Cytotoxicity of sophorolipid-gellan gum-gold nanoparticle conjugates and their doxorubicin loaded derivatives towards human glioma and human glioma stem cell lines†
Sheetal
Dhar
ad,
E. Maheswara
Reddy
b,
Asmita
Prabhune
c,
Varsha
Pokharkar
*d,
Anjali
Shiras
*b and
B. L. V.
Prasad
*a
aMaterials Chemistry Division, National Chemical Laboratory, Pune, 411 008, India. E-mail: pl.bhagavatula@ncl.res.in; Fax: +91 20 25902636; Tel: +91 20 25902013
bNational Centre for Cell Sciences, Pune, 411 007, India. E-mail: anjalishiras@nccs.res.in
cBiochemical Sciences Division, National Chemical Laboratory, Pune, 411 008, India
dPoona College of Pharmacy, Bharati Vidyapeeth University, Pune, 411 038, India. E-mail: varshapokharkar@rediffmail.com
First published on 11th November 2010
Abstract
Biocompatible gold nanoparticles were synthesized by using a naturally occurring gum—Gellan Gum—as a capping and reducing agent. These were further conjugated with sophorolipids which again were accessed through a biochemical transformation of a fatty acid. The cellular uptake of sophorolipid-conjugated gellan gum reduced gold nanoparticles and their cytotoxicity on human glioma cell line LN-229 and human glioma stem cell line HNGC-2 were investigated. Quite surprisingly even the simple sophorolipid-conjugated gellan gum reduced/capped gold nanoparticles showed greater efficacy in killing the glioma cell lines and, gratifyingly, the glioma stem cell lines also. The cytotoxic effects became more prominent once the anti cancer drug doxorubicin hydrochloride was also conjugated to these gold nanoparticles.
1. Introduction
Gliomas are highly vascularized, aggressive and diffusely infiltrating primary brain tumors that are rarely, if ever, cured, despite advances in modern chemo- and radiotherapy.1 Moreover, the failure of typical cytotoxic therapies to completely cure cancer has been attributed to their targeting of rapidly proliferating tumor cells while sparing the tumor stem cell compartment, which has a low proliferation rate and high tumorigenic potential.1 Thus, solid tumors resist current drug therapies and often recur after treatment and the new tumors are much harder to treat.2 Tackling this, we disclose here the efficient manipulation of an established human glioma cell line and more significantly of the human glioma stem cell line by sophorolipid-conjugated gellan gum reduced and capped gold nanoparticles and their doxorubicin hydrochloride loaded derivatives. The sophorolipid (SL) used in this study is a class of glycolipid obtained via biochemical route.3Sophorolipids themselves have been shown to be very interesting for biological and other applications. Sophorolipids have good surfactant properties that have been used in the petroleum and in food industries as emulsifiers.4Sophorolipids and their derivatives have shown immense potential applications as therapeutic agents. Further, they are found to be useful in cosmetics, and as antibacterial, antiviral, spermicidal, and antifungal agents.5 In addition to the above, reports have also indicated therapeutic applications for SLs as immunomodulators for the treatment of septic shock,6 and most importantly as anticancer agents.7 From the literature, it is clear that SL and several of the modified analogues have been extensively investigated by various groups.5b,8 But still their application in nanoparticulate systems has yet not been fully explored. Kasture et al., studied the SL obtained from oleic acid as capping agent for cobalt nanoparticles.9a Singh et al., studied the reducing/capping agent properties of SL derived from oleic acid for the synthesis of water-dispersible silver nanoparticles.9bOn the other hand, gold nanoparticles (AuNPs) have been gaining fame as unique drug delivery vehicles due to their distinctive shape, size, and surface-dependent properties.10 Additionally, their reported biocompatibility and non-cytotoxicity has made drug delivery the most emerging application for AuNPs.11 Further, the ease with which their surfaces can be functionalized also makes them an attractive candidate for the above applications.12 So, here by taking advantage of the surfactant and anticancer properties of SL, we used it for surface functionalization of gellan gum reduced gold nanoparticles (SL-GG-AuNPs). It was hypothesized that SL-GG-AuNPs could efficiently act against the viability of human glioma cells as well as human glioma stem cells. Further, owing to the presence of dimeric sugar moieties in their structure these surface-capped SLs could help the SL-GG-AuNPs to cross the blood-brain barrier and they may become efficient carriers to deliver drugs that are either electrostatically or covalently attached to them. We further hypothesized that loading of doxorubicin hydrochloride (DOX) and SL on GG-Au NPs will have a synergetic effect against glioma cells and glioma stem cells. To demonstrate this, we studied the cellular uptake of SL-GG-AuNPs and the cytotoxicty of SL-GG-AuNPs and DOX-loaded SL-GG-AuNPs (DOX-SL-GG-AuNPs) on human glioma cell line LN-229 and human glioma stem cell line HNGC-2. Quite surprisingly even the simple sophorolipid-conjugated gellan gum reduced/capped gold nanoparticles showed greater efficacy in killing the glioma cell lines. The cytotoxic effects became more prominent once the doxorubicin hydrochloride was also conjugated to these SL-GG-AuNPs. Presented below are the details of the investigation.
