Regio- and stereospecific [2+2] photocyclodimerization of a crown-containing butadienyl dyevia cation-induced self-assembly in solution†
Evgeny N.
Ushakov
*a,
Artem I.
Vedernikov
b,
Michael V.
Alfimov
b and
Sergey P.
Gromov
*b
aDepartment of Nanophotonics, Institute of Problems of Chemical Physics, Chernogolovka, 142432, Moscow Region, Russia. E-mail: en-ushakov@mail.ru; Fax: +7 496 515 5420; Tel: +7 496 524 4346
bPhotochemistry Centre, Novatorov str. 7A-1, Moscow, 119421, Russia. E-mail: spgromov@mail.ru; Fax: +7 495 936 1255; Tel: +7 495 935 0116
First published on 26th October 2010
Abstract
Self-assembled pseudocyclic structures consisting of two molecules of a crown-containing butadienyl dye and two Mg2+ ions readily undergo regio- and stereospecific [2+2] photocycloaddition in MeCN to produce a single cyclobutane stereoisomer in almost quantitative yield.
Self-organization into supramolecular structures via noncovalent interactions such as hydrogen bonding, host–guest interactions, ion association or stacking interactions, makes it possible to control excited-state reactions of organic molecules.1 In the early nineties we reported a dramatic effect of molecular self-assembly on the photochemistry of a styryl dye containing a 15-crown-5 ether fragment and a benzothiazole residue with 3-sulfonatopropylN-substituent (dye 1, Scheme 1).2
![Self-assembly into dimeric complexes leading to stereospecific [2+2] photocycloaddition of styryl dye 1.](/image/article/2011/PP/c0pp00227e/c0pp00227e-s1.gif) | ||
| Scheme 1 Self-assembly into dimeric complexes leading to stereospecific [2+2] photocycloaddition of styryl dye 1. | ||
In aprotic solvents dye 1 binds Mg2+ ions to form pseudocyclic 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 complexes in which the sulfonate anion of one dye molecule coordinates with the metal cation located in the crown ether cavity of another and vice versa. The dye molecules in these complexes undergo selective anti-head-to-tail [2+2] photocycloaddition (PCA) to afford a single cyclobutane stereoisomer (2, Scheme 1). Irradiation at 365 nm led to almost quantitative conversion of dye 1 into cyclobutane 2. In metal-free solutions dye 1 does not undergo PCA even at a relatively high concentration (∼1 mM).
2 complexes in which the sulfonate anion of one dye molecule coordinates with the metal cation located in the crown ether cavity of another and vice versa. The dye molecules in these complexes undergo selective anti-head-to-tail [2+2] photocycloaddition (PCA) to afford a single cyclobutane stereoisomer (2, Scheme 1). Irradiation at 365 nm led to almost quantitative conversion of dye 1 into cyclobutane 2. In metal-free solutions dye 1 does not undergo PCA even at a relatively high concentration (∼1 mM).
The effect of different structural factors on the cation-induced PCA of crown-containing styryl dyes has been studied.3 In particular, it was found that changing to a 4-styrylquinolinium chromophore altered the PCA pattern (syn-head-to-tail vs. anti-head-to-tail).3d
Other supramolecular approaches that have been used to control intermolecular PCA of olefins in solution include duplex formation viahydrogen bonds,4dimerization within the cavities of γ-cyclodextrin,5cucurbit[8]uril6 and calixarenes,7 encapsulation within self-assembled coordination cages,8hydrogen bonding with molecular templates,9self-assembly into pseudorotaxane structures,10 preorganization via multiple noncovalent interactions.11 In a few cases supramolecular assistance to PCA provided almost quantitative conversion of an olefin into a single cyclobutane isomer.
In comparison with olefins, 1,3-butadienes can be involved in a broader spectrum of photochemical reactions.12 This makes it more difficult to control their photochemical behaviour by supramolecular methods. It is known that topochemical PCA of butadienes can result not only in cyclobutanes13 but also in [3]ladderanes through a stepwise process.14 A few studies have been published on supramolecular control of the solid-state intermolecular PCA of butadienes. For instance, the photochemical syntheses of [3]ladderanes from butadienes were reported15 in which proper molecular packing of the reactants were provided by weak noncovalent interactions. However, the potential of supramolecular chemistry with respect to controlling the solution-phase intermolecular PCA of 1,3-butadienes remains unexplored.
