Effective post treatment for preparing highly conductive carbon nanotube/reduced graphite oxide hybrid films†
Ranran
Wang
,
Jing
Sun
*,
Lian
Gao
*,
Chaohe
Xu
,
Jing
Zhang
and
Yangqiao
Liu
The State Key Lab of High Performance Ceramics and Superfine Microstructure, Shanghai Institute of Ceramics, Chinese Academy of Sciences, 1295 Ding Xi Road, Shanghai, 200050, China. E-mail: jingsun@mail.sic.ac.cn; liangao@mail.sic.ac.cn; Fax: +86 21 52413122; Tel: +86 21 52412718
First published on 3rd December 2010
Abstract
SWCNT-reduced graphite oxide hybrid films were prepared by a filtration method. An efficient post-treatment procedure was designed to reduce GO and remove dispersants simultaneously. The sheet resistance decreased significantly after treatment, by a factor of 4–13 times. Films with excellent performance (95.6%, 655 Ω per square) were obtained and had great potential applications.
Transparent conductive films (TCFs) have become more and more important in recent years due to the rapid development of microelectronic techniques. TCFs made from indium tin oxide (ITO) monopolized the market in the last several decades because of ITO’s excellent conductivity and transparency. However, brittleness hinders their application in flexible devices, and replaceable materials need to be developed urgently. Graphene, a single layer of graphite, has recently gained vast interest due to its extraordinary properties.1 Owing to its high transparency, low resistance and high flexibility, graphene has been considered as an alternative to ITO.2 Although a lot of methods have been developed to prepare graphene, the chemical reduction is considered as the most practical and cost effective route, holding the potential of large scale preparation. However, graphene prepared from this method (referred to as reduced graphite oxide here) has high resistance and they tend to restack due to the strong van de Waals attraction. Single-walled carbon nanotubes (SWCNT) are another candidate for replacing ITO. They are more conductive compared with reduced graphite oxide (RGO), but their cost is much higher, and it is very difficult to exfoliate them into individual tubes. Combining RGO with SWCNTs can take advantage of both of their merits. The exfoliation of SWCNTs can be improved by GO, which is amphiphilic and can be used as a dispersing agent.3 At the same time, SWCNTs can hinder the restacking of RGO. Surfactants are often used to enhance the exfoliation of carbon nanotubes4 and graphenes.5 However, they are nonconductive and need to be removed. Biomolecules such as DNA6 and RNA7 are preferable dispersants since they are very efficient for exfoliating SWCNTs and they can be degraded and removed easily. In this work, SWCNTs and GO were dispersed simultaneously in water by RNA. The obtained SWCNTs/GO solution was used to prepare films. A very efficient post-treatment procedure was designed to reduce GO and to remove the residual dispersants simultaneously.
The P3 SWCNTs (from Carbon Solutions Inc.) synthesized by arc-discharge were used as received. GO was prepared by the modified Hummers' method. RNA solution with the concentration of 0.2 mg mL−1 was used to exfoliate SWCNTs and GO. The weight ratio between SWCNTs and GO was 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The detailed experimental procedure is shown in the ESI.† A transmission electron microscope (TEM) image of SWCNTs/GO solution dispersed by RNA is shown in Fig. 1a. SWCNTs were dispersed into thin bundles and GO was exfoliated into thin layers. They mixed together homogeneously. By comparison, SWCNT aggregated into thick bundles when GO was not added, testifying to the promotion of the exfoliation of SWCNTs by GO.
1. The detailed experimental procedure is shown in the ESI.† A transmission electron microscope (TEM) image of SWCNTs/GO solution dispersed by RNA is shown in Fig. 1a. SWCNTs were dispersed into thin bundles and GO was exfoliated into thin layers. They mixed together homogeneously. By comparison, SWCNT aggregated into thick bundles when GO was not added, testifying to the promotion of the exfoliation of SWCNTs by GO.
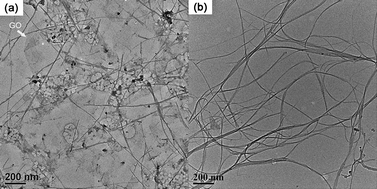 | ||
| Fig. 1 TEM images of (a) SWCNT/GO and (b) SWCNT solution dispersed by RNA. | ||
Films were prepared with the above SWCNT/GO solution by the filtration method. Then the films were post-treated successively by sodium borohydride, sodium hydroxide, and nitric acid. The sheet resistance decreased apparently after each post-treatment step, as seen from Fig. 2. The function of each step will be discussed in detail later. SWCNT/RGO hybrid film with high transmittance (95.6%) and low sheet resistance (655 Ω per square) was obtained after all of the treatment. It was more conductive than pure SWCNT film, which was subjected to the same post-treatment. It was also better than the results reported by Tung (636 Ω per square, 90% transmittance),8 which may be due to the better dispersion state in this work.