2. Experimental
2.1 Materials
Doxorubicin hydrochloride was a gift sample from RPG Life Sciences Limited, Mumbai, India. Sophorolipid was synthesized and characterized at the National Chemical Laboratory, Pune, India.8Chloroauric acid (AuCl4) and Texas red were obtained from Sigma-Aldrich Chemicals. Gellan gum was a gift sample from CP Kelco, USA. The human glioma cell line LN-229 was procured from American type culture collection (ATCC, USA) and the HNGC-2 stem cell line was developed at the National Centre for Cell Sciences, Pune, India.13 The yellow tetrazolium MTT (3-(4, 5-dimethylthiazolyl-2)-2, 5-diphenyltetrazolium bromide) was obtained from Sigma-Aldrich, USA. All the samples were prepared in a Millipore Milli Q water system which was certified to be endotoxin-free.2.2 Conjugation of sophorolipid with gellan gum gold nanoparticles
The GG-AuNPs were prepared and characterized as described earlier.14 In brief, an aqueous solution of HAuCl4 (1 × 10−4 M, 100 μL) was reduced to ruby red colored AuNPs by heating it in 0.02% w/v aqueous solution of GG (100 mL). The AuNP dispersion was thoroughly dialyzed (dialysis tubing 12 kDa cut off) for 24 h to remove the by-products of the reaction. The concentration of gold in the above samples was determined using an atomic absorption spectrophotometer (AA 201, Chemito, India). After dialysis, sophorolipid (10−4 M) was added to the GG-AuNPs dispersion during stirring. The stirring was continued for 24 h at room temperature to ensure optimum conjugation of SL with GG-AuNPs.2.3 Loading of doxorubicin hydrochloride onto sophorolipid gold nanoparticles
A calculated amount of DOX was added to a dispersion of SL-GG-AuNPs obtained as described above, resulting in a final DOX concentration of 10−4 M in solution. The solution was then incubated for 24 h at room temperature and then centrifuged at 37118 g for 0.5 h. The pellets thus obtained after centrifugation were separated from the supernatant solution and redispersed in Milli Q water prior to further characterization. The free DOX present in the supernatant was determined by measurements of its UV absorbance, and the percentage loading of DOX on SL-GG-AuNPs was estimated by following formula:| % Loading efficiency = [(total amount of DOX added − amount of DOX in supernatant)/total amount of DOX added] × 100. |
2.4 Characterization
2.5 In vitro cellular uptake and cytotoxicity assay
MTT assay: After 24 h of incubation, MTT (5 mg mL−1, 20 μL) was added to respective sets of cells and the plates were incubated for an additional 4 h. After 4 h of incubation, the medium was removed and DMSO (200 mL, Sigma-Aldrich, USA) was added to dissolve the formazan crystals resulting from the reduction of the tetrazolium salt only by metabolically active cells. The absorbance of dissolved formazan was measured at 570 nm using a Bio-Rad microplate reader (Model 680, Heraeus, USA). Since the absorbance directly correlated with the number of viable cells, the percent viability was calculated from the absorbance.
