E-beam irradiation and UV photocrosslinking of microemulsion-laden poly(N-vinyl-2-pyrrolidone) hydrogels for “in situ” encapsulation of volatile hydrophobic compounds†
C.
Dispenza
*a,
M.
Ricca
a,
C.
LoPresti‡
a,
G.
Battaglia
b,
M.
La Valle
c,
D.
Giacomazza
d and
D.
Bulone
d
aDipartimento di Ingegneria Chimica dei Processi e dei Materiali, Università degli Studi di Palermo, Edificio 6, Viale delle Scienze, 90128, Palermo, Italy. E-mail: dispenza@dicpm.unipa.it; Fax: +39.091 7025020; Tel: +39 091 23863710
bDepartment of Biomedical Science, The University of Sheffield, Firth Court, S10 2TN, Sheffield, UK
cCNR - Istituto per la Sintesi Organica e la Fotoreattività, Via P. Gobetti 101, 40129, Bologna, Italy
dCNR - Istituto di Biofisica (Palermo unit), Via U. La Malfa 153, 90146, Palermo, Italy
First published on 23rd September 2010
Abstract
Gelled microemulsions are the subject of considerable scientific and commercial interest. Many efforts are currently devoted to improving their toxicological profile and functioning as biocompatible diffusion barrier for the controlled delivery of hydrophobic compounds. In the present investigation, a non-ionic polymeric surfactant was chosen to generate an oil-in-water microemulsion of a model fragrance in the presence of poly(N-vinyl-2-pyrrolidone) (PVP). The microemulsion was then subjected to either electron-beam or UV-irradiation to induce free-radical crosslinking of PVP at low temperature and in the absence of crosslinking agents, catalysts and initiators. Irradiation conditions with the two irradiation sources have been purposely selected to generate PVP hydrogels with similar appearances and mechanical spectra. Despite the macroscopic analogies, specific features are imparted to the two families of hydrogels by the two different irradiation methodologies. A description of the microstructure of both pure PVP and microemulsion-laden hydrogels is given starting from the dynamic light scattering (DLS) characterization of the liquid (unirradiated) formulations, followed by the study the hydrogels' dynamic mechanical, solubility and swelling properties, FTIR analysis of the water insoluble fractions, DLS measurements and cytotoxicity studies.
Introduction
Fragrances are important components of most products in the personal-care, cosmetics, household, dietary and paper market sectors. They are also penetrating other huge market segments, such as fashion, paints, electronics, furniture and automotive, with the aim of involving the consumer in a total experience by eliciting many sensorial reactions at the same time, before, during and after the purchase. Fragrance perception depends, to some extent, on the way that these molecules are released in air and thus transported to the olfactory receptors. The opportunity to control the release profile has led to different strategies of incorporation of these substances into delivery devices.Typically fragrances are aromatic and/or aliphatic esters, ethers, ketones and alcohols with moderate to low polarity (i.e.water solubility) and relatively high vapour pressure.1 Emulsions are commonly used to combine both water-soluble components and oily components into a macroscopically homogeneous formulation.2–4 Depending on the final requirements for the product, emulsions can be either oil-in-water or water-in-oil and imply the recourse to surfactants to ensure both ease of emulsification and stability of the formulation. Microemulsions, in particular, have the unique properties of being optically isotropic and thermodynamically stable. The main problem in the microemulsion application is due their toxic and irritant properties5,6 that limit their use to a narrow range of physiologically acceptable concentration of surfactants and co-surfactants. On the other hand, the large surfactant concentration determines microemulsion's stability under given conditions. Indeed, in emulsions, as well as in liposomes, the surfactant barrier can be mechanically instable and affected by undesired leakage of the incorporated components.
Either to extend the stability range or to improve the toxicological profile of the formulation, low molecular weight surfactant mixtures have often been replaced by polymer/surfactant combinations.7–9Polymers selected for the purpose have to be biocompatible and relatively stable to storage conditions. Poly(ethylene glycol), hydroxylated poly(meth)acrylates, ethylene-vinyl acetate copolymers and polyurethanes are among the most used polymers for the purpose.7 In particular, gelled oil-in-water emulsions have attracted interest as encapsulation devices, especially in drug delivery.10–12 They are often produced using a two-step process in which, firstly, the oil is dispersed in an aqueous polymer solution which is then crosslinked to entrap the oil droplets into a hydrogel matrix.13 The formation of a crosslinked network around the surfactant-stabilised oil droplets or micelles should prevent coalescence phenomena, thus imparting extra-stability, e.g. stability in a wider temperature/pH range. Migration of the surfactant molecules, as either unimers or micelles, may be limited by diffusion constraints or through chemical anchoring of the surfactant to the polymer network, thus improving the toxicological profile of the delivery device. Hydrogels can also be optically transparent and have mechanical properties akin to soft living tissues, such as skin and mucosa. Their high polarity makes them very efficient in retaining and releasing water and/or water-soluble actives (under some circumstances by responding to a stimulus) dissolved in the inner aqueous phase as well as in serving as a rate-limiting secondary diffusion barrier for the release of the oily fragrance contained inside the micelles.14
The aim of the present investigation is to establish if and how the incorporation of a fragrance microemulsion in a hydrogel would affect the organisation and physico-chemical properties of the “in situ” gelling polymer matrix.
Poly(N-vinyl pyrrolidone) has been chosen as water-soluble and cross-linkable polymer in consideration of its hydrolytic stability in a wide range of pH values, as often required for household products. Moreover, the relative low cost of this biocompatible commercial polymer makes it preferable over developmental polymers for consumer applications.
Electron-beam (EB) irradiation and UV-photocrosslinking have been selected as alternative methodologies for generating poly(N-vinyl-2-pyrrolidone) hydrogels at sub-ambient temperature starting from the polymer's aqueous solution. In particular, EB irradiation does not require radical initiators or crosslinking agents and can ensure a simultaneous sterilization, with obvious benefits for the toxicological profile and manufacturing costs of the generated materials. On the other hand, high-energy irradiation facilities have high capital costs and pose safety and regulatory issues.15 Conversely, UV-photocrosslinking requires only relatively inexpensive and accessible equipment but generally imposes the recourse to compounds which form radicals in solution, such as hydrogen peroxide, for the initiation step. It also poses limitations in the thickness of materials to be irradiated because of the relatively lower penetration depth of UV-rays.16–18
Although radiation-crosslinked PVP hydrogels have been subject of many investigations,16–18 the relationships between irradiation processing conditions, their microstructure and macro-functional behaviour are far from being clarified, particularly when the gel is produced “in situ” starting from a heterogeneous colloidal solution.