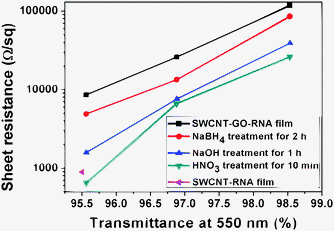 | ||
| Fig. 2 Sheet resistance versus transmittance at 550 nm before and after treatment. | ||
After treatment with sodium borohydride for 2 h, the sheet resistance decreased a lot, by a factor of 1.5–2 times. This was attributed to two reasons. First, GO was reduced by sodium borohydride, which decreased the sheet resistance greatly. The intensity ratio of the D and G bands is a measure of sp2/sp3carbon.9 After treatment, the D peak turned higher, but narrower, while the G peak turned wider. According to the integration of the area under the curve, the intensity ratio of D and G bands decreased from 0.54 to 0.33 after the treatment with sodium borohydride (Fig. S1†). Second, some RNA molecules were removed from the films. X-ray photoelectron spectroscopy (XPS) was used to characterize the content of carbon, oxygen and other elements in the film (Fig. 3). It disclosed that the content of C–O/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups decreased after treatment with NaBH4, testifying to the removal of RNA and the reduction of GO. Phosphorus from the phosphodiester bond represented the existence of RNA. It disappeared after treatment, as seen from Table S1 in the ESI,† again proving the removal of RNA. In order to confirm the above explanation further, SWCNT films without GO were prepared and treated with NaBH4 for 2 h. SWCNTs were not reduced by sodium borohydride since there was no obvious decrease of the ID/IG ratio seen from Raman spectra (Fig. S2†). XPS showed that the content of C–O and C
O groups decreased after treatment with NaBH4, testifying to the removal of RNA and the reduction of GO. Phosphorus from the phosphodiester bond represented the existence of RNA. It disappeared after treatment, as seen from Table S1 in the ESI,† again proving the removal of RNA. In order to confirm the above explanation further, SWCNT films without GO were prepared and treated with NaBH4 for 2 h. SWCNTs were not reduced by sodium borohydride since there was no obvious decrease of the ID/IG ratio seen from Raman spectra (Fig. S2†). XPS showed that the content of C–O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups decreased and the C–C peak shifted down to lower binding energy after treatment (Fig. S3†). The C1s peak would shift toward higher binding energy if CNTs accepted electrons from the surroundings, and vice versa.10RNA could donate electrons to carbon nanotubes and cause the up-shift of the C1s peak according to our previous work.11 The down-shift of the C1s peak of SWCNT-RNA films indicated the removal of RNA after treatment with NaBH4.11 The above analysis disclosed that NaBH4 was effective for removing the dispersant RNA. According to our knowledge, this was the first time to report this phenomenon. There was no surprise that sodium borohydride could reduce GO,12,13 but how can they remove RNA molecules from the films? This may be due to two aspects. First, the sodium borohydride aqueous solution was alkaline, and RNA could be degraded in alkaline condition. Second, hydrogen generated in sodium borohydride solution bubbled and lashed the film, which redounded to the removal of RNA molecules.
O groups decreased and the C–C peak shifted down to lower binding energy after treatment (Fig. S3†). The C1s peak would shift toward higher binding energy if CNTs accepted electrons from the surroundings, and vice versa.10RNA could donate electrons to carbon nanotubes and cause the up-shift of the C1s peak according to our previous work.11 The down-shift of the C1s peak of SWCNT-RNA films indicated the removal of RNA after treatment with NaBH4.11 The above analysis disclosed that NaBH4 was effective for removing the dispersant RNA. According to our knowledge, this was the first time to report this phenomenon. There was no surprise that sodium borohydride could reduce GO,12,13 but how can they remove RNA molecules from the films? This may be due to two aspects. First, the sodium borohydride aqueous solution was alkaline, and RNA could be degraded in alkaline condition. Second, hydrogen generated in sodium borohydride solution bubbled and lashed the film, which redounded to the removal of RNA molecules.