2.6 Cellular uptake and apoptosis studies using confocal laser scanning microscopy
Confocal laser scanning microscopy (CLSM) was used to study the cellular uptake of Texas-red-labeled SL-GG-AuNPs and the apoptotic activity of DOX-SL-GG-AuNPs on various cell lines. After incubation, the cover slips were washed extensively with ice-cold phosphate (PBS, Himedia, Mumbai, India) buffered saline and fixed in 4% paraformaldehyde (Sigma-Aldrich, USA) for 10 min at room temperature. After repeated rinses in PBS, cells were blocked in 5% BSA (ICN biomedicals, Germany) in PBS for 30 min at room temperature. Later the cells were again washed in PBS in the dark and then the nucleus was counterstained with 4′-6-Diamidino-2-phenylindole (DAPI, Molecular probes, USA) for 10 min and the cells were mounted onto glass slides with 1,4-diazobicyclo-2,2,2-octanex (DABCO, Sigma-Aldrich, USA) as the mounting medium. The cover slips were then observed using a Zeiss LSM 510 confocal microscope (Germany). Images were captured using the CCD-4230 camera coupled with the microscope and processed using a computer-based programmable image analyzer KS300 (Carl Zeiss, Germany).3. Results and discussion
To improve the function of AuNPs as a drug delivery vehicle, we studied the synthesis of GG-AuNPs, and subsequently incubated these gellan gum capped AuNPs (GG-AuNPs) with sophorolipid to result in sophorolipid-conjugated GG-AuNPs (SL-GG-AuNPs). The UV/Vis spectra of SL-GG-AuNPs were compared with that of pristine GG-AuNPs in order to monitor any change in the surface plasmon band after conjugation with SL [Scheme 1A]. No broadening and red-shift of the surface plasmon band was observed (Scheme 1B), which is normally associated with the aggregation of the AuNPs as a consequence of surface modification.16![(A) Schematic diagram showing synthesis of gellan gum reduced gold nanoparticles and sophorolipid-conjugated gellan gum reduced gold nanoparticles(B)UV/Vis absorption spectra of sophorolipid-conjugated gold nanoparticles [inset (a) gellan gum reduced gold nanoparticles and (b) sophorolipid-conjugated gellan gum gold nanoparticles].](/image/article/2011/NR/c0nr00598c/c0nr00598c-s1.gif) | ||
| Scheme 1 (A) Schematic diagram showing synthesis of gellan gum reduced gold nanoparticles and sophorolipid-conjugated gellan gum reduced gold nanoparticles(B)UV/Vis absorption spectra of sophorolipid-conjugated gold nanoparticles [inset (a) gellan gum reduced gold nanoparticles and (b) sophorolipid-conjugated gellan gum gold nanoparticles]. | ||
To demonstrate the versatility of SL-GG-AuNPs in biomedical applications, the dispersion stability of SL-GG-AuNPs was evaluated by assessing the formation of aggregates and change in color in the presence of different pH and electrolytic conditions. Detachment of SL from AuNPs under these different conditions would cause the nanoparticles to aggregate, which could be monitored by measuring the disappearance of the characteristic plasmon absorption peak and the appearance of a peak between 600 and 700 nm.17 Quite satisfyingly, the SL-GG-AuNP dispersion did not show any discernible change in position at 520 nm in the pH window of 4–12 [Fig.1 (a) and (c)]. Only when the pH of SL-GG-AuNPs was adjusted to 2, the dispersion showed some instability. The addition of electrolyte (sodium chloride 10−1 M to 10−6 M) also did not cause any aggregation in the SL-GG-AuNPs [Fig. 1 (b)]. The minimal change in the surface plasmon resonance of SL-GG-AuNPs under the above experimental conditions indicated the extra stability imparted to AuNPs due to conjugation with SL, which is in accordance with the literature reports.8,9Fig. 2 shows the representative TEM image and particle size distribution of SL-GG-AuNPs which illustrate that upon modification, the average particle size [∼17 nm; Fig. 2 (b)] slightly increased from that of pristine GG-AuNPs (∼13 nm).14
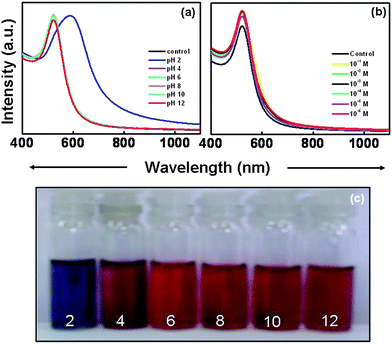 | ||
| Fig. 1 UV/Vis absorption spectra of sophorolipid-conjugated gold nanoparticles. (a, c) are the pH study and (b) is the electrolyte study. | ||
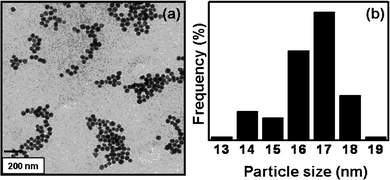 | ||
| Fig. 2 TEM image of sophorolipid-conjugated gold nanoparticles, (a) at t = zero months and (b) particle size distribution of the same. | ||
To understand the cellular uptake of SL-GG-AuNPs, fluorescent marker Texas red was conjugated with nanoparticles. The amount of Texas red conjugated to SL-GG-AuNPs was calculated to be 0.99 ng μL−1. The zeta potential of the SL-GG-AuNPs reduced from −40.6 ± 2.1 mV to −24.1 ± 1.7 mv upon Texas red loading. The decrease in the zeta potential can be taken as an indication of Texas red conjugation to SL-GG-AuNPs. After conjugation, the cellular uptake of labeled SL-GG-AuNPs was studied on human glioma cell line LN-229. The untreated cells were taken as control for the experiment. Observation of cellular uptake using confocal microscopy (Fig. 3) showed that the nanoparticles were efficiently internalized by endocytosis in tumor cells within 3 h of incubation. Gold nanoparticles were clearly observed inside the cells as red dots [Fig. 3 (c)]. The nanoparticles were localized mainly in the cytoplasm and perinuclear region of the cells.18 The internalization of SL-GG-AuNPs was also confirmed by Z-stacking images (Fig. S1 of the ESI†).