Here we have investigated PVP aqueous solutions through dynamic light scattering (DLS) measurements, in order to obtain information on the conformation of the polymer chains at the variance of the polymer concentration in water in the 0.5–8 wt.% range. Irradiation conditions with the two sources were selected in order to obtain high yields of the crosslinking reactions with both the irradiation sources and “rheologically” equivalent, elastic-like hydrogels, i.e. gels with G′ > G′′ and similar dynamic-mechanical spectra.19,20 The possibility of “in situ” incorporation of polymeric micelles and oil-in-water microemulsions in the PVP hydrogels, under the selected irradiation conditions, was also investigated. In some circumstances, this approach may be preferred to the incorporation of the colloidal solution into already formed networks, either in their swollen or dry states, for the following reasons: a minimal interference of the forming network with the oil phase and surfactant organization can be ensured; only the desired release pattern and not the uptake requirements must be considered in the engineering of the network mesh size; a partial or complete immobilization of the surfactant molecules can be obtained; the content of lipophiles and amphiphiles loaded in the hydrogel can exceeds the value dictated by the partition equilibrium for swollen gels or by the rehydration ability of the network as in the case of xerogels; when high energy irradiation is used, as already pointed out, simultaneous sterilization can be achieved.
A highly water insoluble fragrance, tetrahydrogeraniol (THG), was used as the oil phase, upon check up for its UV and E-beam irradiation resistance in the chosen experimental conditions. A non-ionic, fully saturated surfactant was also used. Solubility tests and rheological measurements on the gels were complemented with swelling behaviour investigation, FTIR and DLS measurements carried out on water swollen hydrogel formulations, either in the absence or in the presence of micelles and oil-in-water droplets, with the aim of providing a description of their chemical composition and insight on the microstructural characteristics of their swollen and dry states. Finally, we studied the biocompatibility of the different hydrogel formulations by measuring cell metabolic activity.
Experimental
Materials
PVP k60 (Mn ≈ 160 kDa, Mw ≈ 400 kDa) was purchased as 45 wt.% aqueous solution from Fluka. Brij-58P (polyoxyethylene (20) cetyl ether, HLB = 15.3, average Mw = 1123, CMC = 0.007 mM at 25 °C), tetrahydrogeraniol (3,7-dimethyl-octanol or THG), hydrogen peroxide (30 wt.%), sodium phosphate monobasic, sodium phosphate dibasic, sodium hydroxide, hydrochloric acid and water were purchased from Aldrich. All chemicals were used without any purification.Preparation of PVP solutions and microemulsions and hydrogels
From the original 45 wt.% aqueous solution, PVP–water mixtures in the 0.5–8 wt.% range were prepared and filtered through 0.45 μm pore size filters. To produce PVP/BriJ-58P micellar solution, Brij-58P (0.05 M) was added to the 8 wt.% PVP aqueous solution and stirred at 60 °C to a complete dissolution of the surfactant. Samples containing Brij were filtered through 0.8 μm pore size filters.THG/PVP/Brij-58P microemulsions were prepared according to the procedure described above for the production of the micellar solution with the subsequent addition of 1 wt./vol.% of THG, while stirring at 3000 rpm for 5 min.
When the above systems were prepared to be subjected to UV-irradiation, hydrogen peroxide (0.32M) was added just before irradiation. Conversely, when prepared for EB-irradiation, PVP/BriJ-58P micellar solutions were thoroughly deoxygenated and THG was added under nitrogen atmosphere through a micro-pump.
UV irradiation was carried out with a high-pressure Hg lamp (2 mW cm−2) at 5 ± 1 °C for 5 h. E-beam irradiation was performed at the ISOF-CNR laboratory in Bologna with the 12-MeV Vickers type linear accelerator in the 4–8 °C temperature range throughout the irradiation. Irradiation dose rate was 100 kGy/h and an integrated dose of 40 kGy was selected in order to obtain “rheologically equivalent” PVP hydrogels to those produced by UV-irradiation. A detailed description of the irradiation conditions is reported elsewhere.20
Characterizations
The average molecular weight of the polymer segments between two crosslinks, Mc, was calculated by the well known equations derived by the application of the general theory of rubber elasticity:23,24
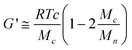 | (1) |
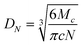 | (2) |
Swelling experiments were carried out at on the insoluble portions of the hydrogels as obtained after extraction by prolonged immersion (72 h) in excess distilled water at room temperature. Freeze-dried hydrogel samples were rehydrated at 5 ± 1 °C and 37 ± 1 °C in phosphate buffers of different pH (2.5, 6.8 and 9) and same ionic strength (0.2 M) or 0.1M aqueous HCl for pH 1. The rehydration ratio is defined as RR = ws/wd, where ws and wd are the measured weight of the hydrogel in the swollen state at fixed time intervals and in the initial dry state, respectively.
Reported values are the average of minimum eight samples from minimum two independent preparations.
Results
Dynamic light scattering measurements on liquid systems
A preliminary dynamic light scattering study was carried out on PVP aqueous solutions in the range 0.5–8 wt.%, for the purpose of choosing the concentration value more appropriate for preparing macroscopic hydrogels (macrogels) by irradiation with the two different sources: a high pressure Hg lamp and an electron accelerator. The results are briefly summarized hereafter (the experimental data are reported in detail as ESI†).DLS measurements on PVP aqueous solutions revealed the presence of one “fast” relaxation associable to the presence of freely moving particles, that may correspond to single PVP chains or small aggregates of few chains. For concentrations above 2 wt.%, there is also evidence of a second “slow” relaxation, typical of cooperative macromolecular motions that may be associated to the presence of a transient polymer network, i.e. a network formed by labile junctions due to van der Waals interactions, water mediated intermolecular hydrogen bonds and/or chain entanglements. In particular, it was observed a progressive reduction of the apparent hydrodynamic radius of the mobile objects associated to small PVP chain aggregates is observed, with values varying from 5.90 nm at 0.50 wt.%, to 4.36 nm at 2 wt.% and to 2.87 nm at 8 wt.%. This behavior, as already observed by Burchard and Eisele25 in aqueous solutions of 560 kDa poly(N-vinyl-pyrrolidone), is typical of polymeric solutions in a semi-dilute regime, e.g. above their overlapping concentration.26 Indeed, under these conditions, the fast relaxation has to be assigned to the random motion of the mass centers of the chain segments according to De Gennes theory.27 The characteristic dimension associated with the fast relaxation is the correlation length corresponding to the mesh size of the transient network. A detailed data analysis (reported as ESI†) has suggested that the “critical” concentration for the system here studied is in the range 1–2 wt. %. The value of the “overlap” concentration was independently estimated by using literature data28 relative to the intrinsic viscosity of poly(N-vinyl-pyrrolidone) solution with different molecular weight (see also ESI†). We obtained c* = 0.01 g ml−1 in good agreement with the estimation derived by DLS data.
