine oxide (TOPO) ligands of conventional TOPO-stabilized CdSe nanoparticles were exchanged with 129 to attach trithiocarbonate groups and these were used in solution RAFT polymerization of a variety of monomers.273 In a similar manner, CdSe/ZnS quantum dots were functionalized with dithiobenzoate groups using 130 and these used to form PSt and PSt-b-PBA CdSe/ZnS quantum dot nanocomposites by miniemulsion polymerization.274
PAN was grafted from hydroxy-functional cadmium sulfide nanoparticles using the process described in Scheme 17.275
 | ||
| Scheme 17 Process used in “grafting from” quantum dots. | ||
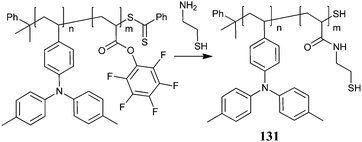
The copolymer 131 was derived from P103-b-P49 (prepared with RAFT agent 3) by reaction with cysteamine as shown in Scheme 18. This copolymer was grafted to CdSe/ZnS quantum dots to prepare hybrid materials for PLEDs.276,277
RAFT polymerization has been used in the synthesis of functional copolymers for use in “grafting to” experiments. Examples include:
• A glycopolymer containing AEMAM units grafted to commercial carboxy-functional CdS(CdTe) quantum dots by carbodiimide coupling.278
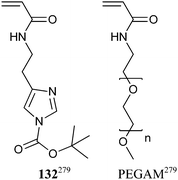
• A polymer containing imidazole functionality (prepared by RAFT copolymerization of monomers 132 and PEGAM with dibenzyl trithiocarbonate (15) and subsequent deprotection) was grafted to CdSe(CdZnS) core(shell) quantum dots by ligand exchange.279
• A RAFT-synthesized dendritic-linear block copolymer based on the 2nd generation dendron RAFT agent 134.263 The PMMA synthesized with 134 was deprotected and the hydroxyl groups reacted with 5-(dioctylphosphoryl)pentanoic anhydride to PMMA with a phosphine oxide functional dendron end-group.
Quantum dot containing nanocomposites or networks have been prepared based on RAFT-synthesized carboxy functional block copolymers such as PBA-b-PMAA (synthesized by macromonomer RAFT)280 or PSt-b-PAA (prepared from PSt-b-PtBA).281
Hydrophobic oleic acid stabilized lead sulfide quantum dots have been transferred from non-polar organic solvents to polar solvents such as alcohols and water by exchanging the oleic acid ligand with RAFT-synthesized PAA.282
Carbon nanotubes, fullerene and graphene
Functionalization of carbon nanotubes using methods based on living radical polymerization (RDRP) and the applications of the materials have been reviewed.283–286The “grafting from” approach has been applied starting with “lightly” oxidized nanotubes with carboxy functionality.287–298 These were transformed to nanotubes with ‘R’ connected RAFT agent functionality as shown in Scheme 19 and then used to prepare nanotubes grafted with MMA,296 St,287NIPAM,288,289HPMAM,290 PS-co-MAH,291 PMMA-b-PS295 or PS-b-PNIPAM297PAA,298 PDMAEMA298 or PMDMAS.298 While there is good evidence for grafting taking place and the mass of polymer was determined, the graft density was not provided. We can note that the approach to nanotube functionalization used in these studies (Scheme 19) was based on substitution of a tertiary bromide. The analogous approach when applied to low molecular weight substrates does not provide high yields.86
 | ||
| Scheme 19 Process used in forming ‘R’-connected macro-RAFT agents from carbon nanotubes. | ||
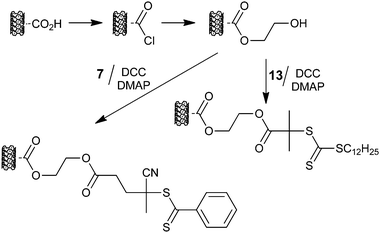
An alternative approach to nanotube functionalization is shown in Scheme 20, in which an acid-functional RAFT agent is coupled to hydroxyl-functional nanotubes with DCC, and has been used to form PHEMA grafts299 or PNVC grafts.300
 | ||
| Scheme 20 Process used in forming ‘R’-connected macro-RAFT agents from carbon nanotubes. | ||
Ellis et al.301 treated carboxy-functional nanotubes as shown in Scheme 21 to attach RAFT agent functionality with proposed structure 133. The use of the functionalized nanotubes in a “grafting from” process with HEMA was presented in a patent application.302 While there was evidence of sulfur incorporation and evidence for grafting after RAFT polymerization, no characterization of the attached polymer or its mode of attachment was provided.
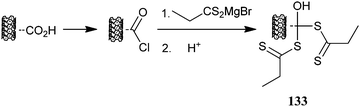 | ||
| Scheme 21 Proposed process for introducing dithioester functionality to carbon nanotubes. | ||
Curran and Ellis303 reported that oxidized nanotubes could be functionalized with dithioester functionality by thiation with phosphorus pentasulfide or Lawesson's reagent; proposed to proceed as shown in Scheme 22. The functionalized nanotubes were used in “grafting from” experiments with styrene.
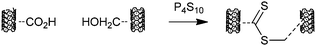 | ||
| Scheme 22 Process for introducing dithioester functionality by thiation with phosphorus pentasulfide. | ||
Single walled carbon nanotubes with Z-connected RAFT agent functionality have also been prepared and used in “grafting from” experiments with AM as shown in Scheme 23.294
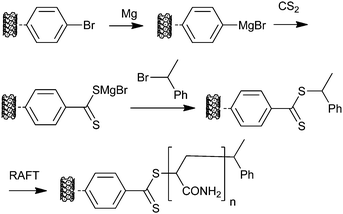 | ||
| Scheme 23 Process used in forming ‘Z’-connected macro-RAFT agents from carbon nanotubes. | ||
There are reports that fullerenes may be incorporated directly in what could be considered a “grafting to” approach.157,304 Heating a solution of RAFT-synthesized PNIPAM with dithiobenzoate ends, C60fullerene and AIBN in N,N-dimethylformamide–chlorobenzene provided PNIPAM that was mono-end capped with fullerene.304 It was proposed that PNIPAM propagating radicals generated by RAFT add to fullerene. The resulting fullerene radicals were trapped by reaction with cyanoisopropyl radicals.
One process for attaching fullerene by a “grafting to” reaction has already been shown in Scheme 12. “Grafting to” processes based on “click” chemistry have been applied to carbon nanotubes305–307 and fullerene derivatives.308
• The thiocarbonylthio end-groups of RAFT-synthesized PNIPAM were converted to thiol end-groups which were in turn coupled to nanotubes functionalized with pyridyl disulfide groups.305,306
• RAFT-synthesized ω-azido(PDMAM-b-PNIPAM) was grafted by copper catalysed “click” reaction to alkyne functional multiwalled nanotubes.307
Covalent attachment to graphene has the drawback that the bonds formed may disrupt the conjugated structure thereby leading to compromised physical or electronic properties. Thus, “grafting to” approaches that involve non-covalent attachment based on π–π stacking seem attractive.309–312
Pyrene end-functional PNIPAM,311PDMAEA312 and PAA312 were prepared using a pyrene functional RAFT agent and then employed in forming graphene composites. A “polysoap” was prepared from RAFT-synthesized PSt-alt-MAH through reaction with 1-aminopyrene and this was used to disperse single-walled carbon nanotubes in aqueous media.310
Inorganic semiconductors
“Grafting from” titania nanoparticles was achieved in two ways. Titania nanoparticles were modified with initiator functionality through reaction with 4,4′-azobis-4-cyanopentanoic acid chloride and “grafting from” of styrene performed in the presence of RAFT agent 3.313 The RAFT agent 12 with an available carboxyl group was used to functionalize the surface of TiO2 nanoparticles and these particles then used in “grafting from” experiments with MMA.314 “Grafting from” indium-tin oxide (ITO) surfaces has also been reported.315
Titania
nanoparticles were functionalized with 3-(trimethoxysilyl)propyl methacrylate. These were copolymerized with MMA and tert-butyldimethylsilyl methacrylate in the presence of RAFT agent 5.316
RAFT polymerization has also been used to synthesize end functional polymer or block copolymer dispersants for TiO2 particles and nanorods. RAFT-synthesized dendritic-linear block copolymers based on the 2nd generation dendron RAFT agent 134 were used to prepare dispersants for TiO2 nanoparticles.263 The PMMA synthesized with 134 was deprotected and the hydroxyl groups reacted with maleic anhydride to give PMMA with a carboxy-functional dendron end-group.
RAFT-synthesized block copolymers based on the active ester 50 (PMMA-b-P50135,317 and PEGMA-b-P50135,317) were functionalized by reaction with dopamine as shown in Scheme 24. These block copolymers were used as dispersants for TiO2 nanorods. The same strategy was used to graft the pendant hole transport polymer P103 to TiO2, SnO2 or ZnO nanorods.137 In this case the precursor polymer was derived from P103-b-P49 prepared with RAFT agent 3.
Films of RAFT-synthesized PEO-b-P102 were used to template the formation of TiO2 in a semiconductor matrix.318 There has also been use of RAFT-synthesized PAA and PAA blocks to form dispersants for TiO2.319
Silicon wafers
The “grafting-from” approach has been widely applied to silicon wafers. Baum and Brittain320 described RAFT polymerization from silicon wafers functionalized with azo-initiator in the presence of RAFT agent 3 and added AIBN. PMMA, PSt and PDMAM homopolymer brushes and PSt-b-PDMAM and PDMAM-b-PMMA diblock brushes were produced. Yu et al.321 used a similar approach to form PCMS brushes (Scheme 25) which were further functionalized with viologen to create a photoresponsive surface. Other examples include PCMS-b-PPFS with cumyl dithiophenylacetate,321 PDMAPS and PDMAPS-b-PSSO3H with 7.321a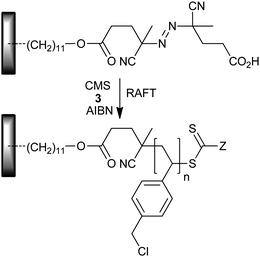 | ||
| Scheme 25 Functionalized silicon wafer with azo-initiator. | ||
Various methods have been used to affix RAFT agent functionality to the surface via ‘Z’ or ‘R’.
