Human serum albumin as key mediator of the differential accumulation of hypericin in normal urothelial cell spheroids versusurothelial cell carcinoma spheroids
Mieke
Roelants
a,
Ben
Van Cleynenbreugel
b,
Evelyne
Lerut
c,
Hendrik
Van Poppel
b and
Peter A. M.
de Witte
*a
aLaboratorium voor Farmaceutische Biologie, Katholieke Universiteit Leuven, Herestraat 49, B-3000, Leuven, Belgium. E-mail: peter.dewitte@pharm.kuleuven.be; Fax: + 32-16-323460; Tel: + 32-16-323432
bAfdeling Urologie, UZ Gasthuisberg, Herestraat 49, B-3000, Leuven, Belgium
cAfdeling Morfologie en Moleculaire Pathologie, Katholieke Universiteit Leuven, Minderbroedersstraat 12, B-3000, Leuven, Belgium
First published on 22nd November 2010
Abstract
Hypericin is a bright red fluorescent compound that can be used in urological medicine as a photodiagnostic to detect non-muscle-invasive bladder cancer lesions. To this end a bladder instillation fluid is prepared in which the water-insoluble hypericin is solubilized by the presence of human serum albumin (HSA) to which the compound binds. In the present study, we explored the possibility that besides acting as a passive hypericin carrier, HSA also actively contributes to the selective localization of the compound. By using multicellular spheroids prepared from normal human urothelial (NHU) cells and from different urothelial carcinoma cell (UCC) lines (T24, RT-112 and RT-4), we simulated three-dimensionally the normal urothelium and urothelial cell carcinomas present in the bladder of patients. The distribution of hypericin in these spheroids was investigated in the presence or absence of HSA. Our data show that when hypericin is solubilized by HSA, an excellent differentiation in distribution of hypericin in normal urothelial spheroids and malignant spheroids is observed, clearly suggesting a key role for albumin in the specific localization of hypericin in non-muscle-invasive bladder tumours. Furthermore, PDT results show that both the hypericin-PDT effect on tumour spheroids and the selective character of the treatment can significantly be increased by the presence of HSA. Interestingly, we also observed that the presence of HSA did not convey tumouritropic characteristics to other photosensitizers like pheophorbide a and mTHPP, implying that both the particular characteristics of the photosensitizer and HSA contribute to the final selective accumulation of the compound in tumoural tissue.
Introduction
Hypericin is a secondary metabolite with a phenanthroperylene quinone structure present in plants of the genus Hypericum. The compound shows a bright red fluorescence1 and is used nowadays in urological medicine as a photodiagnostic to detect non-muscle-invasive bladder cancer lesions, in which the compound accumulates with high sensitivity and specificity.2,3 For that purpose hypericin is instilled intravesically for minimally 2 h in patients suspected of having bladder cancer, followed by a blue-light endoscopic examination of the bladder wall.
Hypericin is a water-insoluble compound that precipitates in an aqueous environment as non-fluorescent aggregates. Hence it is essential that the bladder instillation fluid contains a vehicle that is able to monomerize and solubilize the compound.4 It is well known that hypericin exhibits high affinity for bovine (BSA) and in particular human serum albumin (HSA).5 As a matter of fact, serum albumin presents two binding sites for hypericin of which pocket IIA is the most relevant, and as a result a strong water-soluble 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 hypericin-HSA complex is formed.4,6 This given and the fact that human albumin can be used in a clinical setting urged us to supplement the bladder instillation fluid with HSA to prepare a stable formulation of hypericin.2,3 Importantly, this solution can also be sterilized easily by membrane filtration.
1 hypericin-HSA complex is formed.4,6 This given and the fact that human albumin can be used in a clinical setting urged us to supplement the bladder instillation fluid with HSA to prepare a stable formulation of hypericin.2,3 Importantly, this solution can also be sterilized easily by membrane filtration.
In the present study, we explored in in vitro conditions the possibility that HSA not only acts as a passive carrier of hypericin, but also actively contributes to the selective localization of the compound in non-muscle-invasive urothelial cell carcinomas. By using multicellular spheroids prepared from normal human urothelial cells (NHU) and from different urothelial carcinoma cell lines, we simulated three-dimensionally the normal urothelium and urothelial cell carcinomas present in the bladder of patients. Additionally, we investigated whether two other photosensitizers, i.e.pheophorbide a and the Foscan®-related compound meso-tetra(m-hydroxyphenyl)porphine (mTHPP), accumulate in the presence of HSA in 3-D urothelial cell carcinomas preferentially.7,8
Since hypericin produces singlet oxygen very efficiently, and hence is considered to be an excellent photosensitizing agent9,10 that could be used in the photodynamic management of bladder cancer,11 the present study also focused on the selectivity of the PDT effects of hypericin.
Our data show that when hypericin is solubilized by HSA, an excellent differentiation in distribution of hypericin in normal urothelial spheroids and malignant spheroids is observed. Similar results could not be obtained with the two other photosensitizers. Furthermore, PDT results show that both the efficacy of hypericin-PDT on tumour spheroids and the selective character of the treatment can significantly be increased by the presence of HSA.