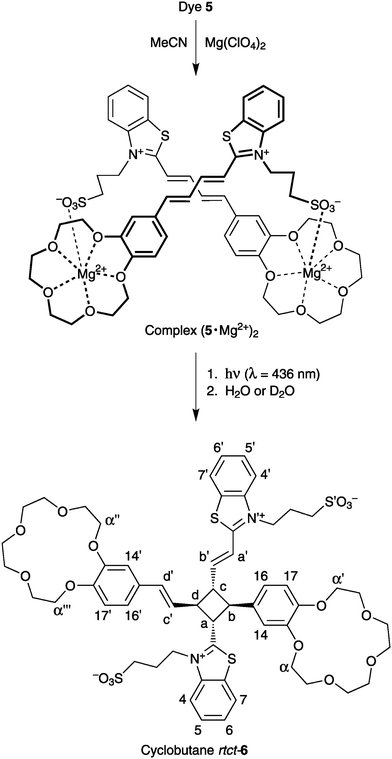
We assumed that for stereospecific PCA of two butadiene molecules to occur in solution, the reactant must be properly preorganized by means of relatively strong noncovalent interactions, such as two-centre ion pairing in complexes (1·Mg2+)2.16 To test this assumption we synthesized a butadiene analogue of styryl dye 1, viz., the crown-containing butadienyl dye 5 (Scheme 2).
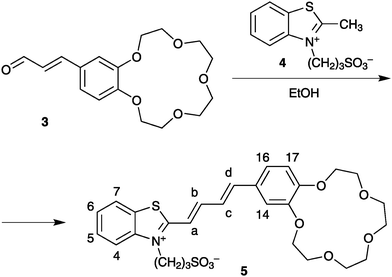 | ||
| Scheme 2 Synthesis of butadienyl dye 5. | ||
Dye 5 was obtained in 43% yield by condensation of cinnamaldehyde 3 (ref. 17) with quaternary salt 4 (ref. 3b) on long-term heating in absolute ethanol. The structure assigned to 5 is confirmed by elemental analysis and 1H NMR spectroscopy.‡
Fig. 1 shows the absorption spectra of dye 5 in MeCN/0.01 M Bu4NClO4 as a function of the concentration of added magnesium perchlorate. The strong hypsochromic effect observed on addition of Mg(ClO4)2 indicates Mg2+ coordination to the 15-crown-5 ether moiety of dye 5.18 The spectrum measured at a 20% excess of Mg(ClO4)2 over the dye (spectrum B in Fig. 1) is attributable to a fully complexed form of 5 because a subsequent increase in the metal salt concentration by an order of magnitude did not induce any spectral changes. This spectrum has a specific profile with a pronounced long-wavelength shoulder, which points to H-type aggregation of dye molecules.19 To interpret this feature, we assume that dye 5, similarly to styryl dye 1, forms 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 complexes with Mg2+via two-centre ion pairing. Factor analysis20 showed that other complexes most likely make an insignificant contribution to the spectra represented in Fig. 1. This enabled us to assign spectrum B to the dimers (5·Mg2+)2.
2 complexes with Mg2+via two-centre ion pairing. Factor analysis20 showed that other complexes most likely make an insignificant contribution to the spectra represented in Fig. 1. This enabled us to assign spectrum B to the dimers (5·Mg2+)2.
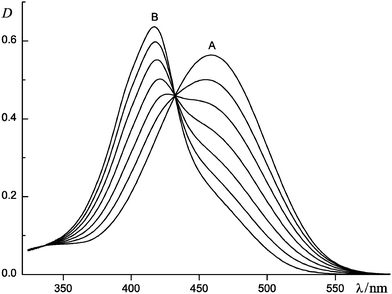 | ||
| Fig. 1 Absorption spectra of dye 5 in MeCN/0.01 M Bu4NClO4 as a function of the concentration of added magnesium perchlorate (CM): 1 cm cell, the concentration of 5CD = 1.2 × 10−5 M, CM/CD = 0 (A), 0.18, 0.36, 0.53, 0.71, 0.87 and 1.2 (B); spectrum B is assigned to complex (5·Mg2+)2. | ||
The main absorption and luminescence characteristics of dye 5 and complex (5·Mg2+)2 are listed in Table 1. Note that the formation of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 complexes between 5 and Mg2+ leads not only to significant changes in the absorption spectrum but also to luminescence quenching.