DLS measurements were also carried out on aqueous solution of PVP at 8 wt.% after the addition of the surfactant (PVP/Brij), on the fragrance microemulsions prepared with the surfactant (Brij/THG), and on the fragrance microemulsions in presence of PVP (PVP/Brij/THG). Fig. 1 shows the field autocorrelation functions measured at 45° angles for the different liquid formulations. The curve for the Brij-58P/water mixture is also reported for comparison. Measurements were taken at different scattering angles to ascertain the type of motion associated to the autocorrelation decay. Two relaxation modes are well visible at all scattering angles for the aqueous Brij-58P as well as for the THG o/w microemulsion, either in the presence or in the absence of PVP. Data were, therefore, analyzed in terms of two exponential decays. Conversely, in the sample of PVP with Brij-58P the contribution of the relaxation mode at longer time is smaller than for all the other systems and visible only at low scattering angles, where it is well represented by a stretched exponential. The average relaxation times of fast (τfast) and slow (τslow) mode are plotted vs. q in panels a and b of Fig. 2. Generally, τ∝q−n and the value assumed by the exponent n in the fit depends on the type of motion, e.g.n = 2 for Brownian diffusion, n = 3 for vibrational motion. In particular, the slope of τfastvs. q is −2 for all systems, thus indicating clearly that the fast motion corresponds to Brownian diffusion. On the other hand, the slow mode shows n = 2 in the case of PVP/Brij/THG and and Brij alone, about n = 2.5 both in the case of Brij/THG and PVP/Brij, probably reflecting some contribution of other type of motion (vibrational).
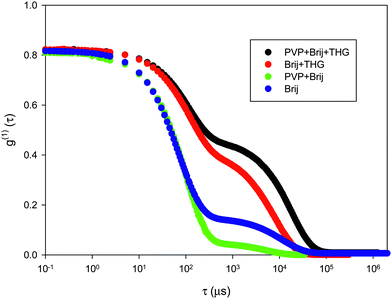 | ||
| Fig. 1 Electric field autocorrelation function at T = 25 °C and 45° angle for liquid samples (prior to irradiation) of PVP/Brij/THG, Brij/THG, PVP/Brij and aqueous Brij. | ||
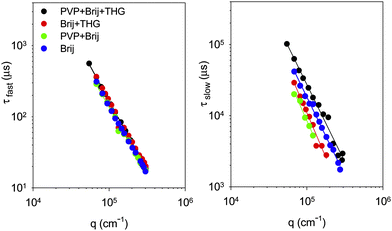 | ||
| Fig. 2 Average relaxation time vs. q-vector of fast and slow decay, as defined in the text, at T = 25 °C for liquid samples of PVP/Brij/THG, Brij/THG, PVP/Brij and aqueous Brij. | ||
According to the above results, aqueous Brij-58P (0.05M) shows two different populations of mobile objects with calculated average hydrodynamic radius of about 3.2 and 380 nm, respectively. The former can be associated with the presence of surfactant micelles whilst the latter can be attributed to micellar aggregates, that can be formed at the high concentration of surfactant here used.29,30 Although the estimated numerical ratio between the small and the large objects is of the order of several millions, their volume ratio is about 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Therefore, their presence has to be taken into account in the description of the network structure and properties. For the Brij/THG mixture, the average dimension associated to the smaller objects is about 4 nm, thus, slightly higher than that of the surfactant only, according to the fact that the micelles in this case are loaded with the fragrance. In the mixture of Brij-58P and PVP the faster decay is due to the diffusion of small objects with a hydrodynamic radius of about 3.5 nm, that can correspond to either surfactant micelles or to PVP coils similarly to those observed in PVP aqueous systems. The second relaxation at longer times can be attributed to cooperative motion of polymer segments forming a transient network, like that observed in the pure PVP solution (see ESI†). The absence of the larger mobile objects in this preparation is likely to be due to a stabilizing effect of PVP on micelles against the formation of larger aggregates.
1. Therefore, their presence has to be taken into account in the description of the network structure and properties. For the Brij/THG mixture, the average dimension associated to the smaller objects is about 4 nm, thus, slightly higher than that of the surfactant only, according to the fact that the micelles in this case are loaded with the fragrance. In the mixture of Brij-58P and PVP the faster decay is due to the diffusion of small objects with a hydrodynamic radius of about 3.5 nm, that can correspond to either surfactant micelles or to PVP coils similarly to those observed in PVP aqueous systems. The second relaxation at longer times can be attributed to cooperative motion of polymer segments forming a transient network, like that observed in the pure PVP solution (see ESI†). The absence of the larger mobile objects in this preparation is likely to be due to a stabilizing effect of PVP on micelles against the formation of larger aggregates.
This stabilizing effect was not observed when the micelles were fragrance-loaded. In fact, in the case of PVP/Brij/THG, two populations of mobile objects were observed with characteristic dimensions of about 4 nm and 600 nm, respectively. It is worth pointing out that the numerical proportion of small to bigger oil droplets is still of the order of magnitude of several millions. Also in this case, though, the oil-phase would equally partition between the internal volume of the micelles and the bigger oil droplets. The presence of small and large reservoirs for the fragrance in the hydrogel may have an impact on the release kinetics of the fragrance.
Rheological analysis of the gelled systems
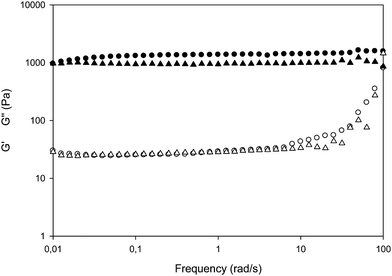 | ||
| Fig. 3 Storage, G′, (solid symbols) and loss, G′′, (empty symbols) moduli as function of angular frequency for PVP-EB (triangles) and PVP-UV (circles) hydrogels. | ||
For PVP-UV, G′ curve slightly increases in the first decade of frequency and it levels off at a higher value (approx. 1400 Pa) than for PVP-EB (approx. 1000 Pa). This behavior suggests that for the PVP-UV there is the possibility to rearrange macromolecular segments between crosslinks under stress at low frequency, so increasing the energy storage ability of the network, i.e. tightening the network. On the contrary, the PVP-EB network in the same conditions does not appreciably change, except at the top end of the frequency range, where G′′ curve rises to approach G′. The average G′ values in the two central decades have been used to calculate Mc and DN values, accordingly to the formula (1) and (2) reported in the experimental section, yielding the values of 57 kDa and 13.2 nm for PVP-EB; 52 kDa and 12.7 nm for PVP-UV, respectively. The average mesh size of the base PVP network, from this estimation, results in larger than the typical Brij-58P micelle but smaller than the bigger surfactant aggregates or oil-laden droplets.