Results and discussion
Influence of HSA on the intraspheroidal distribution of hypericin
Multicellular spheroids were generated from three bladder cancer cell lines (RT-4, RT-112 and T24) or NHU cells derived from different patients. They were incubated with hypericin (10 μM), and in specific cases also with HSA (0.3% [m/v]) for 2 h, thereby mimicking the clinical situation where similar conditions are used for the fluorescent detection of non-muscle-invasive bladder cancer lesions.2,3 The photomicrographs in Fig. 1 represent centrally cut sections of these spheroids. Incubation of normal urothelial spheroids and urothelial cell carcinoma (UCC) spheroids with hypericin in the absence of HSA resulted in a similar hypericin distribution pattern. In both conditions we observed a maximum fluorescence in the outer cell layers and an immediate drop as a result of a very poor penetration. In contrast, there was a pronounced difference noticeable when the UCC spheroids were exposed to hypericin in MEM supplemented with HSA, and in these conditions hypericin is present uniformly throughout the entire spheroid section. Quantitative data as obtained with a KS imaging software are presented in Table 1 and Fig. 2.| Relative fluorescence values | ||||||||
|---|---|---|---|---|---|---|---|---|
| Mean ± SD NHU (+ HSA) | Mean ± SD NHU (− HSA) | Mean ± SD T 24 (+ HSA) | Mean ± SD T 24 (− HSA) | Mean ± SD RT-112 (+ HSA) | Mean ± SD RT-112 (− HSA) | Mean ± SD RT-4 (+ HSA) | Mean ± SD RT-4 (− HSA) | |
| F max | 196 ± 90 | 320 ± 144 | 243 ± 75 | 279 ± 93 | 268 ± 102 | 423 ± 92 | 272 ± 45 | 392 ± 180 |
| F 25 | 33 ± 25 | 26 ± 16 | 119 ± 31 | 35 ± 18 | 169 ± 37 | 68 ± 14 | 141 ± 29 | 63 ± 56 |
| F 50 | 13 ± 11 | 4 ± 3 | 72 ± 11 | 2 ± 2 | 143 ± 32 | 13 ± 3 | 110 ± 23 | 7 ± 5 |
| F min | 8 ± 7 | 3 ± 3 | 30 ± 8 | 0 ± 0 | 97 ± 24 | 8 ± 2 | 84 ± 20 | 0 ± 0 |
| One-way Tukey-Kramer post test | ||||
|---|---|---|---|---|
| F ax | F 25 | F 50 | F min | |
| NHU (− HSA) vs.NHU (+ HSA) | ** | ns | ns | ns |
| T 24 (− HSA) vs. T 24 (+ HSA) | ns | *** | *** | *** |
| RT-112 (− HSA) vs. RT-112 (+ HSA) | ns | *** | *** | *** |
| RT-4 (− HSA) vs.RT-4 (+ HSA) | ns | *** | *** | *** |
| NHU (+ HSA) vs. T 24 (+ HSA) | ns | *** | *** | *** |
| NHU (+ HSA) vs. RT-112 (+ HSA) | ns | *** | *** | *** |
| NHU (+ HSA) vs.RT-4 (+ HSA) | ns | *** | *** | *** |
![Fluorescence photomicrographs of 5 μm centrally cut sections of NHU and UCC (T24, RT-112 and RT-4) spheroids incubated with 10 μM hypericin in the presence or absence of HSA (0.3% [m/v]). Single representative experiments; other images were similar. All photomicrographs were taken at identical gain and were reduced from ×200.](/image/article/2011/PP/c0pp00109k/c0pp00109k-f1.gif) | ||
| Fig. 1 Fluorescence photomicrographs of 5 μm centrally cut sections of NHU and UCC (T24, RT-112 and RT-4) spheroids incubated with 10 μM hypericin in the presence or absence of HSA (0.3% [m/v]). Single representative experiments; other images were similar. All photomicrographs were taken at identical gain and were reduced from ×200. | ||
![Hypericin fluorescence vs. distance from spheroid periphery after curve fitting using nonlinear regression. NHU and UCC (T24, RT-112 and RT-4) spheroids were exposed to 10 μM hypericin in the presence (dashed lines) or absence of HSA (0.3% [m/v]) (solid lines) for 2 h. The quantification was performed in 5.7 μm concentric layers on 5 μm centrally cut sections of the spheroids. Corrections were made for autofluorescence. Values represent the mean ± SEM (n = 6 for UCC spheroids; n = 30 for NHU spheroids [5 different patients]).](/image/article/2011/PP/c0pp00109k/c0pp00109k-f2.gif) | ||
| Fig. 2 Hypericin fluorescence vs. distance from spheroid periphery after curve fitting using nonlinear regression. NHU and UCC (T24, RT-112 and RT-4) spheroids were exposed to 10 μM hypericin in the presence (dashed lines) or absence of HSA (0.3% [m/v]) (solid lines) for 2 h. The quantification was performed in 5.7 μm concentric layers on 5 μm centrally cut sections of the spheroids. Corrections were made for autofluorescence. Values represent the mean ± SEM (n = 6 for UCC spheroids; n = 30 for NHU spheroids [5 different patients]). | ||
The current study therefore confirms the outcome of our previous study showing that the highest intraspheroidal hypericin penetration was observed in urothelial cell carcinoma spheroids, whereas limited permeation was seen in NHU spheroids.12 Besides we have shown evidence that hypericin diffuses mainly via paracellular routes throughout the three-dimensional network before being taken up intracellularly.13 However, also obvious differences can be noticed, as in the former experiments hypericin was never distributed as uniformly as observed in the present study with the hypericin-HSA complex.13 Actually, earlier hypericin distribution studies using spheroids were performed with culture medium supplemented with fetal bovine serum (FBS) which contains a mixture of low and high density lipoproteins (HDL and LDL) and bovine serum albumin (BSA). In these circumstances hypericin mainly associates with the lipoproteins, and virtually no hypericin-BSA complexes are formed.14
Surprisingly, when investigating the total amount of hypericin that accumulated in NHU or T24 spheroids, we did not observe any difference between the spheroids incubated with hypericin in the presence or absence of HSA (Fig. 3). These data therefore seem to conflict with the penetration of hypericin as seen by fluorescence microscopy in centrally cut spheroid sections. Of interest, in the absence of a carrier like HSA, hypericin forms non-fluorescent aggregates in aqueous solutions.4 It is expected that in these conditions the aggregates formed precipitate on the spheroids, after which hypericin is monomerized in the presence of the phosholipid bilayers and starts to diffuse transcellularly. The non-fluorescent aggregates associated with the rim of the spheroids cannot be visualized by fluorescence microscopy, but become solubilized when the spheroids are extracted with an organic solvent, likely explaining the difference in outcome between the two techniques. In contrast, T24 spheroids accumulated about twice the amount of hypericin that was found in the NHU spheroids (Fig. 3), both in the presence or absence of HSA, and obviously the malignant spheroids also seem to be more permeable for aggregated hypericin as compared to the normal spheroids.
![Relative fluorescence of an ethyl acetate–methanol extract of T24 and NHU spheroids incubated for 2 h with 10 μM hypericin in the presence or absence of HSA (0.3% [m/v]). Experiment was executed in triplicate; data represent mean ± SEM.](/image/article/2011/PP/c0pp00109k/c0pp00109k-f3.gif) | ||
| Fig. 3 Relative fluorescence of an ethyl acetate–methanol extract of T24 and NHU spheroids incubated for 2 h with 10 μM hypericin in the presence or absence of HSA (0.3% [m/v]). Experiment was executed in triplicate; data represent mean ± SEM. | ||
Altogether, the significant difference between the distribution of hypericin in UCC vs.NHU spheroids and the very effective penetration of hypericin in UCC spheroids, as observed in this study when combining hypericin with HSA, clearly suggest a key role for albumin in the specific distribution throughout the whole spheroid of hypericin in non-muscle-invasive bladder tumours. HSA also proves to be superior as a paracellular carrier of the compound as compared to lipoproteins as LDL and HDL that were used in previous in vitro studies using spheroids.
Influence of HSA on the average cellular accumulation of hypericin
As shown in Fig. 1, in the absence of HSA, hypericin remains located in the periphery of the UCC spheroids, implying that the intracellular uptake is swift and high in these conditions. To better understand the influence of HSA on the intracellular uptake of hypericin, the average cellular accumulation of hypericin was analyzed in the absence or presence of albumin using T24 cells. To that end monolayer cells were exposed to hypericin (10 μM) in MEM supplemented or not with HSA (0.3% [m/v]) for 2 h and extracted with a mixture of ethyl acetate and methanol. Subsequently the relative amount of hypericin was assessed by fluorescence measurement. The results demonstrate that the addition of HSA decreased the uptake of hypericin by monolayer T24 cells by about a 5-fold, due to competitive binding with the extracellular HSA present (Fig. 4). An inverse correlation therefore seems to exist between the extent of cellular uptake under 2-D conditions and the penetration of the compound in 3-D multicellular spheroids. It is therefore believed that by binding hypericin, albumin dramatically facilitates the paracellular diffusion of the compound throughout the 3-D tumour system, releasing its load slowly to the cellular environment. In fact, this balance between drug penetration in tumour tissue and intracellular uptake is very important for any given anticancer drug.15![Relative fluorescence of an ethyl acetate–methanol extract of monolayer T24 cells incubated with 10 μM hypericin in the presence (checkered) or absence (white) of HSA (0.3% [m/v]). Experiment was executed in triplicate; data represent mean ± SEM. The difference in relative fluorescence was statistically analyzed by Student's (unpaired) t-test (*** p < 0.001).](/image/article/2011/PP/c0pp00109k/c0pp00109k-f4.gif) | ||
| Fig. 4 Relative fluorescence of an ethyl acetate–methanol extract of monolayer T24 cells incubated with 10 μM hypericin in the presence (checkered) or absence (white) of HSA (0.3% [m/v]). Experiment was executed in triplicate; data represent mean ± SEM. The difference in relative fluorescence was statistically analyzed by Student's (unpaired) t-test (*** p < 0.001). | ||
Influence of HSA on the intraspheroidal distribution of pheophorbide a and mTHPP
