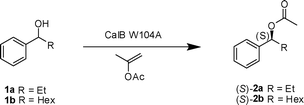
Mutated variant of Candida antarcticalipase B in (S)-selective dynamic kinetic resolution of secondary alcohols†
Karin
Engström
a,
Michaela
Vallin
b,
Per-Olof
Syrén
b,
Karl
Hult
b and
Jan-E.
Bäckvall
*a
aDepartment of Organic Chemistry, Arrhenius Laboratory, Stockholm University, SE-106 91, Stockholm, Sweden. E-mail: jeb@organ.su.se; Fax: (+46) 8-154908; Tel: (+46) 8-6747178
bDepartment of Biochemistry, School of Biotechnology, Royal Institute of Technology (KTH), AlbaNova University Center, SE-106 91 Stockholm, Sweden
First published on 17th November 2010
Abstract
An (S)-selective dynamic kinetic resolution of secondary alcohols, employing a mutated variant of Candida antarcticalipase B (CalB) gave products in 84–88% yield and in 90–97% ee.
There is an increasing demand of enantiomerically pure compounds in organic synthesis, and chiral secondary alcohols are important building blocks in the preparation of natural products, fine chemicals and pharmaceuticals. Enzymatic resolution with lipases has become one of the most popular methods for the preparation of enantiomerically pure alcohols.1Lipases are widely used in organic synthesis due to their high enantioselectivity towards a wide range of substrates and their ability to work efficiently in organic solvent.2 Lipases require no cofactors; they acylate alcohols in organic solvent, and are especially enantioselective towards secondary alcohols. The enantioselectivity of lipases follows Kazlauskas rule, which predicts (R)-selectivity for most substrates.3
Candida antarctica lipase B (CalB) is a thermostable lipase that shows a high natural enantioselectivity towards secondary alcohols and chiral primary amines and has been used as an enantioselective biocatalyst in a large number of transformations.4 The high enantioselectivity can be explained based on the different binding modes for the enantiomers in the active site.5,6 The fast-reacting enantiomer positions its medium-sized group in the so called stereoselectivity pocket and its large group towards the active-site entrance. The size of the stereoselectivity pocket of CalB is limited by the sidechains of three residues; Thr42, Ser47 and Trp104 and the largest alkyl group that can smoothly fit into this pocket is ethyl. The slow-reacting enantiomer needs to put its large group in the stereospecificity pocket to be in the correct position for catalytic activity. It does not fit however, and this steric limitation is what makes CalB highly enantioselective towards secondary alcohols when one of the groups are ethyl or smaller.
A variant of CalB, with a mutation in one of the residues limiting the size of the stereoselectivity pocket, Trp104 was published in 2005.7,8 This variant of CalB, where Trp104 was changed into an alanine (CalB W104A), has an enlarged stereospecificity pocket. As predicted by modelling,5 this variant can accept larger substrates than wild type CalB. Furthermore, it shows (S)-selectivity towards 1-phenylethanol and other secondary alcohols.
A recent study of CalB W104A investigated its use in kinetic resolution of 1-phenylalkanols.9 This study showed that its stereoselectivity pocket can accommodate 1-phenylalkanols with an alkyl chain of up to eight carbon atoms and the (S)-enantiomer was preferred. The enantioselectivity was unsatisfactory for 1-phenylethanol (E = 3) but acceptable for the longer phenylalkanols. The highest E values were obtained for substrates with the long alkyl chains.
Based on these results, we wanted to explore the possibility to apply the (S)-selective CalB W104A in a dynamic kinetic resolution (DKR). (S)-selective DKR's of alcohols are rare and there are only two examples known in the literature.10,11 These examples employ the serine protease Subtilisin Carlsberg as the (S)-selective enzyme. The latter enzyme is quite fragile and can not be used at elevated temperatures. It was therefore highly desirable to develop an (S)-selective DKR with a less sensitive enzyme.