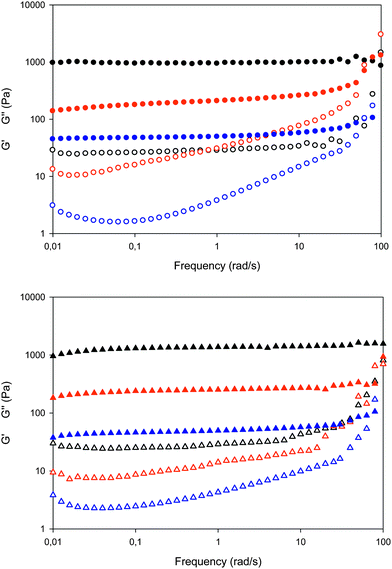 | ||
| Fig. 4 G′ (solid symbols) and G′′ (empty symbols) behaviour recorded as a function of the frequency for e-beam irradiated samples (circles); UV irradiated samples (triangles): PVP (black), PVP/Brij (red) and PVP/Brij/THG (blue). | ||
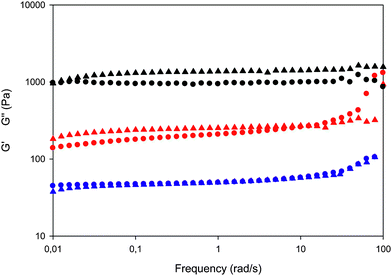 | ||
| Fig. 5 Comparison of G′ vs. frequency curves for e-beam irradiated (circles) and UV irradiated (triangles) systems: PVP (black), PVP/Brij (red) and PVP/Brij/THG (blue). | ||
Solubility of base PVP and microemulsion-laden hydrogels
The gel fraction values, GF, for all irradiated systems are presented in Table 1. Preliminary investigations allowed us to establish that aqueous Brij-58P (0.05M) subjected to irradiation does not lead to macroscopic gelation through either UV-irradiation or EB-irradiation. In turn, irradiation of PVP aqueous solutions at 8 wt.% with both irradiation sources leads to macrogel formation, but UV irradiation requires the presence of a photointiator (hydrogen peroxide). The value of GF for PVP-EB is slightly higher than that for PVP-UV, thus suggesting that, in the chosen experimental conditions, UV irradiation in the presence of hydrogen peroxide is quantitatively less effective than EB irradiation. When both PVP and Brij-58P are present in the formulation, macrogels are formed at a lower insoluble fraction yield with respect to the corresponding base systems, despite the fact that the overall concentration of polymers in water is increasing from 8 wt.%, when only PVP is present, to 13.6 wt.%, when the surfactant is also added. The decrease of the insoluble fraction in the presence of Brij-58P suggests that Brij58-P is less susceptible to irradiation induced crosslinking than PVP. In the calculation of the gel fraction the weight of crosslinked (insoluble) polymer after irradiation is divided by the total weight of polymeric (solid) components, including Brij-58P when present. Considering the weight ratio between PVP and Brij (approximately 1.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), under the assumption that the surfactant is not involved in crosslinking at all, the gel fraction is expected to be reduced of about 40% with respect to the base PVP system. Actually, we measured a gel fraction reduced of only 15% and 25% for PVP/Brij-EB and PVP/Brij-UV, respectively. Therefore, in both cases the surfactant should be partially bound, either chemically or physically, to the chemical network formed upon irradiation. The extent of bonding appears larger when the system is irradiated with accelerated electrons. The gel fraction values for PVP hydrogels containing Brij-58P and the fragrance are similar to those of the system containing PVP and Brij-58P only. Therefore the presence of the fragrance does not affect the yield of crosslinking reactions.
1), under the assumption that the surfactant is not involved in crosslinking at all, the gel fraction is expected to be reduced of about 40% with respect to the base PVP system. Actually, we measured a gel fraction reduced of only 15% and 25% for PVP/Brij-EB and PVP/Brij-UV, respectively. Therefore, in both cases the surfactant should be partially bound, either chemically or physically, to the chemical network formed upon irradiation. The extent of bonding appears larger when the system is irradiated with accelerated electrons. The gel fraction values for PVP hydrogels containing Brij-58P and the fragrance are similar to those of the system containing PVP and Brij-58P only. Therefore the presence of the fragrance does not affect the yield of crosslinking reactions.
| Hydrogel | GF (%) | ERR (stand. dev.) | |||
|---|---|---|---|---|---|
| pH 1 | pH 2.5 | pH 6.8 | pH 9 | ||
| PVP-EB | 73 | 37.5 (0.6) | 31.2 (1.7) | 29.7 (1.4) | 23.4 (1.4) |
| PVP-UV | 65 | 15.1 (1.3) | 14.3 (1.3) | 14.6(1.5) | 12.0 (0.5) |
| PVP/Brij-EB | 62 | 34.9 (3.2) | 26.6 (1.8) | 25.3 (2.4) | 24.0 (4,0) |
| PVP/Brij-UV | 48 | 15.3 (1.1) | 15.0 (0.9) | 16.0 (0.8) | 16.4 (1.3) |
| PVP/Brij/THG-EB | 61 | 31.1 (1.9) | 26.7 (2.9) | 27.6 (1.1) | 25.6 (2.7) |
| PVP/Brij/THG-UV | 49 | 21.7 (1.3) | 25.4 (4.1) | 24.3 (1.7) | 20.4 (2.8) |
Swelling behavior
To compare the swelling behavior of the insoluble fractions of hydrogels at different pH and similar ionic strength, we used phosphate buffers (0.2M) for pH 2.4, 6.8 and 9 and 0.1M aqueous HCl solution for pH = 1. A systematic study of the influence of ionic strength, pH, temperature, nature of the ionic species or temperature falls outside the scope of the present investigation and studies focused on the base PVP hydrogels swelling behavior are well documented in the literature.31–33In Table 1 the rehydration ratios (RR) values at the plateau for the different formulations are displayed. The corresponding swelling curves (not reported) showed that all systems reached plateau values within the first four hours of incubation in the swelling medium. Equilibrium rehydration ratios (ERR) for electron beam irradiated systems are always approximately twice as higher than those for the corresponding UV-irradiated systems.