• Direct modification of the hydroxy functional silicon wafer surface with the appropriate silane-functional RAFT agent (Scheme 26);322 used for PS and PBA grafts.
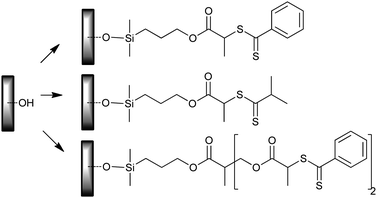 | ||
| Scheme 26 Direct modification of silicon wafer surface with silane-functional RAFT agents. | ||
• Modification via atom transfer radical addition;323 used for PMMA, PDMAEMA, PSt and PSt-b-PMA grafts (Scheme 27).
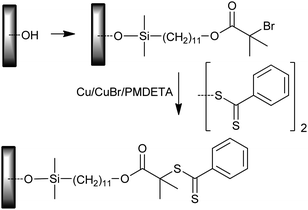 | ||
| Scheme 27 Modification of silicon wafer surface by atom transfer radical addition. | ||
• Modification of the surface with a combination of silane-functional monomer, RAFT agent and initiator (Scheme 28);324 used for PGMA and PEGMA diblock grafts.
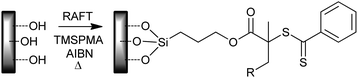 | ||
| Scheme 28 Modification of silicon wafer surface with silane functional methacrylate, RAFT agent, initiator combination. R = –CH(CH3)Ph or –(CH3)2CCN. | ||
• Modification of the surface with amine functionality which is in turn modified using active ester–amine “click” chemistry (Scheme 29);58,66 used for PMMA grafts.
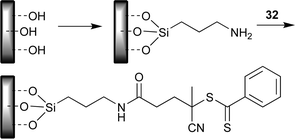 | ||
| Scheme 29 Conversion to amine functional surface and modification by active ester–amine “click” reaction. | ||
• Modification of the chloro-functional silicon wafer surface with sodium ethyl xanthate (Scheme 30);325 used for PMMA grafts. Xanthate RAFT agents are not known to provide control over MMA polymerization.16 It is possible that the xanthate function surface is functioning as a conventional transfer agent in this example.
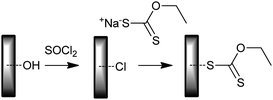 | ||
| Scheme 30 Preparation of xanthate-functional surface. | ||
• Modification of the H functional surface with CMS which is in turn converted to ‘Z’ attached dithiobenzoate functionality (Scheme 31);326 used for PHEMA, PMMA and PHEMA-b-PDMAEMA.
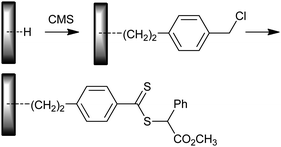 | ||
| Scheme 31 Preparation of dithioester functional surface. | ||
“Grafting to” approaches have also been applied. RAFT-synthesized heterotelechelic NIPAM (–SH and COOH ends) were coupled to silicon wafers with amine functionality (functionalized with 3-aminopropyltrimethoxysilane).327 The thiocarbonyl–diene hetero-Diels–Alder process has also been used to form brushes on silicon wafers.38Styrene units were attached to the surface using silane chemistry. These underwent a hetero-Diels–Alder reaction with RAFT-synthesized poly(isobornyl acrylate) as shown in Scheme 32.
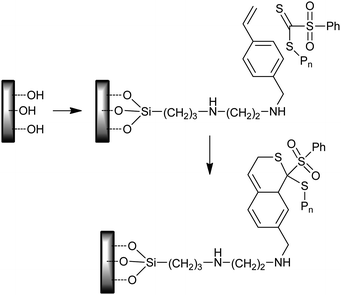 | ||
| Scheme 32 Use of thiocarbonyl–diene hetero-Diels–Alder reaction in surface functionalization. | ||
Silicon wafers or silica particles have been coated sequentially with an amine functional polymer (polyethyleneimine or poly(allylamine hydrochloride)) and RAFT-synthesized PAA-b-PSSO3Na in a layer-by-layer assembly process.328
Photolithography and block copolymer lithography
RAFT synthesized copolymers have found use in photoresist applications.329–332 Uniformity in composition and molecular weight improves rates of dissolution and aids obtaining a low line edge roughness. Acrylate and methacrylate copolymers are used in 193 nm resists while styrenic polymers may be used in 248 nm resists. A complete absence of metal ion contamination is also required.A number of studies have concerned the preparation of polymer films with controlled morphology typically on silicon wafer substrates in what has been called “block copolymer lithography”.333RAFT polymerization has been used both in synthesizing copolymers for so-called surface neutralization layers334 and in making block copolymers designed to give a desired morphology.101,207,334–338 The RAFT-synthesized polymers used in this application include PEO-b-PMMA-b-PS,336 PLA-b-P105207 (prepared by using PEO or PLA macro-RAFT agents respectively), PMMA-b-(PSt-co-4VP),337PMMA-b-(4-(acryloyloxy)phenyl)-dimethylsulfonium 2,2,2-trifluoroacetate338 and P(MMA-co-CMS-co-St).334
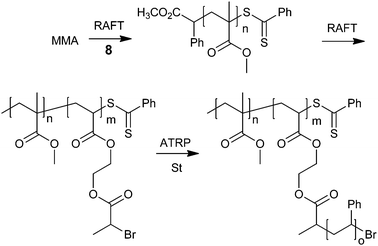
A recent example is PMMA with well-defined PSt grafts and a comb–coil architecture which was synthesized by a combination of RAFT and ATRP (Scheme 33). This copolymer provided films consisting of cylindrical microdomains oriented perpendicular to the film plane.339
Conclusions
The use of synthetic polymers in the field of optoelectronics is currently experiencing marked growth. Well over half of the references cited in this review were published in the last two years (2008–2010) and new developments are being reported on a daily basis. Applications include OPVs, OLEDs, PLEDs, TFTs, sensors, and related devices. RAFT polymerization, in providing the ability to synthesize a wide range of polymers with precise control over molecular weight, molecular weight distribution, architecture and composition and its remarkable tolerance of functionality is already providing benefits and is positioned to play a significant role in the further development of this field. In particular, we predict a bright future for RAFT-synthesized block copolymers as materials or as additives.Significant benefits of RAFT polymerization are the ability to form polymers with narrow molecular weight distributions and to construct block copolymers and other designed architectures with defined composition and end-group functionality. Narrow molecular weight distributions make it possible to eliminate the low molecular weight “impurities” which can act as hole or electron traps while, at the same time, targeting the modest molecular weights that offer advantages in solubility, processing and film forming characteristics. The ability to precisely control polymer architecture should enable control over the morphology of polymer films. However, the relationship between architecture and morphology is difficult to predict for functional polymers.182,186 Thus, the ability to rapidly synthesize a range of structures is extremely important in enabling this space to be explored and may ultimately redress the issue of structure–property prediction. The thiocarbonyl functionality of RAFT-synthesized polymers was once seen as a limitation to the wide-spread application of RAFT. Research on end-group transformation/removal has now shown the thiocarbonyl to be an enabling functionality in addressing the needs of optoelectronic and other fields.
Abbreviations
| AA | acrylic acid |
| AEAM | 2-aminoethyl acrylamide |
| AEMAM | 2-aminoethyl methacrylamide |
| AMPS | sodium 2-acrylamido-2-methyl propane-1-sulfonate |
| AN | acrylonitrile |
| ATRP | atom transfer radical polymerization |
| b | block |
| BA | butyl acrylate |
| CMS | 4-(chloromethyl)styrene |
| DEGMA | (diethylene glycol monomethyl ether) methacrylate or (2-(2-methoxyethoxy)ethyl methacrylate) |
| DHPAM | (2,3-dihydroxypropyl)acrylamide |
| DMAEMA | 2-(dimethylamino)ethyl methacrylate |
| DMAM | N,N-dimethylacrylamide |
| DVB | divinylbenzene |
| GMA | glycidyl methacrylate |
| DMAPS | 3-((2-(methacryloyloxy)ethyl)dimethylammonio) propane-1-sulfonate |
| HEMA | hydroxyethyl methacrylate |
| MDMAS | 3-((3-methacrylamidopropyl)dimethylammonio)propane-1-sulfonate |
| MA | methyl acrylate |
| MAA | methacrylic acid |
| MAH | maleic anhydride |
| MAEDAPS | (3-(2-N-methylacrylamido)ethyl)dimethyl ammoniopropane sulfonate |
| MMA | methyl methacrylate |
| NIPAM | N-isopropyl acrylamide |
| NMP | nitroxide mediated polymerization |
| NVC | N-vinylcarbazole (99) |
| OEGA | oligo(ethylene glycol) acrylate |
| P3HT | poly(3-hexylthiophene) |
| PCBM | [6,6]-phenyl-C61-butyric acid methyl ester |
| PEGA | poly(ethylene glycol) acrylate |
| PEGAM | poly(ethylene glycol) acrylamide |
| PEGMA | poly(ethylene glycol) methacrylate |
| PFS | pentafluorostyrene |
| PLA | polylactic acid |
| Pn | polymer chain of length n |
| RAFT | reversible addition–fragmentation chain transfer |
| RDRP | reversible deactivation radical polymerization |
| SSO3H | styrene-4-sulfonic acid |
| SOH | 4-hydroxystyrene |
| St | styrene |
| StB | 4-(3-butenyl)styrene |
| t BA | tert-butyl acrylate |
| THF | tetrahydrofuran |
| TMSPMA | 3-(trimethoxysilyl)propyl methacrylate |
| VBTAC | (ar-vinylbenzyl) trimethyl ammonium chloride |
| 2VP | 2-vinylpyridine |
| 4VP | 4-vinylpyridine |
References and notes
-
A. Favier, B. de Lambert and M.-T. Charreyre, in Handbook of RAFT Polymerization, ed. C. Barner-Kowollik, Wiley-VCH, Weinheim, Germany, 2008, pp. 483–535 Search PubMed
.