Miskovsky and coworkers demonstrated that a binding of hypericin to HSA can be inferred from a change in the absorption spectral profile of the compound.16 In Fig. 5 we show evidence that the absorption spectra of other photosensitizers like pheophorbide a and mTHPP were also noticeably modified by the presence of HSA in specific wavelength regions. These observations prompted us to investigate whether the use of HSA as a carrier for the tumouritropic delivery of compounds could be expanded to other photosensitizers like pheophorbide a and mTHPP.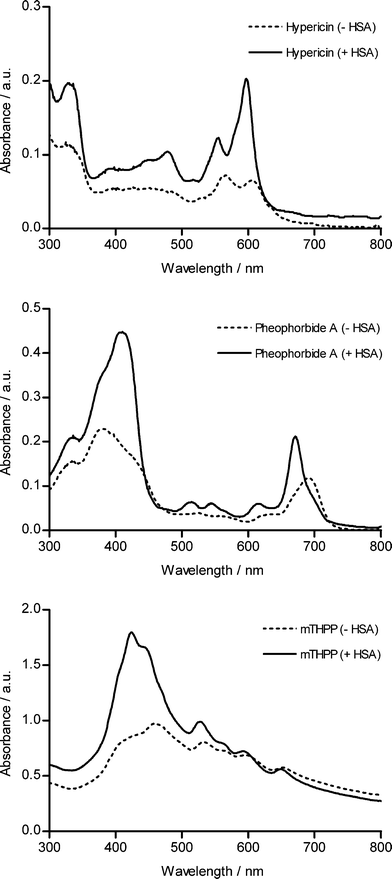 | ||
| Fig. 5 Absorbance spectra of hypericin, pheophorbide A and mTHPP in MEM (without phenol red) at 10, 12.5 and 45 μM respectively in the presence (solid line) or absence (dashed line) of HSA. | ||
As reported in Fig. 6 and Table 2, by supplementing the incubation medium with HSA, spheroids incubated with pheophorbide a showed an increased permeation of the photosensitizer. However, the permeation of pheophorbide a did not only increase in UCC spheroids but, albeit to a lesser extent, also in NHU spheroids. In contrast to pheophorbide a, the mTHPP distribution in spheroids did not differ in NHU and UCC spheroids, independently from the presence or absence of HSA. The data therefore point out that adding HSA to a bladder instillation fluid cannot be regarded as a general strategy to target photosensitizers to non-muscle-invasive bladder cancer lesions. When it comes to affinity for the binding sites of HSA and affinity for cellular membranes, all parameters affecting the outcome of these experiments, hypericin probably features unique physicochemical characteristics which are not present in all compounds, at least not in the photosensitizers tested.
| Pheophorbide a | ||||||||
|---|---|---|---|---|---|---|---|---|
| Relative fluorescence values | ||||||||
| Mean ± SD NHU (+ HSA) | Mean ± SD NHU (− HSA) | Mean ± SD T 24 (+ HSA) | Mean ± SD T 24 (− HSA) | Mean ± SD RT-112 (+ HSA) | Mean ± SD RT-112 (− HSA) | Mean ± SD RT-4 (+ HSA) | Mean ± SD RT-4 (− HSA) | |
| F max | 206 ± 76 | 65 ± 31 | 535 ± 177 | 110 ± 26 | 262 ± 51 | 57 ± 17 | 510 ± 189 | 55 ± 19 |
| F 25 | 144 ± 57 | 37 ± 16 | 268 ± 102 | 60 ± 19 | 205 ± 52 | 37 ± 11 | 303 ± 79 | 29 ± 11 |
| F 50 | 100 ± 37 | 23 ± 13 | 156 ± 61 | 24 ± 10 | 175 ± 52 | 20 ± 8 | 187 ± 70 | 10 ± 6 |
| F min | 39 ± 23 | 10 ± 12 | 53 ± 42 | 4 ± 3 | 110 ± 38 | 3 ± 3 | 83 ± 46 | 3 ± 2 |
| One-way Tukey-Kramer post test | ||||
|---|---|---|---|---|
| F max | F 25 | F 50 | F min | |
| NHU (− HSA) vs.NHU (+ HSA) | *** | *** | *** | ** |
| T 24 (− HSA) vs. T 24 (+ HSA) | *** | *** | *** | * |
| RT-112 (− HSA) vs. RT-112 (+ HSA) | ** | *** | *** | *** |
| RT-4 (− HSA) vs. RT-4 (+ HSA) | *** | *** | *** | *** |
| NHU (+ HSA) vs. T 24 (+ HSA) | *** | *** | * | ns |
| NHU (+ HSA) vs. RT-112 (+ HSA) | ns | ns | *** | *** |
| NHU (+ HSA) vs. RT-4 (+ HSA) | *** | *** | *** | ** |
| mTHPP | ||||||||
|---|---|---|---|---|---|---|---|---|
| Relative fluorescence values | ||||||||
| Mean ± SD NHU (+ HSA) | Mean ± SD NHU (− HSA) | Mean ± SD T 24 (+ HSA) | Mean ± SD T 24 (− HSA) | Mean ± SD RT-112 (+ HSA) | Mean ± SD RT-112 (− HSA) | Mean ± SD RT-4 (+ HSA) | Mean ± SD RT-4 (− HSA) | |
| F max | 47 ± 35 | 60 ± 56 | 96 ± 21 | 45 ± 13 | 73 ± 24 | 22 ± 10 | 47 ± 17 | 51 ± 41 |
| F 25 | 3 ± 3 | 4 ± 5 | 16 ± 4 | 2 ± 2 | 4 ± 3 | 3 ± 2 | 6 ± 6 | 8 ± 5 |
| F 50 | 4 ± 4 | 8 ± 17 | 7 ± 3 | 1 ± 1 | 3 ± 2 | 2 ± 2 | 3 ± 3 | 4 ± 2 |
| F min | 4 ± 4 | 4 ± 9 | 3 ± 2 | 2 ± 2 | 2 ± 2 | 2 ± 1 | 3 ± 3 | 3 ± 2 |
| One-way Tukey-Kramer post test | ||||
|---|---|---|---|---|
| F max | F 25 | F 50 | F min | |
| NHU (− HSA) vs.NHU (+ HSA) | ns | ns | ns | ns |
| T 24 (− HSA) vs. T 24 (+ HSA) | ns | *** | ns | ns |
| RT-112 (− HSA) vs. RT-112 (+ HSA) | ns | ns | ns | ns |
| RT-4 (− HSA) vs. RT-4 (+ HSA) | ns | ns | ns | ns |
| NHU (+ HSA) vs. T 24 (+ HSA) | ns | *** | ns | ns |