When the hydrogel was formed through EB-irradiation either in the presence of the surfactant only or in the presence of both surfactant and THG, a reduction of ERR values with respect to that of PVP-EB was observed. This can be explained with a lower overall hydrophilicity of the network as a result of a partial immobilisation of the surfactant. Conversely, when the hydrogel is produced through UV-irradiation, ERR values are unchanged in the presence of the surfactant alone (PVP/Brij-UV), while they increase when the fragrance distributed between micelles and oil droplets is also present (PVP/Brij/THG-UV).
Furthermore, despite the analogies in the chemical composition of the formulations irradiated with either UV-rays or e-beams, the influence of pH on the rehydration of the two families of systems is different. All EB-irradiated systems tend to have close ERR values at pH 2.5 and 6.8 when networks are essentially non-ionic and to be slightly more swollen at pH 1 and more shrunken at pH 9. The behavior at pH 1 can be explained by invoking the repulsion between fixed charges on the network in the assumption that the network has become slightly polycationic;33,34 the shrinking at pH 9 could be due to a competition between the hydroxyl groups and the polar functional groups grafted to the network for the hydration water.33–35 Conversely, the influence of pH can be considered negligible or non-systematic for all the UV-networks in the whole range of pH investigated. It is worth pointing out that the differences in ERR values are very much attenuated for fragrance-containing hydrogels.
FTIR analysis of the hydrogel insoluble fractions
Fig. 6 shows FTIR spectra of linear PVP and of the insoluble fractions of both PVP-EB and PVP-UV hydrogels. The three spectra show evident similarities: both irradiation methodologies preserve the pyrrolidone moiety that characterizes the linear polymer, whose absorbance is at 1680–1650 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching of R2N–C
O stretching of R2N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). In particular, it is known that for high energy radical polymerization of PVP the preferential sites where the radical is located are the alfa carbon atom of the polymer backbone and the fifth carbon of the five-member pyrrolidone ring.36 Therefore, a significant modification of the linear PVP's spectrum is not to be expected. It can be noticed only an increased intensity of the absorption at 1057 cm−1 (C–OH stretching) particularly for PVP-UV hydrogel, that may be explained with the introduction of primary alcohol groups through photo-oxidation phenomena in the presence of H2O2.18
O). In particular, it is known that for high energy radical polymerization of PVP the preferential sites where the radical is located are the alfa carbon atom of the polymer backbone and the fifth carbon of the five-member pyrrolidone ring.36 Therefore, a significant modification of the linear PVP's spectrum is not to be expected. It can be noticed only an increased intensity of the absorption at 1057 cm−1 (C–OH stretching) particularly for PVP-UV hydrogel, that may be explained with the introduction of primary alcohol groups through photo-oxidation phenomena in the presence of H2O2.18
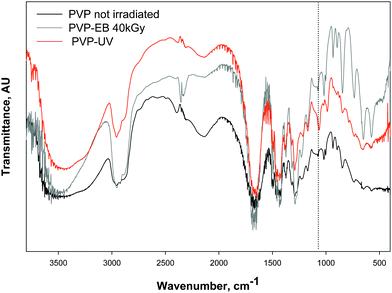 | ||
| Fig. 6 FTIR spectra of the dry insoluble fractions of pure PVP hydrogels produced via EB or UV irradiation in comparison with the spectrum of linear (unirradiated) PVP. Dotted line at 1057 cm−1. | ||
Fig. 7 shows FTIR spectra of UV and EB-irradiated micellar systems. With respect to the corresponding PVP hydrogel spectra, new peaks at 1180 cm−1 for both PVP/Brij and PVP/Brij/THG formulations appear regardless of the irradiation methodology chosen, which can be attributed to ether bonds of the poly(oxyethylene) portion of the Brij-58P (EO20C16) chains. This evidence confirms the above formulated hypothesis that upon irradiation part of the polymeric surfactant becomes irreversibly entrapped in the PVP network and strongly suggest a structural equivalence among all the crosslinked portions of hydrogels obtained with the two irradiation sources.
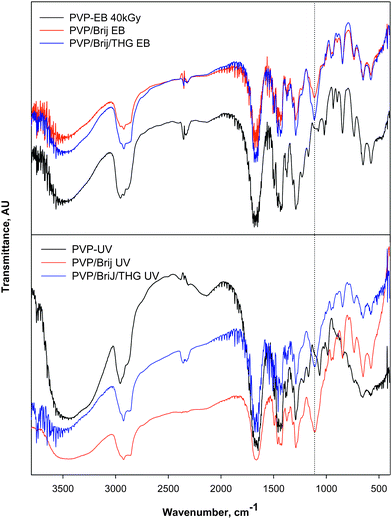 | ||
| Fig. 7 FTIR spectra of the dry insoluble fractions of PVP/Brij and PVP/Brj/THG hydrogels produced via EB-irradiation (top) or UV-irradiation (bottom). Dotted line at 1057 cm−1. | ||
Dynamic light scattering measurements on gelled samples
Dynamic light scattering measurements on gelled samples were performed by collecting the signal on different sample's regions to take into account the non-ergodicity of such systems. In Fig. 8 the autocorrelation functions of a PVP (8 wt.%) sample before and after irradiation are shown.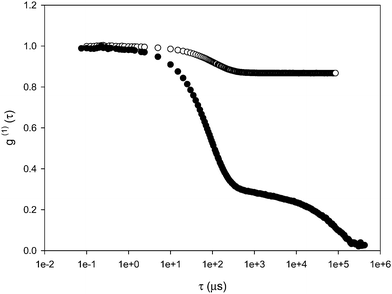 | ||
| Fig. 8 Electric field autocorrelation function of PVP (8 wt.%) at T = 25 °C and 90° angle before (full symbols) and after (empty symbols) UV irradiation. | ||
The autocorrelation function of the hydrogel sample is composed by a small contribution of the fast relaxation mode and a high value of constant signal indicating that the slow relaxation has became completely frozen over the experimental time scale. In Fig. 9(a–b) the average fast relaxation time and constant C vs. q-vector are shown for PVP and PVP/Brij/THG and the corresponding gelled samples, obtained by either UV or EB irradiation. Values of τfast very close to those observed for the unirradiated systems are obtained in all cases except for the hydrogel of PVP-UV, where τfast is quite larger, as it can be appreciated in the comparison shown in the panels c–d of Fig. 9. Slightly lower values are found in gelled emulsions of PVP/Brij/THG with respect to hydrogels of PVP alone. The difference between PVP hydrogels in the presence and absence of fragrance-laden micelles is more marked for the constant C. Indeed, the presence of both the surfactant and the fragrance, lead to a minor extent of dynamical freezing both in UV- and EB-irradiated gels, in agreement with the storage modulus decrements evidenced by dynamic mechanical analysis.