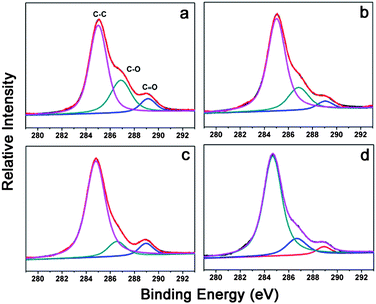 | ||
| Fig. 3 XPS of SWCNT/GO hybrid films before and after treatment: (a) before treatment; (b) after treatment with NaBH4; (c) after subsequent treatment with NaOH; (d) after subsequent treatment with HNO3. | ||
After treatment with sodium borohydride, sodium hydroxide was used to treat the film further. The sheet resistance decreased greatly after treatment, by a factor of 2–3 times, which can be attributed to the further reduction of GO and further removal of RNA molecules. Raman spectra (Fig. S1†) showed that the intensity ratio of D and G band decreased further after treatment with sodium hydroxide, from 0.33 to 0.16. This confirmed the further reduction of GO in sodium hydroxide solution, which was consistent with the report from Fan et al.14 The underlying mechanism of the deoxygenation of GO under alkaline conditions remained unclear. It appeared to be the reverse of the oxidation reaction of graphite in strong acid according to the work of Fan et al.14XPS showed an apparent decrease of C–O peaks after treatment with sodium hydroxide (Fig. 3b and c), testifying to the reduction of GO and the removal of RNA molecules. Besides, a 0.22 eV down-shift occurred after treatment with sodium hydroxide, as seen from Table S2 in the ESI.† This again proved the removal of RNA molecules by treating with sodium hydroxide. The removal of RNA was attributed to the hydrolysis of them in NaOH solution, which was discussed in our previous work.15
Both NaBH4 treatment and the subsequent NaOH treatment had the function of reducing GO and removing RNA molecules. Can we adopt only one of them, and omit the other one? In order to answer this question, fresh SWCNT/GO hybrid films were treated respectively with NaBH4 and NaOH solution. The results showed that the sheet resistance decreased by a factor of 2 times after treatment with NaBH4 and that decreased by 2–3 times after NaOH treatment. However, the sheet resistance decreased by 4–5 times when combined these two post-treatment steps. When we changed the order of these two steps, which meant NaOH treatment was conducted first, the sheet resistance decreased less compared to the previous order. Hydrogen generated in NaBH4 solution lashed the film and weakened the interaction between RNA and carbon nanotubes. Therefore, conducting NaBH4 treatment foremost would redound to removing RNA molecules sufficiently in the following post-treatment steps. According to the above analysis, we found that NaBH4 treatment combining subsequent NaOH treatment was the optimum order to reduce GO and remove RNA molecules.
Nitric acid could remove dispersants from the SWCNT films effectively.10,16 Besides, they could p-dope SWCNTs and increase their conductivity under appropriate conditions.17 Therefore, nitric acid was chosen as the last step to post-treat SWCNT films. The sheet resistance decreased apparently after treatment. Raman spectra showed that the intensity ratio of D and G band was increased to 0.36, and the peak position of G-band blue shifted by 5 cm−1 after treatment with nitric acid (Fig. S1†), indicating slightly p-doping effect. XPS disclosed that the oxygen content decreased a little after treatment, and the C1s peak shifted from 284.8 eV down to 284.68 eV, as seen from Tables S1 and S2 in ESI.† This again proved the removal of RNA and the weak p-doping effect induced by nitric acid.
The removal of RNA molecules was also characterized by scanning electron microscopy. Fig. 4 shows that the film was covered by RNA molecules before post-treatment, which caused the large sheet resistance of the film. After treatment with NaBH4, some RNA molecules were removed and a SWCNT network appeared. The SWCNT network turned more clear after treatment with NaOH solution, testifying to the further removal of RNA molecules. SWCNT network was denser after the acid treatment, which was consistent with the previous report.10 The schematic diagram of each post-treatment step is shown in Fig. 5.
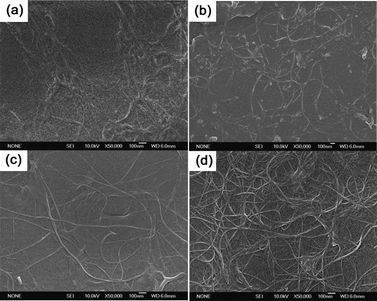 | ||
| Fig. 4 SEM images of SWCNT/GO hybrid films before and after treatment: (a) before treatment; (b) after treatment with NaBH4 for 2 h; (c) after subsequent treatment with NaOH for 1 h; (d) after subsequent treatment with HNO3 for 10 min. | ||
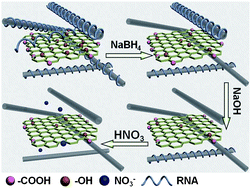 | ||
| Fig. 5 Schematic diagram of the post-treatment of SWCNT/GO hybrid films. | ||
In this communication, SWCNT/GO solution was prepared with RNA as the dispersant and SWCNT/GO hybrid films were prepared by a filtration method. An efficient post treatment procedure was designed to reduce GO and to remove RNA. The optimum post-treatment order was NaBH4 treatment, NaOH treatment and then nitric acid treatment. The sheet resistance decreased significantly after treatment, by a factor of 4–13 times. The function of each procedure was characterized by XPS and Raman spectra. NaBH4 was found to be effective to remove RNA molecules for the first time. This provided an effective route to reduce GO and to remove dispersants simultaneously, which was meaningful for preparing SWCNT/GO hybrid films with high conductivity.