2 complexes between 5 and Mg2+ leads not only to significant changes in the absorption spectrum but also to luminescence quenching.
| Compound | λ max abs/nm | ε/dm3 mol−1 cm−1 | λ max em/nm | φ lum |
|---|---|---|---|---|
| φ lum – luminescence quantum yield. | ||||
| 5 | 459 | 47000 | 626 | 0.017 |
| (5·Mg2+)2 | 417 | 106000 | 587 | 0.0008 |
Fig. 2 shows the effect of irradiation with low-intensity 436 nm light on the absorption spectra of (5·Mg2+)2 in MeCN. The irradiation results in rapid disappearance of the long-wavelength absorption band associated with (5·Mg2+)2. The product obtained after 15 min photolysis was tested for the presence of Z photoisomers of 5 using a four-stage procedure, as described previously for styryl dyes.3d This test showed that the photolysis led to complete consumption of dye 5, presumably owing to very efficient PCA reaction between dye molecules in the dimeric complexes (5·Mg2+)2. The PCA quantum yield was estimated to be about 0.3.
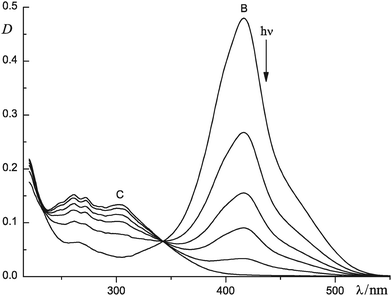 | ||
| Fig. 2 Photolysis of dye 5 (9.1 × 10−6 M) in MeCN in the presence of Mg(ClO4)2 (1 × 10−4 M), as monitored by spectrophotometry: 1 cm cell, irradiation at 436 nm, light intensity 5 × 1014 cm−2c−1, irradiation time 0 (B), 0.5, 1.0, 1.5, 2.5 and 15 (C) min; spectrum C is assigned to complex 6·(Mg2+)2. | ||
The photolysate obtained from (5·Mg2+)2 was studied in a MeCN-d3/D2O solution by 1H NMR spectroscopy.§Water was added in order to preclude complex formation between the photolysate and Mg2+. The 1H NMR spectrum of the uncomplexed photolysate (Fig. 3) revealed that the PCA reaction in complexes (5·Mg2+)2 occurs regio- and stereospecifically to afford a single cyclobutane isomer (cyclobutanertct-6, Scheme 3). The photolysate contained no other detectable components, i.e., the cyclobutane 6 is obtained from dye 5 in almost quantitative yield (>95%).
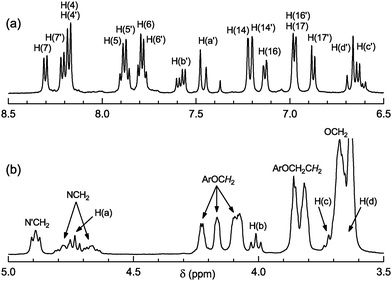 | ||
Fig. 3
1H NMR spectrum of cyclobutanertct-6 in a MeCN-d3/D2O solution (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v): (a) aromatic and (b) aliphatic regions. 1 v/v): (a) aromatic and (b) aliphatic regions. | ||
 | ||
| Scheme 3 Regio- and stereospecific synthesis of cyclobutanertct-6. | ||
In the aromatic region, the 1H NMR spectrum of 6 exhibits four signals from the ethylene protons H(a′)–H(d′) and a set of signals from the protons of two non-equivalent benzothiazole residues and two non-equivalent benzo-15-crown-5 ether fragments. The protons of cyclobutane ring, H(a)–H(d), give four signals between 3.6 and 4.8 ppm.22 These facts indicate that the substituents in the 1,2,3,4-tetrasubstituted cyclobutane 6 differ from each other in structure. The relative position of these substituents was derived from analysis of the 2D COSY and NOE spectra of 6. The cyclobutane protons H(a) and H(b) show two triplet signals at 4.73 and 4.01 ppm with 3J = 9.5 and 9.7 Hz, respectively. The triplet structure of these signals as well as the magnitudes of vicinal coupling constants are indicative of the rtct-configuration of the cyclobutane moiety in 6.3a–c The structure assigned to cycloadduct 6 suggests an anti-head-to-tail alignment of the two dye molecules in the precursor complexes (5·Mg2+)2 (Scheme 3). This alignment is confirmed by molecular mechanics and DFT calculations of (5·Mg2+)2 (see ESI†).