| NHU (+ HSA) vs. RT-112 (+ HSA) | ns | ns | ns | ns |
| NHU (+ HSA) vs. RT-4 (+ HSA) | ns | ns | ns | ns |
![Pheophorbide a and mTHPP fluorescence vs. distance from spheroid periphery after curve fitting using nonlinear regression. NHU and UCC (T24, RT-112 and RT-4) spheroids were exposed to 12.5 μM pheophorbide a or 45 μM mTHPP in the presence (dashed lines) or absence of HSA (0.3% [m/v]) (solid lines) for 2 h. The quantification was performed in 5.7 μm concentric layers on 5 μm centrally cut sections of the spheroids. Corrections were made for autofluorescence. Values represent the mean ± SEM (n = 6 for UCC spheroids; n = 6 × 4 [different patients] for NHU spheroids [pheophorbide a] and n = 6 ×3 [mTHPP]).](/image/article/2011/PP/c0pp00109k/c0pp00109k-f6.gif) | ||
| Fig. 6 Pheophorbide a and mTHPP fluorescence vs. distance from spheroid periphery after curve fitting using nonlinear regression. NHU and UCC (T24, RT-112 and RT-4) spheroids were exposed to 12.5 μM pheophorbide a or 45 μM mTHPP in the presence (dashed lines) or absence of HSA (0.3% [m/v]) (solid lines) for 2 h. The quantification was performed in 5.7 μm concentric layers on 5 μm centrally cut sections of the spheroids. Corrections were made for autofluorescence. Values represent the mean ± SEM (n = 6 for UCC spheroids; n = 6 × 4 [different patients] for NHU spheroids [pheophorbide a] and n = 6 ×3 [mTHPP]). | ||
Photodynamic therapy
The possibility to induce selective PDT effects was subsequently tested by exposing UCC and NHU spheroids to four different concentrations of hypericin for 2 h in the presence or absence of HSA. After washing the spheroids with PBS they were irradiated using a low pressure sodium lamp with a fluence of 4.5 or 27 J cm−2 and a fluence rate of 15 mW cm−2. The total output of this lamp is practically monochromatic and has an emission spectrum which consists of two spectral lines, at 586 nm and 589 nm, which matches very well the maximal absorption peak of hypericin.17Fig. 7 shows the results of the MTT antiproliferation assay. In the absence of HSA we noted a decline in tumour cell survival by increasing the hypericin concentration, an observation which was not made in case of NHU spheroids, and overall the tumour spheroids were more susceptible to PDT than normal human urothelial spheroids in these conditions.
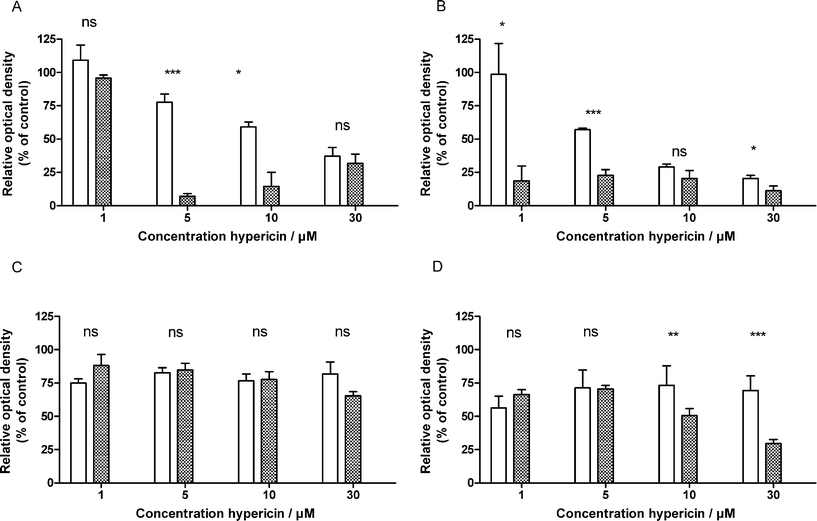 | ||
| Fig. 7 Survival fraction of T24 (A/B) and NHU (C/D) (isolated from 4 different patients) spheroids after hypericin PDT in the presence (checkered) or the absence (white) of HSA. Cells were irradiated with a fluence of 4.5 (A/C) or 27.0 J cm−2 (B/D). Data represent the mean ± SEM. The difference in photocytotoxic effect was statistically analyzed by Student's (unpaired) t-test (ns: not significant; *: p < 0.05; **: p < 0.001; *** p < 0.001). | ||
Results show that due to the presence of HSA, the hypericin-PDT effect on tumour spheroids is increased significantly. This increase can most likely be explained by the difference in intraspheroidal hypericin localization since the HSA supplementation resulted in a more uniformly hypericin localization throughout the T24 spheroids. Additionally, also the selective character of the treatment can dramatically be improved in the presence of HSA. For instance, by using 5 μM hypericin in the presence of HSA, in combination with a low fluence (4.5 J cm−2), a maximal differential photocytotoxic effect can be obtained on the UCC and NHU spheroids. In general in case of the NHU spheroids the effect of HSA is negligible, except when using high concentrations of hypericin combined with a high fluence.
Experimental
Chemicals
Hypericin was synthesized as reported previously.18 Human serum albumin (HSA) (solution for perfusion 50 g L−1) was purchased from Baxter (Vienna, Austria). meso-Tetra(m-hydroxyphenyl)porphine (mTHPP) and pheophorbide a were obtained from Frontier Scientific Europe Ltd (Lancashire, UK). All stock solutions of the photosensitizers were made in dimethylsulfoxide (DMSO) and kept at −20 °C in the dark until needed. All manipulations with hypericin, pheophorbide a and mTHPP were performed under strictly subdued light conditions (<1 μW cm−2).Tumour cell lines
RT-4 and T24 (urothelial cell carcinoma [UCC], urinary bladder, human) were obtained from American Type Culture Collection (Rockville, MD, USA). RT-112 (UCC, urinary bladder, human) was obtained from the German Collection of Micro-organisms and Cell Cultures (DSMZ, Braunschweig, Germany). All cell lines were cultured as monolayer cultures in minimum essential medium (MEM) with Earle's salts containing 2 mM L-glutamine under 5% CO2 at 37 °C. The medium was supplemented with 10% (v/v) foetal bovine serum (FBS), 1% (v/v) non-essential amino acids, 1% (v/v) antibiotic/antimycotic solution and tylosine (60 μg ml−1). All culture medium compounds were purchased from Invitrogen (Merelbeke, Belgium).Normal human urothelial cells (NHU)
Normal human urothelial cells were obtained from the ureter of multiple patients undergoing nephrectomy, following standard departmental procedures. The histology confirmed benign urothelium in each case. The specimens were dissected and NHU cells were isolated as reported previously.19Multicellular spheroid culture
Both UCC and NHU spheroids were prepared using the liquid overlay technique.20 They were initiated by inoculating 5 × 103cells in 200 μl culture medium supplemented with 1% sodium pyruvate (Invitrogen, Merelbeke, Belgium) on 96-well tissue culture plates (BD Labware, Franklin Lakes, NJ, USA) previously coated with 1.5% agarose (Sigma-Aldrich) in MEM. Medium was replaced once during the growth of the spheroids. All experiments were performed on spheroids with an average diameter of 300 μm.Absorbance spectra in MEM
Absorbance spectra of hypericin (10 μM), pheophorbide a (12.5 μM) and mTHPP (45 μM) dissolved in MEM (without phenol red) supplemented or not with 0.3% (m/v) HSA were acquired between 300 nm and 800 nm using a spectrophotometer (Ultrospec 2000, Pharmacia Biotech, Cambridge, UK). Solutions were kept in the dark for 30 min at room temperature before measuring.Influence of HSA on the intraspheroidal photosensitizer distribution
UCC and NHU spheroids (from at least 3 different patients) were incubated with hypericin (10 μM), pheophorbide a (12.5 μM) or mTHPP (45 μM) in the presence or absence of human albumin (0.3% [m/v]) for 2 h, after which they were washed and transferred into medium (TissueTek embedding medium, Miles, Elkhart, Indiana, USA) and immersed in liquid nitrogen. Centrally cut cryostat cross sections (5 μm) were examined by fluorescence microscopy (Axioskop 2 plus fluorescence microscope, Carl Zeiss, Göttingen, Germany) using a 535/25 nm band-pass excitation filter (hypericin) or a 417/20 nm band-pass excitation filter (pheophorbide a and mTHPP) and 590 nm long-pass emission filter. Fluorescence images were obtained using a light-sensitive charge-couples device digital camera (AxioCam HP, Carl Zeiss). A KS imaging software system (Carl Zeiss, Vision, Hallbergmoos, Germany) was used to measure the average fluorescence in concentric layers of 5.7 μm from the rim to the centre. Fluorescence intensities were determined as the mean of 6 spheroids. Corrections were made for autofluorescence.Influence of HSA on the average accumulation of hypericin
T24 cells (9.0 × 105 per well) were seeded onto 6-well tissue culture plates (BD Labware, Franklin Lakes, NJ, USA) After 24 h the monolayer T24 cells were exposed to 10 μM hypericin in MEM supplemented or not with HSA (0.3% [m/v]) for 2 h. After the incubation the cells were washed twice with PBS and subsequently extracted with a mixture of ethyl acetate and methanol (50/50 [v/v]). The extract was dried and dissolved in DMSO. The fluorescence was analyzed using a microplate fluorescence reader (FL600, Biotek, Winooski, VT, USA). The excitation and emission filters were 590/20 and 645/40 nm (peak/bandwidth at half-maximum transmission). For the determination of the effect of HSA on the cellular accumulation of hypericin in 3D conditions, spheroids were handpicked and transferred to a falcon tube (60 spheroids) after which they were exposed to the same conditions as mentioned for the 2D experiments. Following the 2 h incubation the spheroids were washed twice with PBS or PBS supplemented with EDTA in case of T24 and NHU spheroids, respectively. Before performing the extraction as described above, the spheroids were trypsinized.Photodynamic therapy