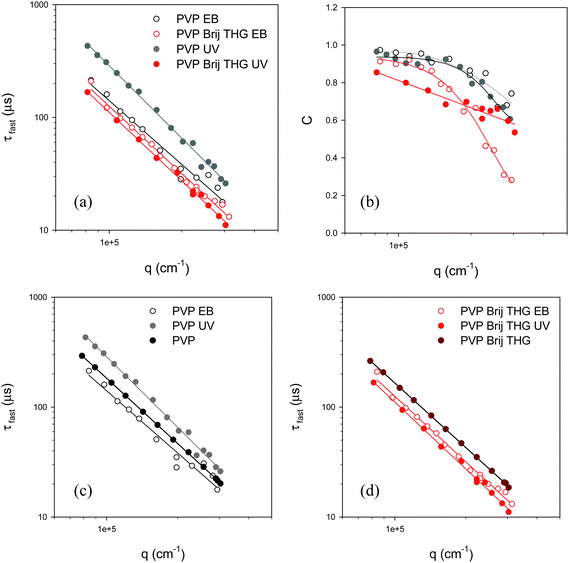 | ||
| Fig. 9 Panels (a) and (b): average relaxation time and constant value of the autocorrelation function measured on gelled samples for e-beam irradiated systems (empty symbols) and UV irradiated systems (solid symbols): PVP (black); PVP/Brij/THG (red). Panel (c): comparison between fast decay relaxation time measured in PVP aqueous solution and in UV or EB irradiated gels. Panel (d): comparison between fast decay relaxation time measured in PVP/Brij/THG aqueous solution and in UV or EB irradiated gels. | ||
Cytotoxicity tests
MTT essay carried out on primary human dermal fibroblasts (HDF) showed a depletion of cell metabolism suggesting cell cytotoxicity in the presence of pure PVP soluble fractions (data not reported). The particular mechanisms of activation of the pathways codifying for cellular death were not specifically investigated, although the amphiphatic nature of PVP would suggest some negative effect on the cell membrane integrity.On the other hand, Fig. 10 shows the viability of cells in direct contact with the crosslinked PVP and PVP/Brij/THG hydrogels obtained with both the irradiation methodologies. All the data are compared to the control (cells not in contact with gel), which is arbitrarily taken to be 100%. Tests were carried out after 24 h, 48 h and 72 h of incubation.
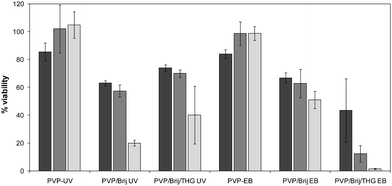 | ||
| Fig. 10 The viability of human dermal fibroblast after 24 h, 48 h and 72 h for the pure PVP hydrogels and the microemulsion-laden hydrogels in the absence and in the presence of the fragrance. | ||
Pure PVP hydrogels present high viability independently of the radiation source used for their production. On the other hand, the presence of Brij in the formulations leads to cell death within 72 h both in the case of UV and EB irradiation. This is likely to be due to the migration of the surfactant not chemically bound to the network from the hydrogel to the outer medium. Surfactant molecules in contact with HDF can penetrate the cell membrane and, eventually, induce cell death.37,38
Discussion
The possibility of generating “strong” PVP hydrogels, i.e. with prevailing elastic-like than viscous-like behavior, yet able to incorporate large quantities of water, is sought by the use of two different irradiation sources: a low power, high pressure Hg-lamp and a linear electron accelerator.20 Under UV irradiation macroscopic gelation of the whole irradiated sample occurred only in the presence of hydrogen peroxide and at a polymer concentration in water of 8 wt.% and above. A “rheologically” equivalent hydrogel, i.e. a gel with a similar dynamic-mechanical spectrum and, therefore, comparable average distance between adjacent crosslinks, was sought by irradiation with accelerated electrons of the same polymer in water, at the same concentration. The conditions that lead to such hydrogels were 40 kGy of absorbed dose at a dose rate of 100 kGy/h, with no recourse to hydrogen peroxide (see Fig. 1). With e-beam irradiation no macroscopic gelation was observed for polymer concentration below 4 wt.%. Upon visual inspection the two hydrogels appeared indistinguishable.From dynamic light scattering experiments carried out on aqueous PVP it was possible to establish that above 1 wt.% of polymer in water an extended transient network is formed, due to physical interactions, water-mediated hydrogen bonds and chain entanglements. Therefore, the irradiation induced macrogel formation appears to be associated with the reinforcement with chemical bonds of the transient net already formed in solution in semi-dilute conditions.
Despite the purposely sought analogies between the two base PVP hydrogels and the close average DN values, some specific features, stemming from the two different irradiation methodologies and conditions, are evident. One subtle difference is in the shape of G′ vs. frequency curve for PVP-UV with respect to PVP-EB. The UV-irradiated system shows a possibility to rearrange its macromolecular segments under the applied stress in the investigated frequency range. This is not shown by PVP-EB system, that despite its lower G′ curve shows a more elastic-like character. It is worth pointing out that the dynamic mechanical response of these materials can be strongly affected by the molecular features of the crosslinked networks as well as by the way the “soluble” fractions either “fill” or surround the network itself. In facts, when it comes to compare properties of the macroscopically insoluble fractions of the EB- and UV-irradiated formulations, the differences prevail on the similarities: twofold higher ERR values for PVP-EB with respect to PVP-UV have been measured. Furthermore, the presence of micelles does not induce changes in ERR in the same direction for the two families of hydrogels: i.e. the EB-network rehydrates less when generated in the presence of the surfactant stabilized oil-phase while the UV-network rehydrates more, with respect to the corresponding pure PVP systems. In addition, the fast relaxation time, associated to “free” polymer coils in the PVP-UV hydrogel, is significantly higher the that of the unirradiated polymer solution, that is very close to that of PVP-EB system, thus reflecting higher local viscosity.
The mechanisms of PVP network forming under either UV and EB irradiation have similarities and specificities that may affect, in turn, the way the microemulsion-laden hydrogels form.