 | ||
| Fig. 3 Confocal images of cellular uptake of Texas red labeled sophorolipid gold nanoparticles in human glioma cell lines LN-229. (a) phase (b) DAPI (c)gold nanoparticles and (d) overlaid images from (b) and (c). | ||
After characterizing the SL-GG-AuNPs, DOX was loaded onto SL-GG-AuNPs. Based on the UV/Vis absorbance studies, the loading efficiency of DOX on SL-GG-AuNPs was determined to be 85% of the DOX concentration taken. Based on TGA analysis this corresponded to nearly 50% of the total weight (Fig. S2 of the ESI†). This reasonably good amount of loading12 can be attributed to the presence of many sugar groups exposed on the SL-GG-AuNP surfaces to which DOX can get conjugated viahydrogen bonding and electrostatic interactions. It was found that even the DOX-SL-GG-AuNPs remained in suspension by their electrostatic repulsion and maintained the negative charge on the surface (−26.7 ± 1.4 mV). Park et al., studied the loading of DOX on porous silicon nanoparticles and reported that the loading of DOX on nanoparticles was by electrostatic forces and that there was a decrease in the zeta potential of the DOX loaded nanoparticles (−32.00 mV) as compared to the blank silicon nanoparticles (−52.00 mV).19 The binding and stability of DOX molecules after loading onto SL-GG-AuNPs was studied using fluorescence spectroscopy. It has been reported that when loaded on bare metal nanoparticles the emission of many fluorophores is quenched.20 Quite satisfyingly, there was no major change in the emission profile from DOX in DOX-SL-GG-AuNPs and the peaks at 597 nm and 635 nm as observed in pure DOX were retained (Fig. S3A of the ESI†). The preservation of the fluorescence signature supports the claim that DOX molecules are effectively screened from the nanoparticles surface by the GG capping. The stability of DOX-SL-GG-AuNPs was also monitored (Fig. S3B of the ESI†) and found to be stable during its storage period of 3 months with no change in any of the fluorescence signature.
After successful synthesis and characterization of SL-GG-AuNPs and DOX-SL-GG-AuNPs, in vitro cytotoxicity of different formulations; namely SL-GG-AuNPs, free DOX solution, DOX-SL-GG-AuNPs and culture media alone, was evaluated using MTT assay.21 The wells that received culture media were regarded as control with a cell viability of 100%. Fig. 4 (a) and 4 (b) show the percent viability of LN-229 and HNGC-2, respectively, after 24 h exposure to SL-GG-AuNPs. At the highest concentration of 12.5 μg mL−1, the cell viability was found to be 80% in both LN-229 [Fig. 4 (a)] and HNGC-2 [Fig. 4 (b)] cell line cases. The most appealing result is that after 48 h of exposure, the cell viability in the SL-GG-AuNPs treated case decreased to ∼50% for both cell lines [(Fig. 4 (c) and (d)]. It was apparent that the LN-229 and HNGC-2 cells when incubated with SL-GG-AuNPs had lower viability than the blank GG-AuNPs.14 This clearly establishes that capping with SL alone on the nanoparticles surface leads to enhanced cytotoxicity towards not just cancerous cells but towards the cancer stem cells also.