- J. Chiefari, Y. K. Chong, F. Ercole, J. Krstina, J. Jeffery, T. P. T. Le, R. T. A. Mayadunne, G. F. Meijs, C. L. Moad, G. Moad, E. Rizzardo and S. H. Thang, Macromolecules, 1998, 31, 5559–5562 CrossRef CAS
.
-
T. P. Le, G. Moad, E. Rizzardo and S. H. Thang, Polymerization with living characteristics, DuPont/CSIRO, WO9801478, 1998
.
- G. Moad, E. Rizzardo and S. H. Thang, Polymer, 2008, 49, 1079–1131 CrossRef CAS
.
- G. Moad, E. Rizzardo and S. H. Thang, Acc. Chem. Res., 2008, 41, 1133–1142 CrossRef CAS
.
- G. Moad, E. Rizzardo and S. H. Thang, Aust. J. Chem., 2005, 58, 379–410 CrossRef CAS
.
- G. Moad, E. Rizzardo and S. H. Thang, Aust. J. Chem., 2006, 59, 669–692 CrossRef CAS
.
- G. Moad, E. Rizzardo and S. H. Thang, Aust. J. Chem., 2009, 62, 1402–1472 CrossRef CAS
.
- A. Favier and M.-T. Charreyre, Macromol. Rapid Commun., 2006, 27, 653–692 CrossRef CAS
.
- S. Perrier and P. Takolpuckdee, J. Polym. Sci., Part A: Polym. Chem., 2005, 43, 5347–5393 CrossRef CAS
.
- C. Boyer, V. Bulmus, T. P. Davis, V. Ladmiral, J. Liu and S.b. Perrier, Chem. Rev., 2009, 109, 5402–5436 CrossRef CAS
.
- A. D. Jenkins, R. I. Jones and G. Moad, Pure Appl. Chem., 2010, 82, 483–491 CrossRef CAS
.
- T. R. Darling, T. P. Davis, M. Fryd, A. A. Gridnev, D. M. Haddleton, S. D. Ittel, R. R. Matheson, Jr, G. Moad and E. Rizzardo, J. Polym. Sci., Part A: Polym. Chem., 2000, 38, 1706–1708 CrossRef CAS
.
- R. P. Quirk and B. Lee, Polym. Int., 1992, 27, 359–367 CrossRef CAS
.
-
E. Rizzardo, G. Moad and S. H. Thang, in Handbook of RAFT Polymerization, ed. C. Barner-Kowollik, Wiley-VCH, Weinheim, Germany, 2008, pp. 189–234 Search PubMed
.
- J. Chiefari, R. T. A. Mayadunne, C. L. Moad, G. Moad, E. Rizzardo, A. Postma, M. A. Skidmore and S. H. Thang, Macromolecules, 2003, 36, 2273–2283 CrossRef CAS
.
- R. T. A. Mayadunne, E. Rizzardo, J. Chiefari, Y. K. Chong, G. Moad and S. H. Thang, Macromolecules, 1999, 32, 6977–6980 CrossRef CAS
.
- M. Benaglia, J. Chiefari, Y. K. Chong, G. Moad, E. Rizzardo and S. H. Thang, J. Am. Chem. Soc., 2009, 131, 6914–6915 CrossRef CAS
.
- M. Benaglia, M. Chen, Y. K. Chong, G. Moad, E. Rizzardo and S. H. Thang, Macromolecules, 2009, 42, 9384–9386 CrossRef CAS
.
- K. Matyjaszewski and J. Xia, Chem. Rev., 2001, 101, 2921–2990 CrossRef CAS
.
- W. A. Braunecker and K. Matyjaszewski, Prog. Polym. Sci., 2007, 32, 93–146 CrossRef CAS
.
- M. Ouchi, T. Terashima and M. Sawamoto, Chem.
Rev., 2009, 109, 4963–5050 CrossRef CAS
.
- B. M. Rosen and V. Percec, Chem. Rev., 2009, 109, 5069–5119 CrossRef CAS
.
- C. J. Hawker, A. W. Bosman and E. Harth, Chem. Rev., 2001, 101, 3661–3688 CrossRef CAS
.
- R. A. Evans, Aust. J. Chem., 2007, 60, 384–395 CrossRef CAS
.
- R. K. Iha, K. L. Wooley, A. M. Nyström, D. J. Burke, M. J. Kade and C. J. Hawker, Chem. Rev., 2009, 109, 5620–5686 CrossRef CAS
.
- W. H. Binder and R. Sachsenhofer, Macromol. Rapid Commun., 2007, 28, 15–54 CrossRef CAS
.
- W. H. Binder and R. Sachsenhofer, Macromol. Rapid Commun., 2008, 29, 952–981 CrossRef CAS
.
- B. S. Sumerlin and A. P. Vogt, Macromolecules, 2009, 43, 1–13
.
- P. L. Golas and K. Matyjaszewski, Chem. Soc. Rev., 2010, 39, 1338–1354 RSC
.
- A. J. Inglis, S. Sinnwell, T. P. Davis, C. Barner-Kowollik and M. H. Stenzel, Macromolecules, 2008, 41, 4120–4126 CrossRef CAS
.
- S. Sinnwell, A. J. Inglis, T. P. Davis, M. H. Stenzel and C. Barner-Kowollik, Chem. Commun., 2008, 2052–2054 RSC
.
- S. Sinnwell, C. V. Synatschke, T. Junkers, M. H. Stenzel and C. Barner-Kowollik, Macromolecules, 2008, 41, 7904–7912 CrossRef CAS
.
- S. Sinnwell, M. Lammens, M. H. Stenzel, F. E. Du Prez and C. Barner-Kowollik, J. Polym. Sci., Part A: Polym. Chem., 2009, 47, 2207–2213 CrossRef CAS
.
- S. Sinnwell, A. J. Inglis, M. H. Stenzel and C. Barner-Kowollik, Macromol. Rapid Commun., 2008, 29, 1090–1096 CrossRef CAS
.
- L. Nebhani, S. Sinnwell, A. J. Inglis, M. H. Stenzel, C. Barner-Kowollik and L. Barner, Macromol. Rapid Commun., 2008, 29, 1431–1437 CrossRef CAS
.
- A. J. Inglis, S. Sinnwell, M. H. Stenzel and C. Barner-Kowollik, Angew. Chem., Int. Ed., 2009, 48, 2411–2414 CAS
.
- L. Nebhani, P. Gerstel, P. Atanasova, M. Bruns and C. Barner-Kowollik, J. Polym. Sci., Part A: Polym. Chem., 2009, 47, 7090–7095 CrossRef
.
- L. Nebhani, S. Sinnwell, C. Y. Lin, M. L. Coote, M. H. Stenzel and C. Barner-Kowollik, J. Polym. Sci., Part A: Polym. Chem., 2009, 47, 6053–6071 CrossRef CAS
.
- C. E. Hoyle and C. N. Bowman, Angew. Chem., Int. Ed., 2010, 49, 1540–1573 CrossRef CAS
.
- A. B. Lowe, Polym. Chem., 2010, 1, 17–36 RSC
.
- C. E. Hoyle, A. B. Lowe and C. N. Bowman, Chem. Soc. Rev., 2010, 39, 1355–1387 RSC
.
- V. Ladmiral, T. M. Legge, Y. L. Zhao and S. Perrier, Macromolecules, 2008, 41, 6728–6732 CrossRef CAS
.
- S. R. Gondi, A. P. Vogt and B. S. Sumerlin, Macromolecules, 2007, 40, 474–481 CrossRef CAS
.
- J. Zhu, X. Zhu, E. T. Kang and K. G. Neoh, Polymer, 2007, 48, 6992–6999 CrossRef CAS
.
- A. Vora, K. Singh and D. C. Webster, Polymer, 2009, 50, 2768–2774 CrossRef CAS
.
- C. Boyer, J. Liu, V. Bulmus, T. P. Davis, C. Barner-Kowollik and M. H. Stenzel, Macromolecules, 2008, 41, 5641–5650 CrossRef CAS
.
- F. Chen, Z. P. Cheng, J. Zhu, W. Zhang and X. L. Zhu, Eur. Polym. J., 2008, 44, 1789–1795 CrossRef CAS
.
- D. Quemener, T. P. Davis, C. Barner-Kowollik and M. H. Stenzel, Chem. Commun., 2006, 5051–5053 RSC
.
-
(a) R. K. O’Reilly, M. J. Joralemon, C. J. Hawker and K. L. Wooley, J. Polym. Sci., Part A: Polym. Chem., 2006, 44, 5203–5217 CrossRef CAS
; (b) N. Akeroyd, R. Pfukwa and B. Klumperman, Macromolecules, 2009, 42, 3014–3018 CrossRef CAS
.
- R. Ranjan and W. J. Brittain, Macromol. Rapid Commun., 2007, 28, 2084–2089 CrossRef CAS
.
- R. Ranjan and W. J. Brittain, Macromol. Rapid Commun., 2008, 29, 1104–1110 CrossRef CAS
.
- T. Zhang, Z. Zheng, X. Ding and Y. Peng, Macromol. Rapid Commun., 2008, 29, 1716–1720 CrossRef CAS
.
- P. J. Roth, K. T. Wiss, R. Zentel and P. Theato, Macromolecules, 2008, 41, 8513–8519 CrossRef CAS
.
- P. J. Roth, F. D. Jochum, R. Zentel and P. Theato, Biomacromolecules, 2010, 11, 238–244 CrossRef CAS
.