In summary, the self-assembly of butadienyl dye 5 into 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 complexes with Mg2+via two-centre ion paring in MeCN leads to very efficient regio- and stereospecific [2+2] photocycloaddition affording cyclobutanertct-6 in almost quantitative yield. This result demonstrates for the first time that intermolecular PCA of 1,3-butadienes can be effectively controlled in solution by supramolecular methods. The approach we developed can be used to design novel supramolecular systems with high functionality.
2 complexes with Mg2+via two-centre ion paring in MeCN leads to very efficient regio- and stereospecific [2+2] photocycloaddition affording cyclobutanertct-6 in almost quantitative yield. This result demonstrates for the first time that intermolecular PCA of 1,3-butadienes can be effectively controlled in solution by supramolecular methods. The approach we developed can be used to design novel supramolecular systems with high functionality.
This work was supported by the Russian Academy of Sciences and the Russian Foundation for Basic Research.
Notes and references
- J.-M. Lehn, Supramolecular chemistry, Concepts and perspectives, VCH, Weinheim, etc., 1995 Search PubMed.
- M. V. Alfimov, S. P. Gromov, O. B. Stanislavskii, E. N. Ushakov and O. A. Fedorova, Crown-containing styryl dyes. 8. Cation-dependent concerted [2+2] autophotocycloaddition of photochromic 15-crown-5 ether betaines, Russ. Chem. Bull., 1993, 42, 1385 Search PubMed.
- (a) S. P. Gromov, O. A. Fedorova, E. N. Ushakov, A. V. Buevich and M. V. Alfimov, Crown ether styryl dyes. 15. Synthesis and two pathways of regio- and stereospecific cation-dependent [2+2] autophotocycloaddition of chromogenic 15-crown-5 ether betaines of quinoline series, Russ. Chem. Bull., 1995, 44, 2131 Search PubMed; (b) S. P. Gromov, E. N. Ushakov, O. A. Fedorova, A. V. Buevich and M. V. Alfimov, Crown ether styryl dyes. 18. Synthesis, anion-”capped” complexes, and ion-selective stereospecific [2+2] autophotocycloaddition of photochromic 18-crown-6 ethers, Russ. Chem. Bull., 1996, 45, 654 Search PubMed; (c) S. P. Gromov, O. A. Fedorova, E. N. Ushakov, I. I. Baskin, A. V. Lindeman, E. V. Malysheva, T. A. Balashova, A. S. Arsen'ev and M. V. Alfimov, Crown ether styryl dyes. 24. Synthesis of multiphotochromic 15-crown-5 ethers with rigid spacers, their anion-”capped” complexes, and stereospecific [2+2] autophotocycloaddition, Russ. Chem. Bull., 1998, 47, 97 Search PubMed; (d) S. P. Gromov, E. N. Ushakov, O. A. Fedorova, I. I. Baskin, A. V. Buevich, E. N. Andryukhina, M. V. Alfimov, D. Johnels, U. G. Edlund, J. K. Whitesell and M. A. Fox, Novel photoswitchable receptors: synthesis and cation-induced self-assembly into dimeric complexes leading to stereospecific [2+2]-photocycloaddition of styryl dyes containing a 15-crown-5 ether unit, J. Org. Chem., 2003, 68, 6115 CrossRef CAS.