There is general consensus on the fact that with both UV and high energy irradiation (both gamma and electron beam) the formed network is always the result of the competition of three main phenomena: chain scission, inter-molecular and intra-molecular crosslinking, that are significantly affected by system's chemical composition, viscosity and irradiation conditions.16,39,40 In particular, the very different energy dose-rates and integrated absorbed doses by the aqueous polymer solution to yield macroscopically similar gels, as well as the presence of hydrogen peroxide as photo-initiator, required for UV-irradiation, may lead to different macromolecular networks.
For UV irradiation with emission prevalently in the UV-A and UV-B spectral range, the direct interaction of radiation with PVP is not very effective in generating quantitatively macromolecular radicals, that can further evolve into intermolecular crosslinking.18,41 Accordingly, macroscopic gelation was not observed in our experiments in the absence of H2O2. When H2O2 is molecularly dissolved in the polymer solution, highly mobile ˙OH radicals are quantitatively generated by the interaction with UV-radiation and they may transfer to the polymer, generating macromolecular radicals. Termination through recombination of polymer radicals, that is effective for crosslinking and macrogel formation, is at the end of a reaction pathway that involves (i) diffusion of ˙OH radicals toward the polymer chains; (ii) radical transfer to the polymer and (iii) local rearrangements of the macromolecular chain segments carrying the unpaired electron. Recombination reaction of ˙OH radicals with polymer radicals produces hydroxylation of the PVP chains, as observed in the FTIR spectra. Their recombination with other ˙OH radicals yields back H2O2, that causes oxidative degradation and potentially competes with intermolecular crosslinking. Therefore, it is likely that macroradicals, formed in the proximity of sites where polymer chains physically overlap, recombine fixing the transient network by chemical bonds. The crosslinked embryo thus formed grows as the reduced mobility of the macromolecular segments improves “resistance” of the polymer chains to degradation.39 On the other hand, dangling chains, chain loops external to the network embryo or whole macromolecules organized into random coils mainly evolve toward degradation or intra-molecular crosslinking. These two main reaction pathways, localized in different regions of the material, are at the basis of the heterogeneities in the crosslinking density.20,40 In this framework, the observed high energy storage ability of the PVP-UV system may be related to the presence of more tightly crosslinked zones and more deformation compliant structures that tie the denser clusters. The uncrosslinked chains fill the looser portions of the network, thus contributing to the elastic response of the material under the applied dynamic mechanical stress. The confinement of PVP coils inside the looser portions of the network may also justify the higher values of τfast.
Upon extraction of the soluble fractions, followed by freeze drying, the looser portions of the network may irreversibly collapse, causing a significant reduction of the overall swelling ability (low ERR values). Therefore, the swelling behavior is mainly attributable to the more densely crosslinked portions of the gel and it is affected, in turn, by their accessibility by the solvent. The presence of the micelles and oil-droplets during the network formation may contribute to enlarge the mesh size of the more tightly crosslinked portions of the network and/or reduce the extent of physical and chemical entanglements of the collapsed portions, thus favoring water uptake.
With EB irradiation, macroradicals can be formed by methylene hydrogen abstraction operated by the hydroxyl radicals generated by water radiolysis, similarly to the hydrogen peroxide mediated mechanism described for UV irradiation. Nevertheless, they can be also generated by direct interaction of the accelerated electrons with polymer chains, as they are delivered in the medium concentrated in spurs.42 As a result, the instant local concentration of the produced macroradicals can be very high, favoring their recombination (inter- but also intra-molecularly). From direct evidence gathered with EB irradiation at the chosen dose-rate (100 kGy/h), macroscopic gelation is observed at ∼20 kGy.20 Therefore, by prolonging irradiation above the gel dose (up to 40 kGy), degradation phenomena on the already formed macroscopic network portions cannot be ruled out. The final structure is, then, the result of continued network building up, evolving toward a progressive densification, and network destruction, not specifically localized into portions of the material at the mesoscale. In this context, the micelles which are not entrapped in the micro-clusters of networked polymer are little influenced by the crosslinking, so their “fast relaxation” is comparable to that of the unirradiated system.
The modest decrease in ERR values can be due to the presence of some entrapped micelles in the network mesh. The different network organizations are represented in Scheme 1.
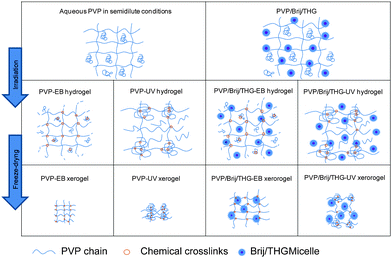 | ||
| Scheme 1 A schematic representation of the polymeric chemical network organization as obtained either through electron beam irradiation or UV irradiation in the absence and in the presence of the fragrance microemulsion Brij/THG. The micelles' radius is ∼4 nm, while the average mesh size of pure PVP hydrogels is ∼13 nm. The rare but much larger oil droplets (with average radius equal to ∼600 nm) are not represented. | ||
Conclusions
Fragrances are important components of many products especially in drugs, personal-care and cosmetic formulations. The possibility of incorporating these lipophilic substances into hydrogel-based systems, at concentrations that exceed the repartition equilibria between the inner and the outer phases of the swelling medium, has often suggested the recourse to emulsification techniques.The feasibility of obtaining chemically crosslinked, gelled microemulsions, through both EB and UV irradiation has been proved here by inducing chemical crosslinking to the linear PVP, purposely added to the microemulsion. The two different irradiation methodologies, despite the sought indistinguishable visual appearance and “rheological equivalence” of the generated pure PVP hydrogels, lead to different chemical network organizations because of their specific features. When carried out in the presence of a microemulsion, irradiation with either of the two sources freezes the dynamics of the physically overlapping PVP chains and of the larger surfactant aggregates. In the microemulsion-laden hydrogels there is evidence of the characteristic motion of the 3–4 nm radii micelles. Indeed, the presence of both the surfactant and the fragrance, lead to a minor extent of dynamical freezing both in UV- and EB-irradiated gels, in agreement with the dynamic mechanical analysis, that evidenced storage modulus decrements.
On the other hand FTIR analysis and swelling studies suggest that part of the surfactant remains entrapped in the water insoluble fractions, especially for the EB-irradiated systems, so improving the rehydration ability of the UV-irradiated xerogels upon freeze-drying. Cytotoxicity tests carried out on the microemulsion-laden hydrogels after extraction of the water-soluble portions in mild conditions suggest that, especially when the surfactant is not engaged at the oil–water interface, it can diffuse out of the network and induce cells death.37,38
References
- A. Herrmann, Angew. Chem., Int. Ed., 2007, 46, 5836 CrossRef CAS.