We first applied CalB W104A in a kinetic resolution of 1-phenylpropanol (1a) and 1-phenylheptanol (1b), to find the proper conditions for the DKR. Isopropenyl acetate was employed as acyl donor. The kinetic resolution (KR) with CalB W104A had previously been reported in cyclohexane. This is not a suitable solvent for DKR as it does not dissolve catalyst 4 used for the racemization of the alcohol. A more suitable solvent for DKR is toluene, and therefore this solvent was used for the KR of 1a and 1b (Table 1). CalB W104A had a significantly higher E value towards 1-phenylpropanol (1a) in toluene at 60 °C (E = 82, Table 1, entry 1) compared to that previously reported in cyclohexane.12 The KR of 1b was run at different temperatures and the enantioselectivity (E) was determined (Table 1, entries 2–5). The enantioselectivity was higher in toluene (E = 176) than in cyclohexane13 at 60 °C. The enantioselectivity increased with temperature in toluene. This temperature effect was previously reported also for 1-phenylethanol with CalB W104A in various solvents,7 but is otherwise quite unusual, and opposite to the temperature effect observed for 1b in cyclohexane.9
|
|
|||||||
|---|---|---|---|---|---|---|---|
| Entry | Substrate | T/°C | Time/h | eep (%)b | ees (%)b | Conv.(%)c | E |
| a Conditions: 1 (0.5 mmol), isopropenyl acetate (1.5 equiv.), CalB W104A (20 mg), toluene (1.0 mL) b Determined by chiral GC. c Determined by 1H NMR. d 1 (0.25 mmol), CalB W104A (5 mg), toluene (0.5 mL). | |||||||
| 1d | 1a | 60 | 24 | 96.4 | 44.2 | 32 | 85 (±10) |
| 2 | 1b | 60 | 6 | 98.4 | 36.0 | 33 | 177 (±25) |
| 3 | 1b | 40 | 24 | 98.0 | 31.5 | 23 | 134 (±15) |
| 4 | 1b | 30 | 72 | 97.7 | 42.6 | 29 | 127 (±15) |
| 5 | 1b | r.t. | 72 | 98.0 | 14.8 | 13 | 114 (±15) |
As both the activity and the enantioselectivity were highest at 60 °C, this was the temperature that was chosen for the initial DKR. The first DKR was performed with 1-phenylpropanol (1a) as substrate. The reaction showed good enantioselectivity and after 36 h full conversion was reached (Table 2, entry 1). The product ester (2a) had an enantiomeric excess of 96.5%, and also contained 10% of the corresponding ketone 3a.
|
|
||||||
|---|---|---|---|---|---|---|
| Entry | Substrate | T/°C | Time/h | eep (%)b | Conv. (%)c | % 3c |
| a Conditions: 1 (0.5 mmol), isopropenyl acetate (1.5 equiv.), Na2CO3 (1 equiv.), Ru-catalyst 4 (0.05 equiv.), tBuOK (0.05 equiv.), CalB W104A (20 mg), toluene (1.0 mL). b Determined by chiral GC. c Determined by 1H NMR. d Isolated yields in parenthesis. e CalB W104A (30 mg). f 350 mg molecular sieves (4 Å) added. | ||||||
| 1 | 1a | 60 | 36 | 96.5 | 99 (85)d | 10 |
| 2 | 1b | 60 | 26 | 94 | 93 | 38 |
| 3 | 1b | 40 | 88 | 95 | 97 | 24 |
| 4e | 1b | 30 | 6 days | 97 | 99 (84)d | 1 |
| 5e,f | 1b | 60 | 24 | 96.5 | 97(84)d | 7 |
| 6e,f | 1c | 60 | 24 | 90 | 99 (88)d | 8 |
DKR of 1-phenylheptanol (1b) was also performed at 60 °C. However, this DKR yielded as much as 38% of the corresponding ketone, 1-phenylheptanone (3b) (Table 2, entry 2). To avoid ketone formation in the DKR of 1-phenylheptanol (1b), the temperature was decreased. At 40 °C, the amount of ketone was reduced to 24%, and at 30 °C, only 1% was formed (Table 2, entries 3–4) However, longer reaction times were required to reach full conversion at decreased temperatures. At 30 °C, 6 days was required to reach full conversion (99%). Under these conditions, (S)-2b was obtained in 97% ee.