 | ||
| Fig. 4 Viability of LN-229 and HNGC-2 cell lines after 24 h (a and b, respectively) and after 48 h (c and d, respectively) of exposure to SL-GG-AuNPs. The four bars represent the four different conditions tried, namely control (culture medium), sophorolipid gold nanoparticles, doxorubicin solution, and doxorubicin loaded sophorolipid gold nanoparticles (from left to right for each concentration). | ||
Encouraged by the results obtained for SL-GG-AuNPs we extended our studies on these cell lines with DOX-SL-GG-AuNPs. At the end of 24 h, the viability of LN-229 cells exposed to DOX-SL-GG-AuNPs effectively decreased to 27% (at DOX concentration of 12.5 μg mL−1) as compared to 59% as found in case of free DOX solution [Fig. 4(a)]. In case of HNGC-2, the cell viability within the concentration range checked (1.0 μg mL−1 to 12.5 μg mL−1) was 79.55–59.07% for free DOX and 60.9–40.01% for DOX-SL-GG-AuNPs. By the end of 48 h of incubation, the viability of LN-229 cells reached 2% for DOX-SL-GG-AuNPs compared to 16% of free DOX solution [Fig. 4(c)]. In case of HNGC-2, the maximum cell viability achieved via 12.5 μg mL−1DOX at the end of 48 h was 32% for free DOX solution and 9% for DOX-SL-GG-AuNPs [Fig. 4(d)]. Thus, the DOX-SL-GG-AuNPs were able to significantly inhibit the cell viability indicating that the combination therapy has a greater potential in eradication of glioma cancer cells and even glioma stem cells.22 The reason behind this could be the better cell penetration of the DOX-SL-nanoparticle conjugate as compared to free DOX alone.23 This may trigger a rapid release of DOX from the DOX-conjugated AuNPs after they are internalized into the tumor cells, thereby greatly enhancing the cell cytotoxicity.24
The better internalization of DOX-SL-GG-AuNPs is clearly established from confocal studies (Fig. 5). We next analyzed the kind of death DOX-SL-GG-AuNPs caused on glioma cell lines. DOX is known to induce apoptosis by blocking the cell cycle and inhibiting the DNA polymerase enzyme.25 Interactions of DOX-SL-GG-AuNPs with cells morphology/architecture was imaged using a confocal microscope. It was possible to visualize DOX directly since DOX (red color) itself is a fluorescent molecule. The apoptosis induced by DOX-SL-GG-AuNPs was clearly visible as the cells shrank to a spherical shape. Because of apoptosis, most of the cells were detached from the cover slips but the main apoptosis features like cell shrinkage, chromatin condensation and nuclei fragmentation were clearly observed. CLSM images clearly demonstrated the apoptosis induced cell death by DOX-SL-GG-AuNPs on human glioma cell line LN-229 and human glioma stem cell line HNGC-2.
 | ||
| Fig. 5 Confocal microscopy images to demonstrate the apoptosis induced by doxorubicin-loaded sophorolipid gold nanoparticles on (A) human glioma cell line LN-229(B) human glioma stem cell line HNGC-2. (a) phase-contrast (b) DAPI staining (c)doxorubicin-loaded sophorolipid gold nanoparticles and (d) overlaid images from (b) and (c). | ||
In conclusion, a completely green process was developed for the preparation of sophorolipid-conjugated—gellan gum capped/reduced—gold nanoparticles. These sophorolipid-conjugated gellan gum reduced/capped gold nanoparticles showed greater efficacy in killing the glioma cell lines and more prominently glioma stem cell lines. The cytotoxic effects became more prominent once the doxorubicin hydrochloride was also conjugated to these gold nanoparticles.
References
- R. Stupp and M. E. Hegi, Nat. Biotechnol., 2007, 25, 193 CrossRef CAS.
- G. Schreiner, Nat. Biotech., 2008, 26, 366.
- (a) S. S. Cameotra and R. S. Makkar, Curr. Opin. Microbiol., 2004, 7, 262–266 CrossRef CAS; (b) A. P. Tulloch, Can. J. Microbiol., 1964, 10, 359 CrossRef CAS.
- I. M. Banat, Bioresour. Technol., 1995, 51, 1 CrossRef CAS.
- (a) K. J. Kim, D. S. Yoo, Y. B. Kim, B. S. Lee, D. D. Shin and E. K. Kim, J. Microbiol. Biotechnol., 2002, 12, 235 CAS; (b) V. Shah, G. F. Doncel, T. Seyoum, K. M. Eaton, I. Zalenskaya, R. Hagver, A. Azim and R. Gross, Antimicrob. Agents Chemother., 2005, 49, 4093 CrossRef CAS; (c) E. Kandil, H. Zhang, R. Schulze, L. Dresner, M. Nowakowski, R. Gross and M. E. Zenilman, J. Am. Coll. Surg., 2003, 197, S40.
- E. Kandil, H. Zhang, R. Schulze, L. Dresner, M. Nowakowski, R. Gross and M. E. Zenilman, J. Am. Coll. Surg., 2003, 197, S40.
- (a) C. Scholz, S. Mehta, K. Bisht, V. Guilmanov, D. Kaplan, R. Nicolosi and R. Gross, Proc. Am. Chem. Soc., 1998, 39, 168 Search PubMed; (b) C. Jing, S. Xin, Z. Hui and Q. Yinbo, Enzyme Microb. Technol., 2006, 39, 501 CrossRef; (c) L. F. