Single-crystalline α-Fe2O3 oblique nanoparallelepipeds: High-yield synthesis, growth mechanism and structure enhanced gas-sensing properties
Xuelian
Li
,
Wenjing
Wei
,
Shaozhen
Wang
,
Long
Kuai
and
Baoyou
Geng
*
College of Chemistry and Materials Science, Anhui Key Laboratory of Functional Molecular Solids, Anhui Laboratory of Molecular-Based Materials, Anhui Normal University, Wuhu, 241000, P. R. China. E-mail: bygeng@mail.ahnu.edu.cn
First published on 12th November 2010
Abstract
In this paper, single-crystalline α-Fe2O3 oblique nanoparallelepipeds are fabricated in high yield via a facile surfactant-free hydrothermal method, which involves oriented aggregation and Ostwald ripening. The obtained nanocrystals have exposed facets of {012}, {01–4} and {−210} with a rhombohedral α-Fe2O3 structure. The gas sensors based on the as-synthesized α-Fe2O3 nanostructures exhibit high sensitivity, short recovery time, and good reproducibility in ethanol and acetone. The superiority of the gas-sensing properties of the obtained nanostructures should be attributed to the surface structure of the nanocrystals. The as-prepared α-Fe2O3 nanocrystals are significant for exploiting their other applications in the future.
1. Introduction
Controlled fabrication of micro/nanostructures has attracted tremendous attention because of their intriguing size/shape-dependent properties. Correspondingly, many techniques have been exploited in the fabrication of various shapes of micro/nanostructures, such as rods, wires, tubes and polyhedra.1–5As an important n-type semiconductor, hematite (α-Fe2O3) is the most stable iron oxide under ambient conditions, and has been extensively used as gas sensors, catalysts, and pigments due to its low cost, non-toxicity and high resistance to corrosion.6–8 In recent years, stimulated by the promising applications of iron oxides and the novel properties of nanomaterials, much effort has been made to synthesize α-Fe2O3 with various morphologies, such as nanoparticles, nanorods, nanowires, and nanotubes.6–9 As expected, the diverse α-Fe2O3 morphologies led to intriguing shape-dependent properties and varied potential applications.6,10 Especially, α-Fe2O3 has been found to be used as an efficient gas-sensing material due to its electrical conductivity that is highly sensitive to the gaseous environment.11 As is well known, most of the metal oxide based sensors operate at elevated temperatures in the range 150–160 °C. However, long-term use of the sensor at high temperatures might degrade its structural properties or, in some cases, lead to irreversible changes in the phase of the sensor material.12 Therefore, it is necessary to exploit good performance and relatively stable metal oxides for gas sensors. Hematite is the most appropriate candidate for gas sensors due to its structural stability, low cost and non-toxicity.
So far, some efforts have been made to study the gas sensitivity of α-Fe2O3 with various morphologies. For example, Jimmy C. Yu and co-workers fabricated α-Fe2O3 nanorings through a wave-assisted hydrothermal process. They found that the thin film sensor made of the α-Fe2O3 nanorings exhibited high sensitivity and good reversibility for gas-sensing of alcohol under ambient conditions.6 Guoxiu Wang et al. reported a surfactant-free hydrothermal process to synthesize the α-Fe2O3 bamboo flute-like porous nanorods and hexapod-like nanostructures with subsequent calcination of the obtained precursors. The obtained materials have been used to detect ethanol, acetone and gasoline.8 Liu and Zhang et al. reported on the synthesis of α-Fe2O3 nanotubes through a carbon nanotube template process. The as-prepared α-Fe2O3 nanotubes exhibited superior sensitivity to hydrogen sulfide (H2S) based on the catalytic chemiluminescence (CL).13 M. V. Reddy and his co-workers used a thermal treatment method to prepare nanoflakes of α-Fe2O3 on Cu foil and so on.14
Despite these achievements, the variety of shapes of α-Fe2O3 nanocrystals still needs to be greatly expanded to meet their growing applications. Particularly, considerable recent researches have focused on the synthesis of different shapes of micro/nanostructural polyhedra, such as cubes, octahedra, dodecahedra, 14-facets polyhedra, icosahedra etc. because of their shape-related potential applications in many fields, such as gas sensors, optics, catalysts, electrode materials and so on.6–10 As a rhombohedral structure of α-Fe2O3, fabrication of its nanocrystals with a defined shape, especially with special exposed facets that would bring with it high and special activities, might exploit its novel applications and widen its application ranges.15 For example, Rodriguez et al. found that the thermolysis of acidic ferric chloride could lead to monodispersed hematite nanocrystallites of rhombohedral shape with facets belonging to the {104} family.16 Very recently, Gao and co-workers reported on the synthesis of unusual tetrakaidecahedral and oblique parallelepiped iron oxide nanocrystals with exposed high index facets, which was found to exhibit unusual magnetic properties.17 To enrich the research in this area, herein, we exploit a facile surfactant-free hydrothermal method to fabricate α-Fe2O3 with a special oblique parallelepiped morphology, which involves oriented aggregation and Ostwald ripening. The obtained α-Fe2O3 nanocrystals have exposed facets of {012}, {01–4} and {−210}, which makes it possible to facilitate their applications. Correspondingly, the obtained nanostructures have been fabricated into gas sensors and used to detect some gases such as ethanol and acetone. The results show that the gas sensors based on the as-synthesized α-Fe2O3 nanostructures exhibit high sensitivity, short recovery time, and good reproducibility in ethanol and acetone, which should be attributed to the surface structure of the nanocrystals. The as-prepared α-Fe2O3 nanocrystals may bring us a new opportunity to exploit their other applications in the future.
2. Experimental
In this experiment, all the chemical reagents are reagent grade and used without further purification.2.1 Synthesis of α-Fe2O3 nanocrystals
In this experiment, 1 mmol ferric chloride hexahydrate (FeCl3·6H2O) and 15 mmol urea (CH4N2O) were added into 25 ml deionized water, and a magnetic stirrer was used for 30 min to form a homogeneous mixture. Then, this homogenous mixture was transferred to a 50 ml Teflon® lined vessel. The Teflon lined vessel was kept in an oven at 120 °C for 15 h. After the reaction system cooled to room temperature naturally, the precipitate was washed repeatedly more than three times with distilled water and absolute alcohol, respectively. The as-prepared precipitate was separated from the solution by centrifugation at 6000 rpm and the final product was dried at 60 °C in a vacuum oven for 12 h for further characterization.2.2 Characterization
X-Ray powder diffraction (XRD) was carried out on an XRD-6000 (Shimadzu Instruments, Japan) X-ray diffractometer with Cu Kα radiation (λ = 1.54060 Å) at a scanning rate of 0.05° s−1. Scanning electron microscopy (SEM) micrographs were taken using a Hitachi S-4800 scanning electron microscope. Transmission electron microscopy (TEM) micrographs and high-resolution transmission electron microscopy (HRTEM) was performed using JEM 2010 F microscopes. Specific surface areas were computed from the results of N2 physisorption at 77 K (model: BECKMAN SA3100 COULTER) using the BET (Brunauer-Emmet-Teller) formalism.2.3 Fabrication and analysis of gas sensors
The α-Fe2O3 microcrystal-based gas sensors were fabricated by coating the α-Fe2O3 powder onto the ceramic tubes of the sensor body without an additional annealing process. The fabrication and measurements of gas sensors were similar to that of gas sensors reported previously.18 Typically, the prepared gas sensor was placed in a shielded chamber, through which air could be pumped out and ethanol/acetone vapour could be injected in. The volume of the chamber is 1000 cm3 (10 cm × 10 cm ×10 cm). The gas sensing experiments were conducted under a pressure of one standard atmosphere (atm) and at relative humidities of 10–20%. The operating temperature is 150 °C, and the heating voltage is 0.65 V, which are the optimized conditions.3. Results and discussion
3.1 Structure and morphology analysis
The corresponding powder X-ray diffraction (XRD) pattern (Fig. 1) provides crystallinity and phase information for the as-obtained products. All the diffraction peaks in the XRD pattern are well indexed to the rhombohedral α-Fe2O3 structure (JCPDS 84-0311) with high purity and crystallinity. | ||
| Fig. 1 XRD pattern of the obtained products; the bottom in the pattern gives the standard peaks of rhombohedral α-Fe2O3 structure (JCPDS 84-0311). | ||
The morphology of the as-prepared products was studied with SEM. The low-magnification SEM image in Fig. 2a shows that the obtained products are high-yield and uniform with the oblique parallelepiped morphology. The corresponding high-magnification SEM image in Fig. 2b demonstrates that the edge-to-edge width of the obtained rhombus is about 160–220 nm and thickness is about 70–120 nm, respectively. From Fig. 2b, it is seen that the six surrounded planes of the oblique parallelepiped could be divided into two types. One type is four rectangular planes with a length of about 180 nm and width of about 80 nm. The other type is two opposite diamonds with a side length of about 180 nm, and the angles between adjoining sides are 75° and 105°, respectively, which means that these oblique parallelepipeds might not be enclosed by {001} as usual.
 | ||
| Fig. 2 Low- (a) and high- (b) magnification SEM images of the obtained products. | ||
More-detailed structural information on the α-Fe2O3 nanocrystals was provided by transmission electron microscopy (TEM). Fig. 3a displays a typical TEM image of an as-prepared product at low magnification, which illustrates that the sample is mainly made up of oblique parallelepipeds with a few cubes. Fig. 3b which shows a high-magnification TEM image of a single α-Fe2O3 nanoparticle also confirms that the obtained product has a perfect oblique parallelepiped shape with edge length of about 180 nm.
 | ||
| Fig. 3 (a) Low-magnification TEM image of the obtained α-Fe2O3 nanocrystals. (b) Typical high-magnification TEM image of an individual α-Fe2O3 nanoparallelepiped. (c) Typical HRTEM image taken from the tip of the α-Fe2O3 nanoparallelepiped; inset is the corresponding SAED pattern of the nanoparallelepiped. (d) Geometrical model of the as-prepared oblique parallelepiped. | ||
HRTEM images (Fig. 3c) further confirm the single-crystal nature of the α-Fe2O3 nanoparticle. The fringes in a typical HRTEM image are separated by 0.266 nm and 0.367 nm with a dihedral angle between them of 85°, which can be indexed to the (01![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) ) and (012) planes of rhombohedral α-Fe2O3 structure, respectively. According to the standard data of the rhombohedral iron oxide crystal structure, (012) and (01
) and (012) planes of rhombohedral α-Fe2O3 structure, respectively. According to the standard data of the rhombohedral iron oxide crystal structure, (012) and (01![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) ) are perpendicular to (
) are perpendicular to (![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) 10), and the dihedral angle between (012) and (01
10), and the dihedral angle between (012) and (01![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) ) is 85°, which is in good agreement with the above crystal planes.17 Herein, the HRTEM image of the oblique parallelepiped nanocrystal is projected from the [100] zone axis of a single crystal of rhombohedral iron oxide. The corresponding selected-area electron diffraction (SAED) pattern (inset in Fig. 3c) taken from a single nanoparallelepiped further ascertains that the sample is single crystalline in nature. The highlighted spots in the SAED pattern are indexed to {012} and {01
) is 85°, which is in good agreement with the above crystal planes.17 Herein, the HRTEM image of the oblique parallelepiped nanocrystal is projected from the [100] zone axis of a single crystal of rhombohedral iron oxide. The corresponding selected-area electron diffraction (SAED) pattern (inset in Fig. 3c) taken from a single nanoparallelepiped further ascertains that the sample is single crystalline in nature. The highlighted spots in the SAED pattern are indexed to {012} and {01![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) } planes. From the above characterizations, we can deduce that these nanocrystals should be bound by {012}, {01
} planes. From the above characterizations, we can deduce that these nanocrystals should be bound by {012}, {01![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) }, and {
}, and {![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) 10} planes. Fig. 3d presents a geometrical model of the as-prepared oblique parallelepiped enclosed by those facets.
10} planes. Fig. 3d presents a geometrical model of the as-prepared oblique parallelepiped enclosed by those facets.
3.2 Growth mechanism of the products
 | ||
| Fig. 4 SEM images of the as-prepared products at different reaction temperatures for 15 h. (a) 80 °C; (b) 100 °C; (c) 140 °C; (d) 180 °C. | ||
In these experiments, initially, when the hydrothermal reaction temperature was maintained at 80 °C for 15 h, the corresponding SEM image (Fig. 4a) of the as-obtained product showed the formation of nanorods. When the reaction temperature was maintained at 100 °C for 15 h, the as-obtained product composed of nanorods, nanoparallelepipeds and nanocubes (Fig. 4b). From Fig. 4c, we found that the as-prepared product composed of nanoparallelepipeds and nanocubes at 140 °C and the particles are not uniform. When the reaction temperature was maintained at 180 °C for 15 h, the obtained product composed of quasi-spherical irregular particles. The above results demonstrate that 120 °C is the most favorable temperature for the growth of nanoparallelepipeds.
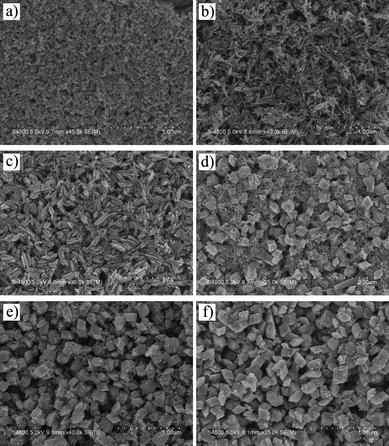 | ||
| Fig. 5 SEM images of the as-prepared products at 120 °C with different reaction times. (a) 1 h; (b) 3 h; (c) 6 h; (d) 8 h; (e) 10 h; (f) 12 h. | ||
The corresponding XRD patterns of the time-dependent products are shown in Fig. 6, which clearly shows that the phases of the products change with the reaction time. The XRD patterns of the products obtained after 1 h and 3 h are well indexed to FeO(OH) (JCPDS No. 75-1594). When the reaction time was prolonged to 6 h or longer, all peaks of the XRD patterns are matched with the rhombohedral phase of α-Fe2O3 (JCPDS No. 84-0311). The time-dependent XRD patterns of the products illustrate that the FeO(OH) is the intermediate product during the growth of the α-Fe2O3 nanocrystals.
 | ||
| Fig. 6 XRD patterns of the as-prepared products at 120 °C with different reaction time. (a) 1 h; (b) 3 h; (c) 6 h; (d) 10 h. | ||
Based on the above results, the probable growth mechanism of the α-Fe2O3 nanoparallelepipeds should be addressed as follow. The reactions involved in the formation of hematite can be summarized as following two reaction procedures:
| (NH2)2CO + H2O → NH3 + H2O + CO2 → NH4+ + OH− + CO32− + H+ | (1) |
| Fe3+ + 3OH− + CO32− + H2O → FeO(OH) + Fe2(CO3)(OH) + H2O → Fe2O3 + H2O | (2) |
In eqn (1), firstly, urea decomposed to form NH3 and CO2 in the hydrothermal condition. In aqueous solution, the obtained ammonia reacted with water and transformed into NH4+ and OH− ions. Meanwhile, the formed CO2 reacted with water to form H+ and CO32− ions. Then, according to eqn (2), the hydroxyl ions released in the reaction would react with Fe3+ ions in the solution to form Fe(OH)3 precipitates, which was unstable in solution and converted to FeO(OH). Besides the formation of FeO(OH) precipitation, there is also a probability for the CO32− ions to react with Fe3+ ions to form Fe2(CO3)(OH), which is very unstable and easily converted into FeO(OH). Thus, FeO(OH) is the main product within a short reaction time (3 h). When the time of the hydrothermal reaction was prolonged to 6 h or longer, the FeO(OH) would decompose to form the stable α-Fe2O3 phase, which has been demonstrated by time-dependent XRD patterns.
Additionally, in this case, the formation of parallelepiped shaped α-Fe2O3 may be attributed to the cooperation of oriented aggregation and Ostwald ripening. The scheme of the growth process can be illustrated as Scheme 1.
 | ||
| Scheme 1 Schematic illustration of the formation mechanism of rhombic α-Fe2O3 nanocrystals. | ||
Initially, FeO(OH) nanoparticles were fabricated in hydrothermal conditions (see SEM image in Fig. 5a). Then, with the increase of reaction time, the oriented attachment of the obtained nanoparticles took place and the nanorods formed (see Scheme 1, step I). The unstable FeO(OH) nanorods transformed into α-Fe2O3 nanorods by dehydration in the successive treatment. This step can be well illustrated by time-dependent SEM images and XRD patterns (Fig. 5 and 6). Fig. 5b shows that the as-prepared products are nanorods within 3 h and the corresponding XRD patterns (see Fig. 6) are well indexed to FeO(OH) (JCPDS No. 75-1594). In comparison, the product after 6 h (Fig. 5c) is also composed of nanorods, however, many nanorods aggregated together to form nanorod bundles. The corresponding XRD pattern (see Fig. 6) clearly reveals that the obtained product is the pure phase of α-Fe2O3, which demonstrates that the phase of nanorods after 6 h is different with that of 1 h and 3 h. Namely, FeO(OH) nanorods have transformed into α-Fe2O3 nanorods by dehydration after a 6 h treatment.
It should be mentioned that the oriented aggregation maybe takes place in two ways (see Scheme 1, step II and III). In step II, α-Fe2O3 nanorods aggregate neatly parallel in three-dimensional space to form a cube shape. In step III, the obtained α-Fe2O3 nanorods show a continuous dislocation arrangement and form a parallelepiped morphology. After that, through Ostwald ripening, the adjacent nanorods would fuse into the core section and create extended cubic or parallelepiped α-Fe2O3 single crystals with a well-defined shape (step IV and V). Noticeably, step II and III are competitive procedures. From the point of the surface energy, the dislocation arrangement possesses lower energy than that of the neat arrangement; therefore, the nanoparallelepipeds are main final products in this case. We have also performed the TEM and HRTEM characterizations on the obtained α-Fe2O3 nanorods. The TEM image of the α-Fe2O3 nanorods in Fig. 7a shows that some nanorods are attached to each other. The corresponding HRTEM of the nanorod in Fig. 7b reveals that the obtained α-Fe2O3 nanorods is crystalline with an interplanar space of 0.266 nm, indicating that the nanorods have a <01![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) > growth direction and are surrounded by (012) planes as side faces.20 The inset in Fig. 7b shows the Fourier transform electron diffraction (FTED) pattern from the image.
> growth direction and are surrounded by (012) planes as side faces.20 The inset in Fig. 7b shows the Fourier transform electron diffraction (FTED) pattern from the image.
 | ||
| Fig. 7 TEM (a) and HRTEM (b) image of the obtained α-Fe2O3 nanorods. Inset in Fig. 7b is the Fourier transform electron diffraction pattern from the image. | ||
3.3 Gas sensing measurements
Considering the important application of α-Fe2O3 as the gas sensing material, we investigated the sensing performance of the as-prepared α-Fe2O3 nanoparallelepipeds by detecting different gases, such as ethanol and acetone. The gas sensing properties of the obtained products were performed through a testing system of gas sensors (Hanwei Electronics Co., Ltd., Henan Province, China).21 The gas sensor was fabricated by coating an aqueous slurry of the prepared products onto a ceramic tube and drying at room temperature. A Ni–Cr alloy coil through the tube was employed as a heater to control the operating temperature.Line 1 in Fig. 8a and c shows the dynamic response curves of the as-fabricated gas sensors based on the α-Fe2O3 nanoparallelepipeds to ethanol and acetone vapor with increasing concentration. The response of our sensor is equal to Vgas/Vair, where Vgas and Vair are the output voltages of contrastive resistor, which is series-wound with the sensor and exposed in the testing gas and dry air, respectively. It can be seen that the response increased abruptly on the injection of the detecting liquids and then decreased rapidly and recovered to their initial value after the vapor was released. After many cycles between the test gas and fresh air, the responses of the reference resistor and the resistance of the sensor could recover their initial state, which indicates that the sensors have good reversibility. The testing results show that the fabricated sensors have a short response time of about 3–5 s and a recovery time of 4–6 s, as well as good reproducibility. Furthermore, we also found that when the sensor based on the α-Fe2O3 nanoparallelepipeds was kept in the corresponding vapor for over 10 days, the response still recovered to the original value, which reveals that the response of the α-Fe2O3 nanoparallelepiped-based gas sensor is excellent for ethanol and acetone.
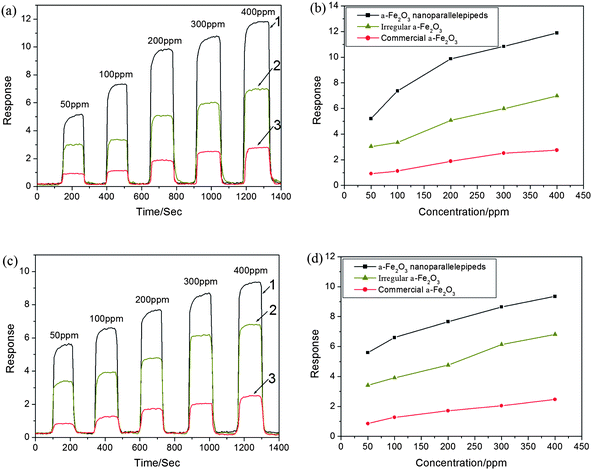 | ||
| Fig. 8 (a) and (c) Sensitivity responses of the gas sensors based on α-Fe2O3 nanoparallelepipeds (line 1), irregular α-Fe2O3 nanoparticles (line 2) and commercial α-Fe2O3 powders (line 3) versus the time for different concentrations of ethanol and acetone, respectively; (b) and (d) Comparison of response of α-Fe2O3 nanoparallelepipeds, irregular α-Fe2O3 nanoparticles and commercial α-Fe2O3 powders versus the concentration of ethanol and acetone, respectively. | ||
In order to illustrate the superiority on the gas sensing properties of the as-prepared α-Fe2O3 nanoparallelepipeds, we investigated the gas sensing properties of the irregular α-Fe2O3 particles (the corresponding SEM is shown in Fig. 4d) and commercial α-Fe2O3 powders (the size ∼ 1 μm) in the same conditions. The corresponding results are shown in line 2 and 3 in Fig. 8a and c, which clearly reveals that the gas sensitivity of the irregular α-Fe2O3 particles and commercial α-Fe2O3 powders based sensors are much lower than that of the α-Fe2O3 nanoparallelepiped-based gas sensors. The corresponding response comparison of the three types of α-Fe2O3 particles versus the concentration of ethanol and acetone are shown in Fig. 8b and d, which clearly show the superiority of the α-Fe2O3 nanoparallelepiped-based gas sensors over both of the others.
The above results can be attributed to the following facts. Firstly, the sizes of the α-Fe2O3 nanoparallelepipeds and irregular α-Fe2O3 particles are obviously less than that of the commercial α-Fe2O3 powders, which leads to the higher response of the α-Fe2O3 nanoparallelepiped-based and irregular particle-based sensors due to their high specific surface area related to the particle size. Then, compared with irregular α-Fe2O3 particles, α-Fe2O3 nanoparallelepiped possesses special surface structure. In order to exclude the effect derived from the difference of the specific surface areas, we obtained the BET specific surface areas of irregular hematite particles and nanoparallelepipeds. The results are shown in Fig. 9. The adsorption curve is of a reverse ‘S’-shape, which is a normal physical adsorption isotherm. The specific surface area was thus evaluated to be 5.43 and 5.86 m2 g−1 for nanoparallelepipeds and irregular hematite particles, respectively, from data points in this pressure range by the BET equation.22 The results illustrate that the difference of the specific surface areas between the nanoparallelepipeds and irregular hematite particles can be negligible.
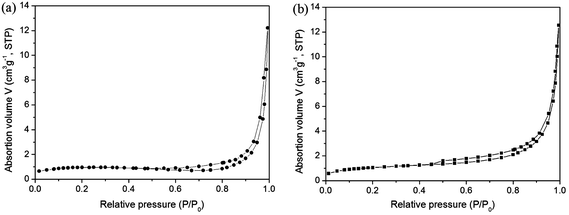 | ||
| Fig. 9 BET spectra of the obtained α-Fe2O3 nanoparallelepipeds (a) and irregular α-Fe2O3 nanoparticles (b). | ||
However, it should be mentioned that one important theory for sensors involves adsorption/desorption phenomena and reactions at the surface. Therefore, the “surface accessibility” is crucial for maintaining the high sensitivity of crystals. For the rhombic α-Fe2O3 crystal, it has large active surface areas of {012}, {01![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) } and {
} and {![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) 10}, which can provide high activity space for the interaction between the obtained nanostructures and the detected gases and thus lead to the higher sensitivity.
10}, which can provide high activity space for the interaction between the obtained nanostructures and the detected gases and thus lead to the higher sensitivity.
4. Conclusions
In summary, we reported a facile hydrothermal route to fabricate well-defined single-crystalline α-Fe2O3 nanoparallelepipeds in high yield. The growth process involved oriented aggregation and Ostwald ripening. FeO(OH) nanorods were first fabricated and then the unstable FeO(OH) nanorods transformed into α-Fe2O3 nanorods by dehydration. The obtained α-Fe2O3 nanorods transformed to nanorhombuses through oriented aggregation and Ostwald ripening. In addition, gas sensors based on α-Fe2O3 nanoparallelepipeds exhibited high sensitivity, short recovery time, and good reproducibility for ethanol and acetone. The as-prepared α-Fe2O3 nanocrystals may lead to novel applications in other fields.Acknowledgements
This work was supported by the National Natural Science Foundation of China (20971003, 20671003), the Key Project of Chinese Ministry of Education (209060), Science and Technological Fund of Anhui Province for Outstanding Youth (10040606Y32), the Education Department of Anhui Province (2006KJ006TD) and the Program for Innovative Research Team in Anhui Normal University.References
- (a) W. K. Koh, A. C. Bartnik, F. W. Wise and C. B. Murray, J. Am. Chem. Soc., 2010, 132, 3909 CrossRef CAS; (b) A. Yella, M. N. Tahir, S. Meuer, R. Zentel, R. Berger, M. Panthöfer and W. Tremel, J. Am. Chem. Soc., 2009, 131, 17566 CrossRef CAS; (c) M. A. Franzman and R. L. Brutchey, Chem. Mater., 2009, 21, 1790 CrossRef CAS.
- (a) C. W. Peng, M. R. Plouet, T. Y. Ke, C. Y. Lee, H. T. Chiu, C. Marhic, E. Puzenat, F. Lemoigno and L. Brohan, Chem. Mater., 2008, 20, 7228 CrossRef CAS; (b) S. D. Luo, W. Y. Zhou, Z. X. Zhang, L. F. Liu, X. Y. Dou, J. X. Wang, X. W. Zhao, D. F. Liu, Y. Gao, L. Song, Y. Y. Xiang, J. J. Zhou and S. S. Xie, Small, 2005, 1(10), 1004 CrossRef CAS.
- (a) S. M. Yoon, I. C. Hwang, K. S. Kim and H. C. Choi, Angew. Chem., Int. Ed., 2009, 48, 2506 CrossRef CAS; (b) C. H. Lee, M. Xie, V. Kayastha, J. S. Wang and Y. K. Yap, Chem. Mater., 2010, 22, 1782 CrossRef CAS.
- (a) B. Y. Geng, J. Z. Ma and J. H. You, Cryst. Growth Des., 2008, 8(5), 1443 CrossRef CAS; (b) B. Y. Geng, C. H. Fang, F. M. Zhan and N. Yu, Small, 2008, 4(9), 1337 CrossRef CAS.
- K. X. Yao, X. M. Yin, T. H. Wang and H. C. Zeng, J. Am. Chem. Soc., 2010, 132, 61314.
- J. Chen, L. N. Xu, W. Y. Li and X. L. Gou, Adv. Mater., 2005, 17, 582 CrossRef CAS.
- X. L. Hu, J. C. Yu, J. M. Gong, Q. Li and G. S. Li, Adv. Mater., 2007, 19, 2324 CrossRef CAS.
- X. L. Gou, G. X. Wang, X. Y. Kong, D. Wexler, J. Horvat, J. Yang and J. Park, Chem.–Eur. J., 2008, 14, 5996 CrossRef CAS.
- Q. Wang, B. Y. Geng, S. Z. Wang, Y. X. Ye and B. Tao, Chem. Commun., 2010, 46, 1899 RSC.
- (a) L. Liu, H. Z. Kou, W. L. Mo, H. J. Liu and Y. Q. Wang, J. Phys. Chem. B, 2006, 110, 15218 CrossRef CAS; (b) C. Z. Wu, P. Yin, X. Zhu, C. Z. OuYang and Y. Xie, J. Phys. Chem. B, 2006, 110, 17806 CrossRef CAS.
- (a) K. Hara and N. Nishida, Sens. Actuators, B, 1994, 20, 181 CrossRef CAS; (b) X. Q. Liu, S. W. Tao and Y. S. Shen, Sens. Actuators, B, 1997, 40, 161 CrossRef; (c) L. H. Luo, X. L. Li, W. Li and S. Q. Xi, Sens. Actuators, B, 2000, 71, 77–81 CrossRef.
- E. Rezlescu, C. Doroftei, N. Rezlescu and P. D. Popa, Phys. Status Solidi A, 2008, 205(8), 1790 CrossRef CAS.
- Z. Y. Sun, H. Q. Yuan, Z. M. Liu, B. X. Han and X. R. Zhang, Adv. Mater., 2005, 17, 2993 CrossRef CAS.
- M. V. Reddy, T. Yu, C. H. Sow, Z. X. Shen, C. T. Lim, G. V. S. Rao and B. V. R. Chowdari, Adv. Mater., 2007, 17, 2792 CAS.
- A. Gurlo, S. Lauterbach, G. Miehe, H. J. Kleebe and R. Riedel, J. Phys. Chem. C, 2008, 112, 9209 CrossRef CAS.
- R. D. Rodriguez, D. Demaille, E. Lacaze, J. Jupille, C. Chaneac and J. P. Jolivet, J. Phys. Chem. C, 2007, 111, 16866 CrossRef CAS.
- J. Z. Yin, Z. N. Yu, F. Gao, J. J. Wang, H. Pang and Q. Y. Lu, Angew. Chem., Int. Ed., 2010, 49(36), 6328 CrossRef CAS.
- (a) J. T. Zhang, J. F. Liu, Q. Peng, X. Wang and Y. D. Li, Chem. Mater., 2006, 18, 867 CrossRef CAS; (b) B. Y. Geng, F. M. Zhan, C. H. Fang and N. Yu, J. Mater. Chem., 2008, 18, 4977 RSC; (c) B. Y. Geng, F. M. Zhan, H. Jiang, Z. J. Xing and C. H. Fang, Cryst. Growth Des., 2008, 8, 3497 CrossRef CAS.
- B. P. Jia and L. Gao, Cryst. Growth Des., 2008, 8(4), 1372 CrossRef CAS.
- B. Jia, L. Gao and J. Sun, J. Am. Ceram. Soc., 2007, 90, 1315 CrossRef CAS.
- J. Liu, S. Z. Wang, Q. Wang and B. Y. Geng, Sens. Actuators, B, 2009, 143, 253 CrossRef.
- S. Brunauer, P. H. Emmett and E. Teller, J. Am. Chem. Soc., 1938, 60, 309 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2011 |