- R. Briquel, J. Mazzolini, T. L. Bris, O. Boyron, F. Boisson, F. Delolme, F. D'Agosto, C. Boisson and R. Spitz, Angew. Chem., Int. Ed., 2008, 47, 9311–9313 CrossRef CAS
.
- K. A. Aamer and G. N. Tew, J. Polym. Sci., Part A: Polym. Chem., 2007, 45, 5618–5625 CrossRef CAS
.
- K. Yuan, L.-L. Lu, Z.-F. Li and X.-N. Shi, Chin. J. Chem., 2008, 26, 1929–1934 CrossRef CAS
.
- X. Zhang, J. Li, W. Li and A. Zhang, Biomacromolecules, 2007, 8, 3557–3567 CrossRef CAS
.
- D. H. Han, L. P. Yang, X. F. Zhang and C. Y. Pan, Eur. Polym. J., 2007, 43, 3873–3881 CrossRef CAS
.
- M. Bathfield, F. D'Agosto, R. Spitz, M. T. Charreyre and T. Delair, J. Am. Chem. Soc., 2006, 128, 2546–2547 CrossRef CAS
.
- M.l. Bathfield, D. Daviot, F. D'Agosto, R. Spitz, C. Ladaviere, M.-T. Charreyre and T. Delair, Macromolecules, 2008, 41, 8346–8353 CrossRef CAS
.
- T. J. V. Prazeres, M. Beija, M.-T. Charreyre, J. P. S. Farinha and J. M. G. Martinho, Polymer, 2010, 51, 355–367 CrossRef CAS
.
- A. Postma, T. P. Davis, R. A. Evans, G. Li, G. Moad and M. O'Shea, Macromolecules, 2006, 39, 5293–5306 CrossRef CAS
.
- A. Postma, T. P. Davis, G. Li, G. Moad and M. O'Shea, Macromolecules, 2006, 39, 5307–5318 CrossRef CAS
.
- K. Yuan, Z. F. Li, L. L. Lu and X. N. Shi, Mater. Lett., 2007, 61, 2033–2036 CrossRef CAS
.
- J. P. S. Farinha, P. Relogio, M.-T. Charreyre, T. J. V. Prazeres and J. M. G. Martinho, Macromolecules, 2007, 40, 4680–4690 CrossRef CAS
.
- M. Chen, K. P. Ghiggino, E. Rizzardo, S. H. Thang and G. J. Wilson, Chem. Commun., 2008, 1112–1114 RSC
.
- A. D. Kitchin, S. Velate, M. Chen, K. P. Ghiggino, T. A. Smith and R. P. Steer, Photochem. Photobiol. Sci., 2007, 6, 853–856 RSC
.
- S. Kato and M. Ishida, Sulfur Rep., 1988, 8, 155–323 CrossRef CAS
.
-
R. Mayer and S. Scheithauer, in Methoden den Organischen Chemie, ed. K. H. Buechel, J. Falbe, H. Hagemann and M. Hanack, Thieme, Stuttgart, 1985, pp. 891–930 Search PubMed
.
- B. Quiclet-Sire and S. Z. Zard, Top. Curr. Chem., 2006, 264, 201–236 CAS
.
- S. Z. Zard, Aust. J. Chem., 2006, 59, 663–668 CrossRef CAS
.
- B. Chong, G. Moad, E. Rizzardo, M. Skidmore and S. H. Thang, Aust. J. Chem., 2006, 59, 755–762 CrossRef CAS
.
- A. Postma, T. P. Davis, G. Moad and M. S. O'Shea, Macromolecules, 2005, 38, 5371–5374 CrossRef CAS
.
- A. Postma, T. P. Davis, G. Moad and M. S. O'Shea, React. Funct. Polym., 2006, 66, 137–147 CrossRef CAS
.
- Y. K. Chong, G. Moad, E. Rizzardo and S. H. Thang, Macromolecules, 2007, 40, 4446–4455 CrossRef CAS
.
- S. Perrier, P. Takolpuckdee and C. A. Mars, Macromolecules, 2005, 38, 2033–2036 CrossRef CAS
.
- M. Chen, G. Moad and E. Rizzardo, J. Polym. Sci., Part A: Polym. Chem., 2009, 47, 6704–6714 CrossRef CAS
.
- M. Dietrich, M. Glassner, T. Gruendling, C. Schmid, J. Falkenhagen and C. Barner-Kowollik, Polym. Chem., 2010, 1, 634–644 RSC
.
- T. Gruendling, M. Dietrich and C. Barner-Kowollik, Aust. J. Chem., 2009, 62, 806–812 CrossRef CAS
.
- H. Willcock and R. K. O'Reilly, Polym. Chem., 2010, 1, 149–157 RSC
.
- G. Moad, Y. K. Chong, E. Rizzardo, A. Postma and S. H. Thang, Polymer, 2005, 46, 8458–8468 CrossRef CAS
.
- G. Moad, E. Rizzardo and S. H. Thang, Polym. Int., 2010 Search PubMed
, in press.
-
L. Barner and S. Perrier, in Handbook of RAFT Polymerization, ed. C. Barner-Kowollik, Wiley-VCH, Weinheim, Germany, 2008, pp. 455–482 Search PubMed
.
- Y. K. Chong, J. Krstina, T. P. T. Le, G. Moad, A. Postma, E. Rizzardo and S. H. Thang, Macromolecules, 2003, 36, 2256–2272 CrossRef CAS
.
- A. Alberti, M. Benaglia, M. Laus and K. Sparnacci, J. Org. Chem., 2002, 67, 7911–7914 CrossRef CAS
.
- J. W. Chan, B. Yu, C. E. Hoyle and A. B. Lowe, Chem. Commun., 2008, 4959–4961 RSC
.
- J. M. Spruell, B. A. Levy, A. Sutherland, W. R. Dichtel, J. Y. Cheng, J. F. Stoddart and A. Nelson, J. Polym. Sci., Part A: Polym. Chem., 2009, 47, 346–356 CrossRef CAS
.
- C. Boyer, V. Bulmus and T. P. Davis, Macromol. Rapid Commun., 2009, 30, 493–497 CrossRef
.
- P. J. Roth, D. Kessler, R. Zentel and P. Theato, Macromolecules, 2008, 41, 8316–8319 CrossRef CAS
.
- X.-P. Qiu and F. M. Winnik, Macromol. Rapid Commun., 2006, 27, 1648–1653 CrossRef CAS
.
- H. Li, B. Yu, H. Matsushima, C. E. Hoyle and A. B. Lowe, Macromolecules, 2009, 42, 6537–6542 CrossRef CAS
.
- J. Xu, L. Tao, C. Boyer, A. B. Lowe and T. P. Davis, Macromolecules, 2009, 43, 20–24
.
- P. J. Roth, D. Kessler, R. Zentel and P. Theato, J. Polym. Sci., Part A: Polym. Chem., 2009, 47, 3118–3130 CrossRef CAS
.
- S. P. S. Koo, M. M. Stamenovic, R. A. Prasath, A. J. Inglis, F. E. Du Prez, C. Barner-Kowollik, W. V. Camp and T. Junkers, J. Polym. Sci., Part A: Polym. Chem., 2010, 48, 1699–1713 CrossRef CAS
.
- J. A. Johnson, M. G. Finn, J. T. Koberstein and N. J. Turro, Macromol. Rapid Commun., 2008, 29, 1052–1072 CrossRef CAS
.
- D. Quemener, M. Le Hellaye, C. Bissett, T. P. Davis, C. Barner-Kowollik and M. H. Stenzel, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 155–173 CrossRef CAS
.
- A. Krieg, C. R. Becer, R. Hoogenboom and U. S. Schubert, Macromol. Symp., 2009, 275–276, 73–81 CrossRef CAS
.
- R. K. O'Reilly, M. J. Joralemon, C. J. Hawker and K. L. Wooley, Chem.–Eur. J., 2006, 12, 6776–6786 CrossRef CAS
.
- J. Stadermann, S. Fleischmann, M. Messerschmidt, H. Komber and B. Voit, Macromol. Symp., 2009, 275–276, 35–42 CrossRef CAS
.
- X. Zhang, X. Lian, L. Liu, J. Zhang and H. Zhao, Macromolecules, 2008, 41, 7863–7869 CrossRef CAS
.
- F. Cavalieri, A. Postma, L. Lee and F. Caruso, ACS Nano, 2009, 3, 234–240 CrossRef CAS
.
- G. K. Such, E. Tjipto, A. Postma, A. P. R. Johnston and F. Caruso, Nano Lett., 2007, 7, 1706–1710 CrossRef CAS
.
- G. K. Such, J. F. Quinn, A. Quinn, E. Tjipto and F. Caruso, J. Am. Chem. Soc., 2006, 128, 9318–9319 CrossRef CAS
.
- L. A. Canalle, S. S. van Berkel, L. T. de Haan and J. C. M. van Hest, Adv. Funct. Mater., 2009, 19, 3464–3470 CrossRef CAS
.
- Y. Li and B. C. Benicewicz, Macromolecules, 2008, 41, 7986–7992 CrossRef CAS
.
- Y. Li, J. W. Yang and B. C. Benicewicz, J. Polym. Sci., Part A: Polym. Chem., 2007, 45, 4300–4308 CrossRef CAS
.
- X. Z. Jiang, J. Y. Zhang, Y. M. Zhou, J. Xu and S. Y. Liu, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 860–871 CrossRef CAS
.
- G. Li, H. Zheng and R. Bai, Macromol. Rapid Commun., 2009, 30, 442–447 CrossRef CAS
.
- L. He, E. S. Read, S. P. Armes and D. J. Adams, Macromolecules, 2007, 40, 4429–4438 CrossRef CAS
.
- K. L. Thompson, E. S. Read and S. P. Armes, Polym. Degrad. Stab., 2008, 93, 1460–1466 CrossRef CAS
.
- Y. Li, B. S. Lokitz and C. L. McCormick, Angew. Chem., Int. Ed., 2006, 45, 5792–5795 CrossRef CAS
.