- F. D. Lewis, T. Wu, E. L. Burch, D. M. Bassani, J.-S. Yang, S. Schneider, W. Jaeger and R. L. Letsinger, Hybrid oligonucleotides containing stilbene units. Excimer fluorescence and photodimerization, J. Am. Chem. Soc., 1995, 117, 8785 CrossRef CAS.
- W. Herrmann, S. Wehrle and G. Wenz, Supramolecular control of the photochemistry of stilbenes by cyclodextrins, Chem. Commun., 1997, 1709 RSC.
- (a) S. Y. Jon, Y. H. Ko, S. H. Park, H.-J. Kim and K. Kim, A facile, stereoselective [2+2] photoreaction mediated by cucurbit[8]uril, Chem. Commun., 2001, 1938 RSC; (b) S. P. Gromov, A. I. Vedernikov, L. G. Kuz'mina, D. V. Kondratuk, S. K. Sazonov, Yu. A. Strelenko, M. V. Alfimov and J. A. K. Howard, Photocontrolled molecular assembler based on cucurbit[8]uril: [2+2]-autophotocycloaddition of styryl dyes in the solid state and in water, Eur. J. Org. Chem., 2010, 2587 CrossRef CAS.
- R. Kaliappan, L. S. Kaanumalle, A. Natarajan and V. Ramamurthy, Templating photodimerization of stilbazoles with water-soluble calixarenes, Photochem. Photobiol. Sci., 2006, 5, 925 RSC.
- M. Yoshizawa, Y. Takeyama, T. Kusukawa and M. Fujita, Cavity-directed, highly stereoselective [2+2] photodimerization of olefins within self-assembled coordination cages, Angew. Chem., Int. Ed., 2002, 41, 1347 CrossRef CAS.
- (a) D. M. Bassani, V. Darcos, S. Mahony and J.-P. Desvergne, Supramolecular catalysis of olefin [2+2] photodimerization, J. Am. Chem. Soc., 2000, 122, 8795 CrossRef CAS; (b) W. G. Skene, E. Couzigné and J.-M. Lehn, Supramolecular control of the template-induced selective photodimerization of 4-methyl-7-O-hexylcoumarin, Chem.–Eur. J., 2003, 9, 5560 CrossRef CAS.
- D. G. Amirsakis, A. M. Elizarov, M. A. Garcia-Garibay, P. T. Glink, J. F. Stoddart, A. J. P. White and D. J. Williams, Diastereospecific photochemical dimerization of a stilbene-containing daisy chain monomer in solution as well as in the solid state, Angew. Chem., Int. Ed., 2003, 42, 1126 CrossRef CAS.
- (a) Y. Vida Pol, R. Suau, E. Perez-Inestrosa and D. M. Bassani, Synergistic effects in controlling excited-state photodimerisation using multiple supramolecular interactions, Chem. Commun., 2004, 1270 RSC; (b) S. P. Gromov, A. I. Vedernikov, Yu. V. Fedorov, O. A. Fedorova, E. N. Andryukhina, N. E. Shepel', Yu. A. Strelenko, D. Johnels, U. Edlund, J. Saltiel and M. V. Alfimov, Self-assembly of a (benzothiazolyl)ethenylbenzocrown ether into a sandwich complex and stereoselective [2+2] photocycloaddition, Russ. Chem. Bull., 2005, 54, 1569 Search PubMed; (c) A. I. Vedernikov, S. K. Sazonov, P. S. Loginov, N. A. Lobova, M. V. Alfimov and S. P. Gromov, Hydrogen bonding- and stacking-induced stereospecific [2+2]-photocycloaddition within a pseudodimeric complex of two styryl dyes, Mendeleev Commun., 2007, 17, 29 Search PubMed.
- B. H. O. Cook and W. J. Leigh, Photopericyclic reactions of conjugated dienes and trienes, in The chemistry of dienes and polyenes, ed. Z. Rappoport, John Wiley & Sons, Chichester, etc., 2000, vol. 2, ch. 3, pp. 197-255 Search PubMed.
- G. M. J. Schmidt, Photodimerization in the solid state, Pure Appl. Chem., 1971, 27, 647 CAS.
- H. Hopf, H. Greiving, P. G. Jones and P. Bubenitschek, Topochemical reaction control in solution, Angew. Chem., Int. Ed. Engl., 1995, 34, 685 CrossRef CAS.