- K. Bauer, D. Garbe, H. Surburg, Common Fragrance and Flavor Materials: Properties and Uses, VCH Verlagsgesellschaft, Weinheim, Germany, 1990 Search PubMed.
- S. Magdassi, Colloids Surf., A, 1997, 123–124, 671 CrossRef CAS.
- N. A. Peppas and L. Brannon-Peppas, J. Controlled Release, 1996, 40, 245 CrossRef CAS.
- B. Gabard, E. Chatelain, E. Bieli and S. Haas, Skin Res. Technol., 2001, 7(1), 49 CrossRef CAS.
- B. Bridges, Flavour Fragrance J., 2002, 17, 361 CrossRef CAS.
- S. E. Friberg, Int. J. Cosmet. Sci., 1997, 19, 75 CrossRef CAS.
- T. Jin, P. Pennefather and P. I. Lee, FEBS Lett., 1996, 397, 70 CrossRef CAS.
- A. Sansukcharearnpon, S. Wanichwecharungruang, N. Leepipatpaiboon, T. Kerdcharoen and S. Arayachukeat, Int. J. Pharm., 2010, 391(1–2), 267 CrossRef CAS.
- D. Gilsen and A. Chauhan, Int. J. Pharm., 2005, 292, 95 CrossRef.
- Y. Kapoor, J. C. Thomas, G. Tan, V. T. John and A. Chauhan, Biomaterials, 2009, 30(5), 867 CrossRef CAS.
- G. Y. Bachhav and V. B. Patravale, Int. J. Pharm., 2009, 365(1–2), 175 CrossRef CAS.
- Y. Lapitsky and E. W. Kaler, Soft Matter, 2006, 2, 779 RSC and references therein.
- C. Dispenza, G. Battaglia, G. Giammona, M. Licciardi and G. Spadaro, Colloid Polym. Sci., 2005, 284(2), 151 CrossRef CAS.
- J. M. Rosiak and F. Yoshii, Nucl. Instrum. Methods Phys. Res., Sect. B, 1999, 151, 56 CrossRef CAS.
- J. M. Rosiak and J. Olejniczak, Radiat. Phys. Chem., 1993, 42, 903 CrossRef CAS.
- J. M. Rosiak and P. Ulanski, Radiat. Phys. Chem., 1999, 55, 139 CrossRef CAS.
- G. J. M. Fechine, J. A. G. Barros and L. H. Catalani, Polymer, 2004, 45, 4705 CrossRef CAS.
- G. M. Kavanagh and S. B. Ross-Murphy, Prog. Polym. Sci., 1998, 23, 533 CrossRef CAS.
- M. Ricca, V. Foderà, D. Giacomazza, M. Leone, G. Spadaro and C. Dispenza, Colloid Polym. Sci., 2010, 288, 969 CrossRef CAS.
- P. Pusey and W. van Megen, Phys. A, 1989, 157, 705 CrossRef CAS.
- A. B. Rodd, D. E. Dunstan, D. V. Boger, J. Schmidt and W. Burchard, Macromolecules, 2001, 34, 3339 CrossRef CAS.
- P. J. Flory, Principles of PolymerChemistry, Cornell University Press, New York, 1953 Search PubMed.
- J. Schurz, Prog. Polym. Sci., 1991, 16(1), 1 CrossRef CAS.
- W. Burchard and M. Eisele, Pure Appl. Chem., 1984, 56, 1379 CrossRef CAS.
- G. Stroble, The Physics of Polymers, Springer-Verlag, Berlin, 1987, ch. 3 Search PubMed.
- P.-G. De Gennes, Cornell University Press, Ithaca, 1979.
- M. Takano, K. Ogata, S. Kawauchi, M. Satoh and J. Komiyama, Polym. Gels Networks, 1998, 6, 217 CrossRef CAS.
- M. Sen, Ő. Kantoglu and O. Güven, Polymer, 1999, 40, 913 CrossRef CAS.
- J. Schefer, R. McDaniel and B. P. Schoenbomt, J. Phys. Chem., 1988, 92, 729 CrossRef CAS.
- S. Borbély, Langmuir, 2000, 16, 5540 CrossRef CAS.
- V. B. Bueno, I. M. Cuccovia, H. Chaimovich and L. H. Catalani, Colloid Polym. Sci., 2009, 287, 705 CrossRef CAS.
- A. Bozkurt and W. H. Meyer, J. Polym. Sci., Part B: Polym. Phys., 2001, 39, 1987 CrossRef CAS.
- J. Rička and T. Tanaka, Macromolecules, 1984, 17(12), 2916 CrossRef CAS.
- C. Dispenza, G. Tripodo, C. LoPresti, G. Spadaro and G. Giammona, React. Funct. Polym., 2009, 69(8), 565 CrossRef CAS.
- J. M. Rosiak, I. Janik, S. Kadlubowski, M. Kozicki, P. Kujawa, P. Stasica, P. Ulanski IAEA TECDOC Series No. 1324, 2002, p. 4.
- A. Jelinek and H.-P. Klocking, Exp. Toxicol. Pathol., 1998, 50(4–6), 472 CAS.
- M. Corazza, M. M. Lauriola, M. Zappaterra, A. Bianchi and A. Virgili, J. Eur. Acad. Dermatol. Venereol., 2010, 24, 1 CrossRef CAS and references therein.
- A. Chapiro and C. Legris, Radiat. Phys. Chem., 1986, 28, 143 CrossRef CAS.
- A. D'Errico, M. De Lellis, G. Mangiapia, A. Tedeschi, O. Ortona, S. Fusco, A. Borzacchiello and L. Ambrosio, Biomacromolecules, 2008, 9, 231 CrossRef.
- L. Lopergolo, A. B. Lugao and L. H. Catalani, Polymer, 2003, 44, 6217 CrossRef CAS.
- J. Bednar, Generalized Model of Radiolysis in Theoretical Foundations of Radiation Chemistry, Kluwer Academic Publishers, Dordrecht, The Nederlands, 1990, ch. 3 Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Dynamic light scattering experiments on aqueous solutions of PVP at different concentrations. See DOI: 10.1039/c0py00161a |
| ‡ Current address: Merck Serono S.p.A., Pharmaceutical Dev. Biotech Products- Technical Operations Via Luigi Einaudi 11 – 00012 Guidonia Montecelio (Roma) Italy |
| This journal is © The Royal Society of Chemistry 2011 |