- Z. C. Deng, H. Bouchekif, K. Babooram, A. Housni, N. Choytun and R. Narain, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 4984–4996 CrossRef CAS
.
- A. W. York, Y. Zhang, A. C. Holley, Y. Guo, F. Huang and C. L. McCormick, Biomacromolecules, 2009, 10, 936–943 CrossRef CAS
.
- Y. Zhou, K. Jiang, Y. Chen and S. Liu, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 6518–6531 CrossRef CAS
.
- Z. Jia, L. Wong, T. P. Davis and V. Bulmus, Biomacromolecules, 2008, 9, 3106–3113 CrossRef CAS
.
- L. Wong, C. Boyer, Z. Jia, H. M. Zareie, T. P. Davis and V. Bulmus, Biomacromolecules, 2008, 9, 1934–1944 CrossRef CAS
.
- M. J. Kade, D. J. Burke and C. J. Hawker, J. Polym. Sci., Part A: Polym. Chem., 2010, 48, 743–750 CrossRef CAS
.
- J. Ma, C. Cheng, G. Sun and K. L. Wooley, Macromolecules, 2008, 41, 9080–9089 CrossRef CAS
.
- J. Ma, C. Cheng and K. L. Wooley, Macromolecules, 2009, 42, 1565–1573 CrossRef CAS
.
- J. Ma, C. Cheng and K. L. Wooley, Aust. J. Chem., 2009, 62, 1507–1519 CrossRef CAS
.
- K. M. Yeh and Y. Chen, J. Polym. Sci., Part A: Polym. Chem., 2006, 44, 5362–5377 CrossRef CAS
.
- K.-M. Yeh and Y. Chen, J. Polym. Sci., Part A: Polym. Chem., 2007, 45, 2259–2272 CrossRef CAS
.
- K. M. Yeh and Y. Chen, Synth. Met., 2008, 158, 411–416 CrossRef CAS
.
- M. J. Yanjarappa, K. V. Gujraty, A. Joshi, A. Saraph and R. S. Kane, Biomacromolecules, 2006, 7, 1665–1670 CrossRef CAS
.
- H. Kakwere and S.b. Perrier, J. Am. Chem. Soc., 2009, 131, 1889–1895 CrossRef CAS
.
- K. V. Gujraty, M. J. Yanjarappa, A. Saraph, A. Joshi, J. Mogridge and R. S. Kane, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 7249–7257 CrossRef CAS
.
- M. D. Rowe, D. H. Thamm, S. L. Kraft and S. G. Boyes, Biomacromolecules, 2009, 10, 983–993 CrossRef CAS
.
- Y. Li, I. Akiba, S. Harrisson and K. L. Wooley, Adv. Funct. Mater., 2008, 18, 551–559 CrossRef CAS
.
- X. Lou, G. Zhang, I. Herrera, R. Kinach, O. Ornatsky, V. Baranov, M. Nitz and M. A. Winnik, Angew. Chem., Int. Ed., 2007, 46, 6111–6114 CrossRef CAS
.
- N. Metz and P. Theato, Eur. Polym. J., 2007, 43, 1202–1209 CrossRef CAS
.
- M. Barz, M. Tarantola, K. Fischer, M. Schmidt, R. Luxenhofer, A. Janshoff, P. Theato and R. Zentel, Biomacromolecules, 2008, 9, 3114–3118 CrossRef CAS
.
- M. Eberhardt and P. Théato, Macromol. Rapid Commun., 2005, 26, 1488–1493 CrossRef CAS
.
- S. Meuer, P. Oberle, P. Theato, W. Tremel and R. Zentel, Adv. Mater., 2007, 19, 2073–2078 CrossRef CAS
.
- M. Zorn, S. Meuer, M. N. Tahir, Y. Khalavka, C. Sonnichsen, W. Tremel and R. Zentel, J. Mater. Chem., 2008, 18, 3050–3058 RSC
.
- M. Zorn and R. Zentel, Macromol. Rapid Commun., 2008, 29, 922–927 CrossRef CAS
.
- R. C. Li, J. Hwang and H. D. Maynard, Chem. Commun., 2007, 3631–3633 RSC
.
- J. Y. Hwang, R. C. Li and H. D. Maynard, J. Controlled Release, 2007, 122, 279–286 CrossRef CAS
.
- K. Nilles and P. Theato, Eur. Polym. J., 2007, 43, 2901–2912 CrossRef CAS
.
- K. Nilles and P. Theato, J. Polym. Sci., Part A: Polym. Chem., 2009, 47, 1696–1705 CrossRef CAS
.
- G. R. Whittell and I. Manners, Adv. Mater., 2007, 19, 3439–3468 CrossRef CAS
.
- M. Chen, K. P. Ghiggino, A. Launikonis, A. W. H. Mau, E. Rizzardo, W. H. F. Sasse, S. H. Thang and G. J. Wilson, J. Mater. Chem., 2003, 13, 2696–2700 RSC
.
- M. Chen, K. R. Ghiggino, S. H. Thang and G. J. Wilson, Angew. Chem., Int. Ed., 2005, 44, 4368–4372 CrossRef CAS
.
- M. Chen, K. P. Ghiggino, S. H. Thang and G. J. Wilson, J. Chin. Chem. Soc. (Taipei), 2006, 53, 79–83 CAS
.
- G. E. Southard, K. A. Van Houten and G. M. Murray, Macromolecules, 2007, 40, 1395–1400 CrossRef CAS
.
- G. E. Southard, K. A. Van Houten, E. W. Ott, Jr and G. M. Murray, Anal. Chim. Acta, 2007, 581, 202–207 CrossRef CAS
.
- N. Zhou, Z. Zhang, J. Zhu, Z. Cheng and X. Zhu, Macromolecules, 2009, 42, 3898–3905 CrossRef CAS
.
- A. O. Moughton and R. K. O'Reilly, Macromol. Rapid Commun., 2010, 31, 37–52 CrossRef CAS
.
- M. Chiper, R. Hoogenboom and U. S. Schubert, Macromol. Rapid Commun., 2009, 30, 565–578 CrossRef CAS
.
- M. Chiper, D. Fournier, R. Hoogenboom and U. S. Schubert, Macromol. Rapid Commun., 2008, 29, 1640–1647 CrossRef CAS
.
- R. Geagea, R. Stefak, S. Mazieres and M. Destarac, Polym. Prepr. (Am. Chem. Soc., Div. Polym. Chem.), 2008, 49, 250–251 CAS
.
- C.-L. Chen, Y.-H. Lo, C.-Y. Lee, Y.-H. Fong, K.-C. Shih and C.-C. Huang, Inorg. Chem. Commun., 2010, 13, 603–605 CrossRef CAS
.
- A. O. Moughton, K. Stubenrauch and R. K. O'Reilly, Soft Matter, 2009, 5, 2361–2370 RSC
.
- G. Zhou, J. He and I. I. Harruna, J. Polym. Sci., Part A: Polym. Chem., 2007, 45, 4225–4239 CrossRef CAS
.
- G. C. Zhou and I. I. Harruna, Anal. Chem., 2007, 79, 2722–2727 CrossRef CAS
.
- G. C. Zhou, J. B. He, I. I. Harruna and K. E. Geckeler, J. Mater. Chem., 2008, 18, 5492–5501 RSC
.
- G. Zhou and I. I. Harruna, Macromolecules, 2004, 37, 7132–7139 CrossRef CAS
.
- G. C. Zhou, I. I. Harruna and C. W. Ingram, Polymer, 2005, 46, 10672–10677 CrossRef CAS
.
- G. Zhou and I. I. Harruna, Macromolecules, 2005, 38, 4114–4123 CrossRef CAS
.
- L. W. Zhang, Y. H. Zhang and Y. M. Chen, Eur. Polym. J., 2006, 42, 2398–2406 CrossRef CAS
.
- L. Munuera and R. K. O'Reilly, Dalton Trans., 2010, 39, 388–391 RSC
.
- R. Shunmugam and G. N. Tew, Macromol. Rapid Commun., 2008, 29, 1355–1362 CrossRef CAS
.
- B. Happ, C. Friebe, A. Winter, M. D. Hager and U. S. Schubert, Eur. Polym. J., 2009, 45, 3433–3441 CrossRef CAS
.
- W. Y. Tam, C. S. K. Mak, A. M. C. Ng, A. B. Djurisic and W. K. Chan, Macromol. Rapid Commun., 2009, 30, 622–626 CrossRef CAS
.
- L.-P. Wang, L.-M. Zhao and W.-Z. Li, React. Funct. Polym., 2010, 70, 35–40 CrossRef CAS
.
- M. Shi, A.-L. Li, H. Liang and J. Lu, Macromolecules, 2007, 40, 1891–1896 CrossRef CAS
.
- S. J. Li, M. N. Lin, J. Lu and H. Liang, Macromolecules, 2009, 42, 1258–1263 CrossRef CAS
.
- Y. Jin, J. Zhu, Z. Zhang, Z. Cheng, W. Zhang and X. Zhu, Eur. Polym. J., 2008, 44, 1743–1751 CrossRef CAS
.
- D. Li, J. Zhu, Z. Cheng, W. Zhang and X. Zhu, React. Funct. Polym., 2009, 69, 240–245 CrossRef CAS
.
- P. Papaphilippou, L. Loizou, N. C. Popa, A. Han, L. Vekas, A. Odysseos and T. Krasia-Christoforou, Biomacromolecules, 2009, 10, 2662–2671 CrossRef CAS
.
- C. Ulbricht, C. R. Becer, A. Winter and U. S. Schubert, Macromol. Rapid Commun., 2010, 31, 827–833 CrossRef CAS
.
- D. Zhou, X. L. Zhu, J. Zhu and Z. P. Cheng, Polymer, 2008, 49, 3048–3053 CrossRef CAS
.
- G. N. Tew, K. A. Aamer and R. Shunmugam, ACS Symp. Ser., 2006, 928, 126–140 CAS
.
- K. A. Aamer and G. N. Tew, Macromolecules, 2004, 37, 1990–1993 CrossRef CAS
.
- J. H. Alstrum-Acevedo, J. M. DeSimone, C. K. Schauer and J. M. Papanikolas, Mater. Res. Soc. Symp. Proc., 2005, 874, EE8.10.11–EE18.10.17
.
- M. Destarac, D. Taton, S. Z. Zard, T. Saleh and Y. Six, ACS Symp. Ser., 2003, 854, 536–550 CAS
.
- C. Park, J. Yoon and E. L. Thomas, Polymer, 2003, 44, 6725–6760 CrossRef CAS
.
- H.-C. Kim, S.-M. Park and W. D. Hinsberg, Chem. Rev., 2009, 110, 146–177
.
- I. Botiz and S. B. Darling, Mater. Today (Oxford, UK), 2010, 13, 42–51 CrossRef CAS
.
- F. S. Bates and G. H. Fredrickson, Annu. Rev. Phys. Chem., 1990, 41, 525–557 CrossRef CAS
.
- S. B. Darling, Energy Environ. Sci., 2009, 2, 1266–1273 RSC
.
- K. Sivula, Z. T. Ball, N. Watanabe and J. M. J. Fréchet, Adv. Mater., 2006, 18, 206–210 CrossRef CAS
.
- S. Rajaram, P. B. Armstrong, B. J. Kim and J. M. J. Frechet, Chem. Mater., 2009, 21, 1775–1777 CrossRef CAS
.
- A. Pron, P. Gawrys, M. Zagorska, D. Djurado and R. Demadrille, Chem. Soc. Rev., 2010, 39, 2577–2632 RSC
.
- R. A. Segalman, B. McCulloch, S. Kirmayer and J. J. Urban, Macromolecules, 2009, 42, 9205–9216 CrossRef CAS
.
- U. Scherf, A. Gutacker and N. Koenen, Acc. Chem. Res., 2008, 41, 1086–1097 CrossRef CAS
.
- B. D. Olsen and R. A. Segalman, Mater. Sci. Eng., R, 2008, 62, 37–66 CrossRef
.
-
M. Chen, G. Moad, E. Rizzardo,R. A. Evans and M. Haeussler, Conducting and Semiconducting Organic Materials, CSIRO, WO2009155657A1, 2009
.
- M. Chen, K. P. Ghiggino, A. W. H. Mau, E. Rizzardo, W. H. F. Sasse, S. H. Thang and G. J. Wilson, Macromolecules, 2004, 37, 5479–5481 CrossRef CAS
.
- J. B. McLeary, F. M. Calitz, J. M. McKenzie, M. P. Tonge, R. D. Sanderson and B. Klumperman, Macromolecules, 2004, 37, 2383–2394 CrossRef CAS
.
- G. Moad, Y. K. Chong, R. Mulder, E. Rizzardo and S. H. Thang, ACS Symp. Ser., 2009, 1024, 3–18 CAS
.
- M. C. Iovu, C. R. Craley, M. Jeffries-El, A. B. Krankowski, R. Zhang, T. Kowalewski and R. D. McCullough, Macromolecules, 2007, 40, 4733–4735 CrossRef CAS
.
- J. Xu, W. Zhang, N. C. Zhou, J. Zhu, Z. P. Cheng and X. L. Zhu, e-Polymer, 2008, 24 Search PubMed
.
- C. Yang, J. K. Lee, A. J. Heeger and F. Wudl, J. Mater. Chem., 2009, 19, 5416–5423 RSC
.
- J. W. Fu, Z. B. Zhang, Z. P. Cheng, J. Zhu, W. Zhang and X. L. Zhu, Polym. Bull., 2008, 61, 287–297 CrossRef CAS
.
- Y. Lee, J. K. Kim, K. Fukukawa, J. Bang and C. J. Hawker, Polym. Prepr. (Am. Chem. Soc., Div. Polym. Chem.), 2006, 47(2), 679–680 CAS
.
- X. Xiao, Y. Q. Fu, J. J. Zhou, Z. S. Bo, L. Li and C. M. Chan, Macromol. Rapid Commun., 2007, 28, 1003–1009 CrossRef CAS
.
- G. K. Such, R. A. Evans and T. P. Davis, Macromolecules, 2006, 39, 9562–9570 CrossRef CAS
.
- D. L. Patton, P. Taranekar, T. Fulghum and R. Advincula, Macromolecules, 2008, 41, 6703–6713 CrossRef CAS
.
- H. Mori, H. Ookuma and T. Endo, Macromolecules, 2008, 41, 6925–6934 CrossRef CAS
.
- H. Mori, H. Ookuma, S. Nakano and T. Endo, Macromol. Chem. Phys., 2006, 207, 1005–1017 CrossRef CAS
.
- H. Mori and S. Okabayashi, React. Funct. Polym., 2009, 69, 441–449 CrossRef CAS
.
- H. Mori, S. Nakano and T. Endo, Macromolecules, 2005, 38, 8192–8201 CrossRef CAS
.
- P. Zhao, Q. D. Ling, W. Z. Wang, J. Ru, S. B. Li and W. Huang, J. Polym. Sci., Part A: Polym. Chem., 2007, 45, 242–252 CrossRef CAS
.
- M. Häussler, P. Lok, M. Chen, J. Jasieniak, R. Adhikari, S. King, S. Haque, C. M. Forsyth, K. Winzenberg, S. E. Watkins, E. Rizzardo and G. J. Wilson, Macromolecules, 2010, 43, 7101–7110 CrossRef CAS
.
- E. J. W. Crossland, P. Cunha, S. Scroggins, S. Moratti, O. Yurchenko, U. Steiner, M. A. Hillmyer and S. Ludwigs, ACS Nano, 2010, 4, 962–966 CrossRef CAS
.
- L. Tao, C. S. Kaddis, R. R. O. Loo, G. N. Grover, J. A. Loo and H. D. Maynard, Chem. Commun., 2009, 2148–2150 RSC
.
- Q. Fu, L. L. Cheng, Y. Zhang and W. F. Shi, Polymer, 2008, 49, 4981–4988 CrossRef CAS
.
- Y. Zhang, Z. Cheng, X. Chen, W. Zhang, J. Wu, J. Zhu and X. Zhu, Macromolecules, 2007, 40, 4809–4817 CrossRef CAS
.
- Y. Li, Y. Tang, K. Yang, X. Chen, L. Lu and Y. Cai, Macromolecules, 2008, 41, 4597–4606 CrossRef CAS
.
- H. Z. Cao, W. Zhang, J. Zhu, X. R. Chen, Z. P. Cheng, J. H. Wu and X. L. Zhu, eXPRESS Polym. Lett., 2008, 2, 589–601 Search PubMed
.
- P. Zhao, J. Jiang, B. Leng and H. Tian, Macromol. Rapid Commun., 2009, 30, 1715–1718 CrossRef CAS
.
- J. Jiang, B. Leng, X. Xiao, P. Zhao and H. Tian, Polymer, 2009, 50, 5681–5684 CrossRef CAS
.
- A. Nagai, K. Kokado, J. Miyake and Y. Chujo, J. Polym. Sci., Part A: Polym. Chem., 2009, 48, 627–634
.
- F. Cheng and F. Jakle, Chem. Commun., 2010, 46, 3717–3719 RSC
.
- H. Mori, S. Masuda and T. Endo, Macromolecules, 2006, 39, 5976–5978 CrossRef CAS
.
- C.-F. Huang, J. A. Yoon and K. Matyjaszewski, Can. J. Chem., 2009, 88, 228–235
.
- N. Suchao-in, S. Chirachanchai and S. Perrier, Polymer, 2009, 50, 4151–4158 CrossRef CAS
.
- D. Kessler, M. C. Lechmann, S. Noh, R. Berger, C. Lee, J. S. Gutmann and P. Theato, Macromol. Rapid Commun., 2009, 30, 1238–1242 CrossRef CAS
.
- S. M. Lindner and M. Thelakkat, Macromolecules, 2004, 37, 8832–8835 CrossRef CAS
.
- S.b. Maria, A. S. Susha, M. Sommer, D. V. Talapin, A. L. Rogach and M. Thelakkat, Macromolecules, 2008, 41, 6081–6088 CrossRef CAS
.
- M. Sommer, S. M. Lindner and M. Thelakkat, Adv. Funct. Mater., 2007, 17, 1493–1500 CrossRef CAS
.
- S. Huttner, M. Sommer, U. Steiner and M. Thelakkat, Appl. Phys. Lett., 2010, 96, 073503–073503 CrossRef
.
- E. Kaul, V. Senkovskyy, R. Tkachov, V. Bocharova, H. Komber, M. Stamm and A. Kiriy, Macromolecules, 2009, 43, 77–81
.
- Q. Zhang, A. Cirpan, T. P. Russell and T. Emrick, Macromolecules, 2009, 42, 1079–1082 CrossRef CAS
.
- L.-Y. Wang, K.-C. Li and H.-C. Lin, Polymer, 2010, 51, 75–83 CrossRef CAS
.
- Y. Tian, C.-Y. Chen, H.-L. Yip, W.-C. Wu, W.-C. Chen and A. K. Y. Jen, Macromolecules, 2009, 43, 282–291
.
- L. Zhang, Q.-F. Xu, J.-M. Lu, N.-J. Li, F. Yan and L.-H. Wang, Polymer, 2009, 50, 4807–4812 CrossRef CAS
.
- K.-Y. Pu, X.-Y. Qi, Y.-L. Yang, X.-M. Lu, T.-C. Li, Q.-L. Fan, C. Wang, B. Liu, H. S. O. Chan and W. Huang, Chem.–Eur. J., 2008, 14, 1205–1215 CrossRef CAS
.
- S. P. Economopoulos, C. L. Chochos, V. G. Gregoriou, J. K. Kallitsis, S. Barrau and G. Hadziioannou, Macromolecules, 2007, 40, 921–927 CrossRef CAS
.
- S. Lu, T. Liu, L. Ke, D.-G. Ma, S.-J. Chua and W. Huang, Macromolecules, 2005, 38, 8494–8502 CrossRef CAS
.
- S. P. Economopoulos, A. K. Andreopoulou, V. G. Gregoriou and J. K. Kallitsis, Chem. Mater., 2005, 17, 1063–1071 CrossRef CAS
.
- C. L. Chochos, P. K. Tsolakis, V. G. Gregoriou and J. K. Kallitsis, Macromolecules, 2004, 37, 2502–2510 CrossRef CAS
.
- H. Mori, K. Takano and T. Endo, Macromolecules, 2009, 42, 7342–7352 CrossRef CAS
.
- Y.-K. Lan, C. H. Yang and H.-C. Yang, Polym. Int., 2010, 59, 16–21 CrossRef CAS
.
-
J. Pyan and T. Emrick, in Macromolecular Engineering, ed. K. Matyjaszewski, Y. Gnanou and L. Liebler, Wiley-VCH, Weinheim, 2007, pp. 2409–2449 Search PubMed
.
-
Y. Li, L. S. Schadler and B. C. Benicewicz, in Handbook of RAFT Polymerization, ed. C. Barner-Kowollik, Wiley-VCH, Weinheim, Germany, 2008, pp. 423–453 Search PubMed
.
- P. Liu, e-Polymer, 2007, 62 Search PubMed
.
- R.l. Barbey, L. Lavanant, D. Paripovic, N. Schüwer, C. Sugnaux, S. Tugulu and H.-A. Klok, Chem. Rev., 2009, 109, 5437–5527 CrossRef CAS
.
- B. Radhakrishnan, R. Ranjan and W. J. Brittain, Soft Matter, 2006, 2, 386–396 RSC
.
- M. H. Stenzel, Macromol. Rapid Commun., 2009, 30, 1603–1624 CrossRef CAS
.
- A. B. Lowe, B. S. Sumerlin, M. S. Donovan and C. L. McCormick, J. Am. Chem. Soc., 2002, 124, 11562–11563 CrossRef CAS
.
- M.-Q. Zhu, L.-Q. Wang, G. J. Exarhos and A. D. Q. Li, J. Am. Chem. Soc., 2004, 126, 2656–2657 CrossRef CAS
.
- J. Shan, M. Nuopponen, H. Jiang, E. Kauppinen and H. Tenhu, Macromolecules, 2003, 36, 4526–4533 CrossRef CAS
.
- J. Shan, J. Chen, M. Nuopponen and H. Tenhu, Langmuir, 2004, 20, 4671–4676 CrossRef CAS
.
- A. Aqil, H. Qiu, J.-F. Greisch, R. Jérôme, E. De Pauw and C. Jérôme, Polymer, 2008, 49, 1145–1153 CrossRef CAS
.
- M. Nuopponen and H. Tenhu, Langmuir, 2007, 23, 5352–5357 CrossRef CAS
.
- A. D. Celiz, T.-C. Lee and O. A. Scherman, Adv. Mater., 2009, 21, 3937–3940 CrossRef CAS
.
- A. Aqil, C. Detrembleur, B. Gilbert, R. Jerome and C. Jerome, Chem. Mater., 2007, 19, 2150–2154 CrossRef CAS
.
- D. Zhao, X. Chen, Y. Liu, C. Wu, R. Ma, Y. An and L. Shi, J. Colloid Interface Sci., 2009, 331, 104–112 CrossRef CAS
.
- M. Toyoshima and Y. Miura, J. Polym. Sci., Part A: Polym. Chem., 2009, 47, 1412–1421 CrossRef CAS
.
- D.-H. Han and C.-Y. Pan, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 341–352 CrossRef CAS
.
- S.-i. Yusa, K. Fukuda, T. Yamamoto, Y. Iwasaki, A. Watanabe, K. Akiyoshi and Y. Morishima, Langmuir, 2007, 23, 12842–12848 CrossRef CAS
.
- A. S. Duwez, P. Guillet, C. Colard, J. F. Gohy and C. A. Fustin, Macromolecules, 2006, 39, 2729–2731 CrossRef
.
- J. W. Hotchkiss, A. B. Lowe and S. G. Boyes, Chem. Mater., 2007, 19, 6–13 CrossRef CAS
.
- C. Boyer, M. R. Whittaker, C. Nouvel and T. P. Davis, Macromolecules, 2010, 43, 1792–1799 CrossRef CAS
.
- M. Liang, I. C. Lin, M. R. Whittaker, R. F. Minchin, M. J. Monteiro and I. Toth, ACS Nano, 2009, 4, 403–413
.
- C. Boyer, M. R. Whittaker, K. Chuah, J. Liu and T. P. Davis, Langmuir, 2009, 26, 2721–2730
.
- Z. Merican, T. L. Schiller, C. J. Hawker, P. M. Fredericks and I. Blakey, Langmuir, 2007, 23, 10539–10545 CrossRef CAS
.
- B. J. Kim, J. Bang, C. J. Hawker, J. J. Chiu, D. J. Pine, S. G. Jang, S. M. Yang and E. J. Kramer, Langmuir, 2007, 23, 12693–12703 CrossRef CAS
.
- B. J. Kim, S. Given-Beck, J. Bang, C. J. Hawker and E. J. Kramer, Macromolecules, 2007, 40, 1796–1798 CrossRef CAS
.
- R. Vestberg, A. M. Piekarski, E. D. Pressly, K. Y. V. Berkel, M. Malkoch, J. Gerbac, N. Ueno and C. J. Hawker, J. Polym. Sci., Part A: Polym. Chem., 2009, 47, 1237–1258 CrossRef CAS
.
- J. Raula, J. Shan, M. Nuopponen, A. Niskanen, H. Jiang, E. I. Kauppinen and H. Tenhu, Langmuir, 2003, 19, 3499–3504 CrossRef CAS
.
- Z.-P. Xiao, K.-M. Yang, H. Liang and J. Lu, J. Polym. Sci., Part A: Polym. Chem., 2010, 48, 542–550 CrossRef CAS
.
- W.-C. Wang, K.-G. Neoh and E.-T. Kang, Macromol. Rapid Commun., 2006, 27, 1665–1669 CrossRef CAS
.
- C. Boyer, V. Bulmus, P. Priyanto, W. Y. Teoh, R. Amal and T. P. Davis, J. Mater. Chem., 2009, 19, 111–123 RSC
.
- A. Aqil, S. Vasseur, E. Duguet, C. Passirani, J. P. Benoit, R. Jerome and C. Jerome, J. Mater. Chem., 2008, 18, 3352–3360 RSC
.
- R. Narain, M. Gonzales, A. S. Hoffman, P. S. Stayton and K. M. Krishnan, Langmuir, 2007, 23, 6299–6304 CrossRef CAS
.
- J. J. Lai, J. M. Hoffman, M. Ebara, A. S. Hoffman, C. Estournes, A. Wattiaux and P. S. Stayton, Langmuir, 2007, 23, 7385–7391 CrossRef CAS
.
- Y. Li, X. Li, J. Chu, C. Dong, J. Qi and Y. Yuan, Environ. Pollut., 2010, 158, 2317–2323 CrossRef CAS
.
- R. Matsuno, T. Konno, M. Takai and K. Ishihara, Curr. Appl. Phys., 2009, 9, e284–e286 CrossRef
.
- H. Skaff and T. Emrick, Angew. Chem., Int. Ed., 2004, 43, 5383–5386 CrossRef CAS
.
- A. C. C. Esteves, P. Hodge, T. Trindade and A. M. M. V. Barros-Timmons, J. Polym. Sci., Part A: Polym. Chem., 2009, 47, 5367–5377 CrossRef CAS
.
- M. Feng, Y. Chen, L. Gu, N. He, J. Bai, Y. Lin and H. Zhan, Eur. Polym. J., 2009, 45, 1058–1064 CrossRef CAS
.
- M. Zorn, W. K. Bae, J. Kwak, H. Lee, C. Lee, R. Zentel and K. Char, ACS Nano, 2009, 3, 1063–1068 CrossRef CAS
.
- J. Kwak, W. K. Bae, M. Zorn, H. Woo, H. Yoon, J. Lim, S. W. Kang, S. Weber, H.-J. Butt, R. Zentel, S. Lee, K. Char and C. Lee, Adv. Mater., 2009, 21, 5022–5026 CrossRef CAS
.
- X. Jiang, M. Ahmed, Z. Deng and R. Narain, Bioconjugate Chem., 2009, 20, 994–1001 CrossRef CAS
.
- W. Liu, A. B. Greytak, J. Lee, C. R. Wong, J. Park, L. F. Marshall, W. Jiang, P. N. Curtin, A. Y. Ting, D. G. Nocera, D. Fukumura, R. K. Jain and M. G. Bawendi, J. Am. Chem. Soc., 2009, 132, 472–483
.
- S. Yang, Q. Li, L. Chen and S. Chen, J. Mater. Chem., 2008, 18, 5599–5603 RSC
.
- B. L. Sanchez-Gaytan, W. Cui, Y. Kim, M. A. Mendez-Polanco, T. V. Duncan, M. Fryd, B. B. Wayland and S.-J. Park, Angew. Chem., Int. Ed., 2007, 46, 9235–9238 CrossRef CAS
.
- W. Lin, K. Fritz, G. Guerin, G. R. Bardajee, S. Hinds, V. Sukhovatkin, E. H. Sargent, G. D. Scholes and M. A. Winnik, Langmuir, 2008, 24, 8215–8219 CrossRef CAS
.
- C. M. Homenick, G. Lawson and A. Adronov, Polym. Rev., 2007, 47, 265–290 Search PubMed
.
- C. McClory, S. J. Chin and T. McNally, Aust. J. Chem., 2009, 62, 762–785 CrossRef CAS
.
- P. Liu, Eur. Polym. J., 2005, 41, 2693–2703 CrossRef CAS
.
- Z. Spitalsky, D. Tasis, K. Papagelis and C. Galiotis, Prog. Polym. Sci., 2010, 35, 357–401 CrossRef CAS
.
- J. Cui, W. Wang, Y. You, C. Liu and P. Wang, Polymer, 2004, 45, 8717–8721 CrossRef CAS
.
- C.-Y. Hong, Y.-Z. You and C.-Y. Pan, Chem. Mater., 2005, 17, 2247–2254 CrossRef CAS
.
- G. Xu, W.-T. Wu, Y. Wang, W. Pang, P. Wang, Q. Zhu and F. Lu, Nanotechnology, 2006, 17, 2458–2465 CrossRef CAS
.
- C. Y. Hong, Y. Z. You and C. Y. Pan, J. Polym. Sci., Part A: Polym. Chem., 2006, 44, 2419–2427 CrossRef CAS
.
- C.-Y. Hong, Y.-Z. You and C.-Y. Pan, Polymer, 2006, 47, 4300–4309 CrossRef CAS
.
- C. Y. Hong and C. Y. Pan, J. Mater. Chem., 2008, 18, 1831–1836 RSC
.
- X. W. Pei, W. M. Liu and J. C. Hao, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 3014–3023 CrossRef CAS
.
- G.-J. Wang, S.-Z. Huang, Y. Wang, L. Liu, J. Qiu and Y. Li, Polymer, 2007, 48, 728–733 CrossRef CAS
.
- G. Xu, W.-T. Wu, Y. Wang, W. Pang, Q. Zhu, P. Wang and Y. You, Polymer, 2006, 47, 5909–5918 CrossRef CAS
.
- G. Y. Xu, Y. S. Wang, W. M. Pang, W. T. Wu, Q. R. Zhu and P. H. Wang, Polym. Int., 2007, 56, 847–852 CrossRef CAS
.
- G. Y. Xu, W. T. Wu, Y. S. Wang, W. M. Pang, Q. R. Zhu and P. H. Wang, Nanotechnology, 2007, 18, 145606 CrossRef
.
- Y.-Z. You, C.-Y. Hong and C.-Y. Pan, Nanotechnology, 2006, 17, 2350–2354 CrossRef CAS
.
- X. Pei, J. Hao and W. Liu, J. Phys. Chem. C, 2007, 111, 2947–2952 CrossRef CAS
.
- B. Zhang, J. Wang, Y. Chen, D. Früchtl, B. Yu, X. Zhuang, N. He and W. J. Blau, J. Polym. Sci., Part A: Polym. Chem., 2010, 48, 3161–3168 CrossRef CAS
.
- A. V. Ellis, M. R. Waterland and J. Quinton, Chem. Lett., 2007, 1172–1173 CrossRef CAS
.
-
A. V. Ellis, Functionalised carbon nanotubes and methods of preparation, Industrial Research Limited, New Zealand, WO2007067079A1, 2007
.
-
S. A. Curran and A. V. Ellis, Thiation of carbon nanotubes and composite formation, Arrowhead Center, Inc., U.S. Pat., 7
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 713
713![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 508, 2010
508, 2010 .
- G. Zhou, I. I. Harruna, W. L. Zhou, W. K. Aicher and K. E. Geckeler, Chem.–Eur. J., 2007, 13, 569–573 CrossRef CAS
.
- Y. Z. You, C. Y. Hong and C. Y. Pan, Macromol. Rapid Commun., 2006, 27, 2001–2006 CrossRef CAS
.
- Y.-Z. You, C.-Y. Hong and C.-Y. Pan, Adv. Funct. Mater., 2007, 17, 2470–2477 CrossRef CAS
.
- J. Liu, Z. Nie, Y. Gao, A. Adronov and H. Li, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 7187–7199 CrossRef CAS
.
- A. J. Inglis, P. Pierrat, T. Muller, S. Brase and C. Barner-Kowollik, Soft Matter, 2010, 6, 82–84 RSC
.
- W. Zhou, S. Lv and W. Shi, Eur. Polym. J., 2008, 44, 587–601 CrossRef CAS
.
- D. Wang, W.-X. Ji, Z.-C. Li and L. Chen, J. Am. Chem. Soc., 2006, 128, 6556–6557 CrossRef CAS
.
- J. Liu, W. Yang, L. Tao, D. Li, C. Boyer and T. P. Davis, J. Polym. Sci., Part A: Polym. Chem., 2010, 48, 425–433 CrossRef CAS
.
- J. Liu, L. Tao, W. Yang, D. Li, C. Boyer, R. Wuhrer, F. Braet and T. P. Davis, Langmuir, 2010, 26, 10068–10075 CrossRef CAS
.
- B. J. Lowes, A. G. Bohrer, T. Tran and D. A. Shipp, Polym. Bull., 2009, 62, 281–289 CrossRef CAS
.
- B. Hojjati and P. A. Charpentier, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 3926–3937 CrossRef CAS
.
- J. Liu, W. Yang, H. M. Zareie, J. J. Gooding and T. P. Davis, Macromolecules, 2009, 42, 2931–2939 CrossRef CAS
.
- V. G. Ngo, C. Bressy, C. Leroux and A. Margaillan, Polymer, 2009, 50, 3095–3102 CrossRef CAS
.
- S. Meuer, K. Fischer, I. Mey, A. Janshoff, M. Schmidt and R. Zentel, Macromolecules, 2008, 41, 7946–7952 CrossRef CAS
.
- M. C. Lechmann, D. Kessler and J. S. Gutmann, Langmuir, 2009, 25, 10202–10208 CrossRef CAS
.
- J. Loiseau, N. Doërr, J. M. Suau, J. B. Egraz, M. F. Llauro, C. Ladaviere and J. Claverie, Macromolecules, 2003, 36, 3066–3077 CrossRef CAS
.
- M. Baum and W. J. Brittain, Macromolecules, 2002, 35, 610–615 CrossRef CAS
.
-
(a) G. Q. Zhai, W. H. Yu, E. T. Kang, K. G. Neoh, C. C. Huang and D. J. Liaw, Ind. Eng. Chem. Res., 2004, 43, 1673–1680 CAS
; (b) W. H. Yu, E. T. Kang and K. G. Neoh, Ind. Eng. Chem. Res., 2004, 43, 5194–5202 CrossRef
.
- D. Li, Y. Luo, B.-G. Li and S. Zhu, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 970–978 CrossRef CAS
.
- M. D. Rowe-Konopacki and S. G. Boyes, Macromolecules, 2007, 40, 879–888 CrossRef CAS
.
- L. Li, E. Kang and K. Neoh, Appl. Surf. Sci., 2008, 254, 2600–2604 CrossRef CAS
.
- L. P. Wang, Y. P. Wang, K. Yuan and Z. Q. Lei, Polym. Adv. Technol., 2008, 19, 285–290 CrossRef CAS
.
- Q. Peng, D. M. Y. Lai, E. T. Kang and K. G. Neoh, Macromolecules, 2006, 39, 5577–5582 CrossRef CAS
.
- G. Demirel, Z. Rzaev, S. Patir and E. Piskin, J. Nanosci. Nanotechnol., 2009, 9, 1865–1871 CrossRef CAS
.
- H. P. Yap, X. Hao, E. Tjipto, C. Gudipati, J. F. Quinn, T. P. Davis, C. Barner-Kowollik, M. H. Stenzel and F. Caruso, Langmuir, 2008, 24, 8981–8990 CrossRef CAS
.
- T.-Y. Lee, C.-Y. Yu, M.-Y. Hsu, R. Hayashi, T. Iwai, J.-H. Chen and B.-C. Ho, J. Photopolym. Sci. Technol., 2003, 16, 483–487 CrossRef CAS
.
- M. T. Sheehan, W. B. Farnham, H. Okazaki, J. R. Sounik and G. Clark, Proc. SPIE-Int. Soc. Opt. Eng., 2008, 6923, 69232E/69231–69232E/69239
.
- S. S. Kim, J. W. Kim, J. Y. Lee, S. K. Oh, S. H. Lee, J. W. Kim, J. W. Lee, D.b. Kim, J. Kim, K. D. Ban, C. K. Bok and S.-C. Moon, Proc. SPIE-Int. Soc. Opt. Eng., 2007, 6519, 65191W/65191–65191W/65112
.
- M. Shirai, M. Manabe, S. Tsuji and T. Itani, J. Vac. Sci. Technol., B, 2006, 24, 3021–3024 CrossRef CAS
.
- M. Park, C. Harrison, P. M. Chaikin, R. A. Register and D. H. Adamson, Science, 1997, 276, 1401–1404 CrossRef CAS
.
- J. Bang, J. Bae, P. Löwenhielm, C. Spiessberger, S. A. Given-Beck, T. P. Russell and C. J. Hawker, Adv. Mater., 2007, 19, 4552–4557 CrossRef CAS
.
- Q. Lou, M. A. Kishpaugh and D. A. Shipp, J. Polym. Sci., Part A: Polym. Chem., 2010, 48, 943–951 CrossRef CAS
.
- J. Bang, S. H. Kim, E. Drockenmuller, M. J. Misner, T. P. Russell and C. J. Hawker, J. Am. Chem. Soc., 2006, 128, 7622–7629 CrossRef CAS
.
- C. Tang, E. M. Lennon, G. H. Fredrickson, E. J. Kramer and C. J. Hawker, Science, 2008, 322, 429–432 CrossRef CAS
.
- Y. J. Kim, H. Kang, M. Leolukman, P. F. Nealey and P. Gopalan, Chem. Mater., 2009, 21, 3030–3032 CrossRef CAS
.
- M. Seo, S. Shin, S. Ku, S. Jin, J.-B. Kim, M. Ree and S. Y. Kim, J. Mater. Chem., 2010, 20, 94–102 RSC
.
| This journal is © The Royal Society of Chemistry 2011 |