2-day old NHU and 5-day old T24 spheroids were incubated (10 spheroids per condition) with different concentrations of hypericin (1, 5, 10 and 30 μM) in the presence or absence of HSA (0.3% [m/v]). After 2 h of incubation, the spheroids were washed with PBS and subsequently irradiated for 5 or 30 min with a low pressure sodium lamp (SOX-E36W; Philips, Eindhoven, the Netherlands) using a fluence rate of 15 mW cm−2 as measured with an IL 1400 radiometer (International light, Newburyport, MA, USA). After the photo-activation period PBS was replaced by fresh supplemented MEM or KSFM for T24 and NHU spheroids, respectively. An MTT (Sigma-Aldrich, Bornem, Belgium) antiproliferation assay was used 24 h later to determine the survival fraction. Before solubilizing the formazan in isopropanol, the T24 spheroids were left in trypsin for 30 min. The control group consisted of spheroids that were irradiated in the absence of hypericin.Statistical analysis
Student's t-test using Graph path® software was used to investigate the significance of difference between 2 groups and 1-way ANOVA analysis with the Tukey–Kramer post test using GraphPad InStat was performed to determine the significance of differences among the means of more than 2 groups. Significance was considered at p < 0.05.Conclusion
Hypericin has proven to be an excellent tool for the clinical detection of non-muscle-invasive bladder cancer lesions. A high sensitivity and specificity combined with a high photostability2,3,21 have been reported for fluorescence-guided cystoscopy with hypericin. Since HSA as a carrier is typically present in the bladder instillation fluid to solubilize hypericin, the goal of this work was to investigate the role of albumin in the tumour-selective localization of hypericin. The results clearly show that, at least in the in vitro conditions used, HSA is a key mediator in this process.This study provides evidence of a balance between cellular uptake and paracellular transport of hypericin that dramatically can be altered by binding the compound to HSA. Upon binding, aggregated hypericin complexes, present in aqueous solutions become monomerized. These large complexes impair penetration resulting in a reduced fluorescence as observed in spheroids. During this passage hypericin is slowly released from its complex and finally taken up intracellularly (Fig. 8). A different histo-architecture, due to a higher expression level of E-cadherin in NHU spheroids as compared to UCC spheroids, results in a lesser available paracellular route,12 a situation which results in a differential accumulation of hypericin in UCC and NHU spheroids. Since these spheroids reproduce well the histology of normal and malignant urothelium, our in vitro results also shed some light on the underlying mechanism of the selective uptake of hypericin in non-muscle-invasive urothelial cell carcinomas present in the bladder of patients.
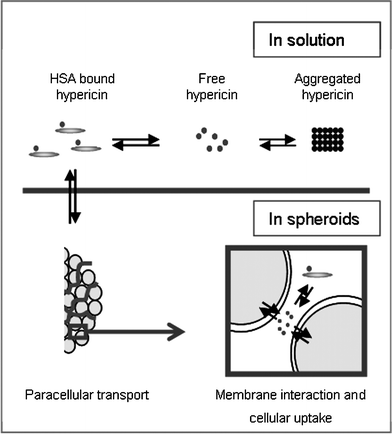 | ||
| Fig. 8 Schematic illustration of the paracellular transport of hypericin in the presence of HSA throughout a spheroid. Free hypericin can associate with HSA or form high molecular non-fluorescent aggregates in aqueous solutions. When hypericin interacts with HSA, paracellular transport is favored over cellular uptake, resulting in an equal distribution of hypericin throughout the whole UCC spheroid. | ||
Interestingly, we also observed that the presence of HSA did not convey tumouritropic characteristics to other photosensitizers like pheophorbide a and mTHPP, implying that both the particular characteristics of the photosensitizer and HSA contribute to the final selective accumulation of the compound in tumoural tissue.
As a result of the more uniform distribution of hypericin in the presence of HSA, we further found that an optimal differentiation between PDT effects on spheroidal tumour and NHU cells can be obtained using a low light fluence. These findings will be important in determining the ideal settings for the clinical use of hypericin as PDT agent for the treatment of non-muscle-invasive bladder cancer lesions.
Acknowledgements
This work was supported by grants awarded by “Fonds voor Wetenschappelijk Onderzoek-Vlaanderen” (F.W.O.-Vlaanderen) and by a grant (Onderzoekstoelage) awarded by the K.U. Leuven.References
- G. Lavie, Y. Mazur, D. Lavie and D. Meruelo, The chemical and biological properties of hypericin–a compound with a broad spectrum of biological activities, Med. Res. Rev., 1995, 15, 111–119 CAS.
- M. A. D'Hallewin, A. R. Kamuhabwa, T. Roskams, P. A. De Witte and L. Baert, Hypericin-based fluorescence diagnosis of bladder carcinoma, BJU Int., 2002, 89, 760–763 CrossRef.