- (a) K. Vishnumurthy, T. N. Guru, Row and K. Venkatesan, Fluorine in crystal engineering: photodimerization of (1E,3E)-1-phenyl-4-pentafluorophenylbuta-1,3-dienes in the crystalline state, Photochem. Photobiol. Sci., 2002, 1, 427 RSC; (b) X. Gao, T. Friščić and L. R. MacGillivray, Supramolecular construction of molecular ladders in the solid state, Angew. Chem., Int. Ed., 2004, 43, 232 CrossRef CAS.
- For dye 1 and its analogues having a sulfonatobenzylN-substituent, the dissociation of dimers (dye·Mg2+)2 into monomers dye·Mg2+ in anhydrous MeCN at a temperature of ca. 20 °C occurs within a characteristic time ranging from a few tens of seconds to a few minutes, which attests relatively strong intermolecular bonding in the dimers: (a) E. N. Ushakov, O. B. Stanislavskii, S. P. Gromov, O. A. Fedorova and M. V. Alfimov, Crown-containing styryl dyes. 6. Photoinduced and dark cation-dependent dimerization of betaines of photochromic 15-crown-5 ethers, Dokl. Phys. Chem., 1992, 323, 164; (b) E. N. Ushakov, S. P. Gromov, A. V. Buevich, I. I. Baskin, O. A. Fedorova, A. I. Vedernikov, M. V. Alfimov, B. Eliasson and U. Edlund, Crown-containing styryl dyes: Cation-induced self-assembly of multiphotochromic 15-crown-5 ethers into photoswitchable molecular devices, J. Chem. Soc., Perkin Trans. 2, 1999, 601 RSC.
- A. I. Vedernikov and S. P. Gromov, Convenient method for the preparation of crown ether cinnamaldehydes, Synthesis, 2001, 889 CrossRef CAS.
- S. P. Gromov, A. I. Vedernikov, E. N. Ushakov, L. G. Kuz'mina, A. V. Feofanov, V. G. Avakyan, A. V. Churakov, Yu. S. Alaverdyan, E. V. Malysheva, M. V. Alfimov, J. A. K. Howard, B. Eliasson and U. G. Edlund, Synthesis, structure, spectroscopic studies, and complexation of novel crown ether butadienyl dyes, Helv. Chim. Acta, 2002, 85, 60 CrossRef CAS.
- H-type dye aggregation commonly results in absorption band splitting; in many cases, the weaker, lower-energy band is unresolved and reveals itself as a shoulder of the more intense, higher-energy band: (a) V. I. Yuzhakov, Association of dye molecules and its spectroscopic manifestation, Russ. Chem. Rev., 1979, 48, 1076 Search PubMed; (b) E. N. Ushakov, S. P. Gromov, O. A. Fedorova and M. V. Alfimov, Crown-containing styryl dyes. 19. Complexation and cation-induced aggregation of chromogenic aza-15-crown-5 ethers, Russ. Chem. Bull., 1997, 46, 463 Search PubMed.
- The set of experimental spectra shown in Fig. 1 was reconstructed within a residual error of 0.0012 optical density units by a factor analysis method under the assumption that there are only two absorbing components in the system; the small value of residual error suggests that contribution from a third component to the total spectral variation can be neglected: (a) J. J. Kankare, Computation of equilibrium constants for multicomponent systems from spectrophotometric data, Anal. Chem., 1970, 42, 1322 CrossRef CAS; (b) E. N. Ushakov, S. P. Gromov, O. A. Fedorova, Yu. V. Pershina, M. V. Alfimov, F. Barigelletti, L. Flamigni and V. Balzani, Sandwich-type complexes of alkaline-earth metal cations with a bisstyryl dye containing two crown ether units, J. Phys. Chem. A, 1999, 103, 11188 CrossRef CAS.