This work was supported by National Natural Science Foundation of China (No. 50932153 and 51072215).
References
- K. S. Novoselov, A. K. Geim, S. V. Morozov, D. Jiang, Y. Zhang, S. V. Dubonos, I. V. Grigorieva and A. A. Firsov, Science, 2004, 306, 666–669 CrossRef CAS.
- P. S. Weiss and M. Dresselhaus, ACS Nano, 2009, 3, 2434–2440 CrossRef CAS.
- J. Kim, L. J. Cote, F. Kim, W. Yuan, K. R. Shull and J. X. Huang, J. Am. Chem. Soc., 2010, 132, 8180–8186 CrossRef CAS.
- E. M. Doherty, S. De, P. E. Lyons, A. Shmeliov, P. N. Nirmalraj, V. Scardaci, J. Joimel, W. J. Blau, J. J. Boland and J. N. Coleman, Carbon, 2009, 47, 2466–2473 CrossRef CAS.
- M. Lotya, P. J. King, U. Khan, S. De and J. N. Coleman, ACS Nano, 2010, 4, 3155–3162 CrossRef CAS.
- M. Zheng, A. Jagota, E. D. Semke, B. A. Diner, R. S. McLean, S. R. Lustig, R. E. Richardson and N. G. Tassi, Nat. Mater., 2003, 2, 338–342 CrossRef CAS.
- J. C. G. Jeynes, E. Mendoza, D. C. S. Chow, P. C. R. Watts, J. McFadden and S. R. P. Silva, Adv. Mater., 2006, 18, 1598–1602 CrossRef CAS.
- V. C. Tung, L. M. Chen, M. J. Allen, J. K. Wassei, K. Nelson, R. B. Kaner and Y. Yang, Nano Lett., 2009, 9, 1949–1955 CrossRef CAS.
- C. Gomez-Navarro, R. T. Weitz, A. M. Bittner, M. Scolari, A. Mews, M. Burghard and K. Kern, Nano Lett., 2007, 7, 3499–3503 CrossRef CAS.
- H. Z. Geng, K. K. Kim, K. P. So, Y. S. Lee, Y. Chang and Y. H. Lee, J. Am. Chem. Soc., 2007, 129, 7758–7759 CrossRef CAS.
- R. R. Wang, J. Sun and L. Gao, J. Phys. Chem. C, 2010, 114, 4923–4928 CrossRef CAS.
- Y. Si and E. T. Samulski, Nano Lett., 2008, 8, 1679–1682 CrossRef CAS.
- H. J. Shin, K. K. Kim, A. Benayad, S. M. Yoon, H. K. Park, I. S. Jung, M. H. Jin, H. K. Jeong, J. M. Kim, J. Y. Choi and Y. H. Lee, Adv. Funct. Mater., 2009, 19, 1987–1992 CrossRef CAS.
- X. B. Fan, W. C. Peng, Y. Li, X. Y. Li, S. L. Wang, G. L. Zhang and F. B. Zhang, Adv. Mater., 2008, 20, 4490–4493 CrossRef CAS.
- R. R. Wang, J. Sun, L. A. Gao and J. Zhang, ACS Nano, 2010, 4, 4890–4896 CrossRef CAS.
- H. Z. Geng, K. K. Kim, C. Song, N. T. Xuyen, S. M. Kim, K. A. Park, D. S. Lee, K. H. An, Y. S. Lee, Y. Chang, Y. J. Lee, J. Y. Choi, A. Benayad and Y. H. Lee, J. Mater. Chem., 2008, 18, 1261–1266 RSC.
- F. Hennrich, R. Wellmann, S. Malik, S. Lebedkin and M. M. Kappes, Phys. Chem. Chem. Phys., 2003, 5, 178–183 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Further experimental details. See DOI: 10.1039/c0nr00655f |
| This journal is © The Royal Society of Chemistry 2011 |