- H. G. Sim, W. K. Lau, M. Olivo, P. H. Tan and C. W. Cheng, Is photodynamic diagnosis using hypericin better than white-light cystoscopy for detecting superficial bladder carcinoma?, BJU Int., 2005, 95, 1215–1218 CrossRef.
- G. Lavie, Y. Mazur, D. Lavie, A. M. Prince, D. Pascual, L. Liebes, B. Levin and D. Meruelo, Hypericin as an inactivator of infectious viruses in blood components, Transfusion, 1995, 35, 392–400 CrossRef CAS.
- J. Hritz, S. Kascakova, J. Ulicny and P. Miskovsky, Influence of structure of human, rat, and bovine serum albumins on binding properties of photoactive drug hypericin, Biopolymers, 2002, 67, 251–254 CrossRef.
- P. Miskovsky, J. Hritz, S. Sanchez-Cortes, G. Fabriciova, J. Ulicny and L. Chinsky, Interaction of hypericin with serum albumins: surface-enhanced Raman spectroscopy, resonance Raman spectroscopy and molecular modeling study, Photochem. Photobiol., 2001, 74, 172–183 CrossRef CAS.
- W. T. Li, H. W. Tsao, Y. Y. Chen, S. W. Cheng and Y. C. Hsu, A study on the photodynamic properties of chlorophyll derivatives using human hepatocellular carcinoma cells, Photochem. Photobiol. Sci., 2007, 6, 1341–1348 RSC.
- L. Ma, J. Moan and K. Berg, Evaluation of a new photosensitizer, meso-tetra-hydroxyphenyl-chlorin, for use in photodynamic therapy: a comparison of its photobiological properties with those of two other photosensitizers, Int. J. Cancer, 1994, 57, 883–888 CrossRef CAS.
- P. Jardon and R. Gautron, Propriétés photophysiques de l'hypercine en solution et en dispersion micellaire, J. Chim. Phys., 1989, 86, 2173–2190 CAS.
- A. P. Darmanyan, L. Burel, D. Eloy and P. Jardon, Singlet oxygen production by hypericin in various solvents, J. Chim. Phys., 1994, 91, 1174–1785.
- T. J. Dougherty, C. J. Gomer, B. W. Henderson, G. Jori, D. Kessel, M. Korbelik, J. Moan and Q. Peng, Photodynamic therapy, J. Natl. Cancer Inst., 1998, 90, 889–905 CrossRef CAS.
- A. Huygens, A. R. Kamuhabwa, T. Roskams, B. Van Cleynenbreugel, H. Van Poppel and P. A. de Witte, Permeation of hypericin in spheroids composed of different grade transitional cell carcinoma cell lines and normal human urothelial cells, J. Urol., 2005, 174, 69–72 CrossRef CAS.
- A. Huygens, I. Crnolatac, J. Develter, B. Van Cleynenbreugel, T. Van Der Kwast and P. A. de Witte, Differential accumulation of hypericin in spheroids composed of T-24 transitional cell carcinoma cells expressing different levels of E-cadherin, J. Urol., 2008, 179, 2014–2019 Search PubMed.
- E. M. Delaey, R. Obermueller, I. Zupko, D. De Vos, H. Falk and P. A. de Witte, In vitro study of the photocytotoxicity of some hypericin analogs on different cell lines, Photochem. Photobiol., 2001, 74, 164–171 CrossRef CAS.
- N. S. Bryce, J. Z. Zhang, R. M. Whan, N. Yamamoto and T. W. Hambley, Accumulation of an anthraquinone and its platinum complexes in cancer cell spheroids: the effect of charge on drug distribution in solid tumour models, Chem. Commun., 2009, 2673–2675 RSC.
- P. Miškovský, D. Jancura, S. Sánchez-Cortés, E. Koišová and L. Chinsky, Antiretrovirally Active Drug Hypericin Binds the IIA Subdomain of Human Serum Albumin: Resonance Raman and Surface-Enhanced Raman Spectroscopy Study, J. Am. Chem. Soc., 1998, 120, 6374–6379 CrossRef CAS.
- A. Boiy, R. Roelandts, J. Van Den Oord and P. A. de Witte, Photosensitizing activity of hypericin and hypericin acetate after topical application on normal mouse skin, Br. J. Dermatol., 2007, 158, 360–369 CrossRef.
- H. Falk, J. Meyer and M. Oberreiter, A convenient semisynthetic route to hypericin, Monatsh. Chem., 1993, 124, 339–341 CAS.
- J. Southgate, J. R. W. Masters and L. K. Trejdosiewicz, in Culture of specialized cells: Culture of epithelial cells, ed. I. Freshney and M. G. Freshney, Wiley-Liss, New York, 2nd edn, 2002, ch. 12, pp. 381–399 Search PubMed.
- J. M. Yuhas, A. P. Li, A. O. Martinez and A. J. Ladman, A simplified method for production and growth of multicellular tumor spheroids, Cancer Res., 1977, 37, 3639–3643 CAS.
- M. A. D'Hallewin, P. A. De Witte, E. Waelkens, W. Merlevede and L. Baert, Fluorescence detection of flat bladder carcinoma in situ after intravesical instillation of hypericin, J. Urol., 2000, 164, 349–351 CrossRef CAS.
| This journal is © The Royal Society of Chemistry and Owner Societies 2011 |