- MeCN solutions of dye 5 and complex (5·Mg2+)2 were prepared in a darkroom under red light. Spectral experiments were conducted in quartz cells with ground stoppers to avoid MeCN evaporation during the experiments. The atmospheric oxygen contained in solutions was not removed. Luminescence spectra were corrected taking into account the spectral sensitivity of the photomultiplier tube. Luminescence quantum yields φlum were determined using [Ru(bpy)3]Cl2 in air-equilibrated water as the reference compound. The φlum value for the reference is 0.028: K. Nakamaru, Synthesis, luminescence quantum yields, and lifetimes of trischelated ruthenium (II) mixed-ligand complexes including 3,3-dimethyl-2,2-bipyridyl, Bull. Chem. Soc. Jpn., 1982, 55, 2697 Search PubMed.
- The signals from cyclobutane protons H(c) and H(d) are overlapped by an intense signal from the protons of CH2O groups.
Footnotes |
| † Electronic supplementary information (ESI) available: Absorption spectroscopy data for a cationic analogue of dye 5; global analysis of spectrophotometric titration data (factor analysis, discussion on complexation stoichiometry, parameterized modelling of absorption spectra); molecular mechanics and DFT modelling of dimers (5·Mg2+)2. See DOI: 10.1039/c0pp00227e |
| ‡ A mixture of cinnamaldehyde 3 (100 mg, 0.31 mmol) and quaternary salt 4 (76 mg, 0.28 mmol) in abs. EtOH (12 mL) was heated at 80–85 °C (oil bath) for 100 h and then cooled to 5 °C. The precipitate formed was filtered, washed with abs. EtOH (3 mL), benzene (2 × 10 mL), hexane (15 mL) and dried in air to yield compound 5 (72 mg, 43%) as a brown-red powder (Found: C, 56.87; H, 5.79; N, 2.30. Calc. for C28H33NO8S2·H2O: C, 56.64; H, 5.94; N, 2.36%); mp 213–216 °C; 1H NMR (Bruker DRX500; DMSO-d6; 25 °C): δ 2.16 (m, 2H, CH2CH2SO3), 2.64 (t, J = 6.8 Hz, 2H, CH2SO3), 3.63 (m, 8H, 4 CH2O), 3.80 (m, 4H, 2 CH2CH2OAr), 4.13 (m, 4H, 2 CH2OAr), 4.91 (m, 2H, CH2N), 7.04 (d, J = 8.4 Hz, 1H, H(17)), 7.21 (br.d, J = 8.4 Hz, 1H, H(16)), 7.29 (s, 1H, H(14)), 7.30 (dd, J = 15.3 Hz, J = 10.7 Hz, 1H, H(c)), 7.43 (d, J = 15.3 Hz, 1H, H(d)), 7.59 (d, J = 14.7 Hz, 1H, H(a)), 7.74 (m, 1H, H(6)), 7.84 (m, 1H, H(5)), 8.02 (dd, J = 14.7 Hz, J = 10.7 Hz, 1H, H(b)), 8.35 (d, J = 8.8 Hz, 1H, H(4)), 8.37 (d, J = 8.4 Hz, 1H, H(7)). The spin–spin coupling constants JH(a),H(b) = 14.7, JH(b),H(c) = 10.7 and JH(c),H(d) = 15.3 Hz point to E,E configuration and s-trans conformation of the butadiene fragment in 5. |