Formation of ketone has previously been observed during racemization by catalyst 4 for bicyclic diols at elevated temperatures.14 This ketone formation is believed to be a result of substitution of the intermediate substrate ketone on the metal center by acetone, a byproduct from the acyl donor. Cross-over experiments have shown that this kind of ketone–ketone exchange takes place to some extent if the racemization is left after completion at elevated temperatures.15
In an attempt to reduce the ketone-formation by reducing the amounts of by-product acetone present in the solution from the acyl donor, a DKR was run at 60 °C in the presence of 4 Å molecular sieves. This reduced the amount of ketone to 7% (Table 2, entry 5). These conditions were then also employed to the DKR of 1-phenylpentanol (1c), which gave the product 2c in 88% yield and 90% ee (Table 2, entry 6).
To conclude, an efficient (S)-selective DKR of 1-phenylalkanols has been developed. 1-Phenylpropanol was efficiently acylated at 60 °C to give the product in 96.5% enantiomeric excess. DKR of 1-phenylheptanol required lower temperatures (and longer reaction times) or addition of molecular sieves to suppress ketone formation. The product was obtained in 97% ee in high yield. This is the first (S)- selective DKR of a sec-alcohol performed with an enzyme that can be used at elevated temperatures.
Acknowledgements
Financial support from the Swedish Research Council, the Swedish Foundation for Strategic Research and the K&A Wallenberg Foundation is gratefully acknowledged.Notes and references
- G. Carrea, S. Riva, Organic synthesis with enzymes in non-aqueous media, Wiley-Vch Verlag GmbH & Co., 2008, Weinheim Search PubMed.
- T. Ema, Curr. Org. Chem., 2004, 8, 1009–1025 CrossRef CAS.
- When the medium-sized group has a lower priority than the large group, the compound will have (R)-stereochemistry according to the Cahn–Ingold–Prelog rules: R. J. Kazlauskas, A. N. E. Weissfloch, A. T. Rappaport and L. A. Cuccia, J. Org. Chem., 1991, 56, 2656–2665 Search PubMed.
- E. M. Anderson, K. M. Larsson and O. Kirk, Biocatal. Biotransform., 1998, 16, 181–204 CrossRef CAS.
- J. Uppenberg, N. Ohmer, M. Norin, K. Hult, G. J. Kleywegt, S. Patkar, V. Waagen, T. Anthomen and T. A. Jones, Biochemistry, 1995, 34, 16838–16851 CrossRef CAS.
- D. Rotticci, F. Haeffner, C. Orrenius, T. Norin and K. Hult, J. Mol. Catal. B: Enzym., 1998, 5, 267 CrossRef CAS.
- A. O. Magnusson, M. Takwa, A. Hamberg and K. Hult, Angew. Chem., Int. Ed., 2005, 44, 4582–4585 CrossRef CAS.
- A. O. Magnusson, J. C. Rotticci-Mulder, A. Santagostino and K. Hult, ChemBioChem, 2005, 6, 1051–1056 CrossRef CAS.
- M. Vallin, P.-O. Syrén and K. Hult, ChemBioChem, 2010, 11, 411–416 CrossRef CAS.
- M.-J. Kim, Y. Chung, Y. Choi, H. Lee, D. Kim and J. Park, J. Am. Chem. Soc., 2003, 125, 11494–11495 CrossRef CAS.
- L. Borén, B. Martín-Matute, Y. Xu, A. Córdova and J.-E. Bäckvall, Chem.–Eur. J., 2006, 12, 225–232 CrossRef CAS.
- The E value was previously reported as 28 at 56 °C in cyclohexane9.
- The E value in cyclohexane at 60 °C under the same conditions was determined to 76.
- P. Krumlinde, K. Bogár and J.-E. Bäckvall, Chem. Eur. J., 2010, 16, 4031–4036 CAS.
- B. Martín-Matute, M. Edin, K. Bogar, F. B. Kaynak and J.-E. Bäckvall, J. Am. Chem. Soc., 2005, 127, 8817–8825 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Supplementary data. See DOI: 10.1039/c0ob00748j |
| This journal is © The Royal Society of Chemistry 2011 |