§ Dye 5 (10.0 μmol) and Mg(ClO4)2 (10.5 μmol) were dissolved in 50 mL of MeCN (water content < 0.03% v/v). The solution was irradiated with 436 nm light (glass-filtered light of a high-pressure Hg lamp); after complete consumption of the dye (monitoring by spectrophotometry), the solvent was evaporated in vacuo. The solid residue was dissolved in a MeCN-d3/D2O mixture (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) and analyzed by 1H NMR spectroscopy. This analysis revealed that the solute contained a single detectable component, viz., cyclobutane isomer rtct-6 (Scheme 3); 1H NMR (Bruker DRX500; MeCN-d3/D2O (10 1 v/v) and analyzed by 1H NMR spectroscopy. This analysis revealed that the solute contained a single detectable component, viz., cyclobutane isomer rtct-6 (Scheme 3); 1H NMR (Bruker DRX500; MeCN-d3/D2O (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v); 25 °C): δ 2.08 and 2.25 (2 m, 2H, CH2CH2SO3), 2.27 (m, 2H, CH2CH2S′O3), 2.67 (br.t, J = 6.3 Hz, 2H, CH2SO3), 2.91 (t, J = 6.2 Hz, 2H, CH2S′O3), 3.60–3.70 (m, 17H, 8 CH2O, H(d)), 3.72 (m, 1H, H(c)), 3.82 (m, 4H, 2 CH2CH2OAr), 3.86 (m, 4H, 2 CH2CH2OAr), 4.01 (t, J = 9.7 Hz, 1H, H(b)), 4.08 (m, 2H, α′′′-CH2OAr), 4.10 (m, 2H, α′-CH2OAr), 4.17 (m, 2H, α′′-CH2OAr), 4.23 (m, 2H, α-CH2OAr), 4.67 and 4.77 (2 m, 2H, CH2N), 4.73 (t, J = 9.5 Hz, 1H, H(a)), 4.89 (m, 2H, CH2N′), 6.62 (dd, J = 15.8 Hz, J = 7.9 Hz, 1H, H(c′)), 6.68 (d, J = 15.8 Hz, 1H, H(d′)), 6.88 (d, J = 8.3 Hz, 1H, H(17′)), 6.98 (br.d, J = 7.5 Hz, 2H, H(17), H(16′)), 7.13 (br.d, J = 8.2 Hz, 1H, H(16)), 7.20 (br.s, 1H, H(14′)), 7.22 (br.s, 1H, H(14)), 7.46 (d, J = 15.5 Hz, 1H, H(a′)), 7.58 (dd, J = 15.5 Hz, J = 7.6 Hz, 1H, H(b′)), 7.78 (m, 1H, H(6′)), 7.79 (m, 1H, H(6)), 7.87 (m, 1H, H(5′)), 7.89 (m, 1H, H(5)), 8.18 (d, J = 8.6 Hz, 2H, H(4), H(4′)), 8.21 (d, J = 8.1 Hz, 1H, H(7′)), 8.30 (d, J = 8.2 Hz, 1H, H(7)). 1 v/v); 25 °C): δ 2.08 and 2.25 (2 m, 2H, CH2CH2SO3), 2.27 (m, 2H, CH2CH2S′O3), 2.67 (br.t, J = 6.3 Hz, 2H, CH2SO3), 2.91 (t, J = 6.2 Hz, 2H, CH2S′O3), 3.60–3.70 (m, 17H, 8 CH2O, H(d)), 3.72 (m, 1H, H(c)), 3.82 (m, 4H, 2 CH2CH2OAr), 3.86 (m, 4H, 2 CH2CH2OAr), 4.01 (t, J = 9.7 Hz, 1H, H(b)), 4.08 (m, 2H, α′′′-CH2OAr), 4.10 (m, 2H, α′-CH2OAr), 4.17 (m, 2H, α′′-CH2OAr), 4.23 (m, 2H, α-CH2OAr), 4.67 and 4.77 (2 m, 2H, CH2N), 4.73 (t, J = 9.5 Hz, 1H, H(a)), 4.89 (m, 2H, CH2N′), 6.62 (dd, J = 15.8 Hz, J = 7.9 Hz, 1H, H(c′)), 6.68 (d, J = 15.8 Hz, 1H, H(d′)), 6.88 (d, J = 8.3 Hz, 1H, H(17′)), 6.98 (br.d, J = 7.5 Hz, 2H, H(17), H(16′)), 7.13 (br.d, J = 8.2 Hz, 1H, H(16)), 7.20 (br.s, 1H, H(14′)), 7.22 (br.s, 1H, H(14)), 7.46 (d, J = 15.5 Hz, 1H, H(a′)), 7.58 (dd, J = 15.5 Hz, J = 7.6 Hz, 1H, H(b′)), 7.78 (m, 1H, H(6′)), 7.79 (m, 1H, H(6)), 7.87 (m, 1H, H(5′)), 7.89 (m, 1H, H(5)), 8.18 (d, J = 8.6 Hz, 2H, H(4), H(4′)), 8.21 (d, J = 8.1 Hz, 1H, H(7′)), 8.30 (d, J = 8.2 Hz, 1H, H(7)). |
| This journal is © The Royal Society of Chemistry and Owner Societies 2011 |
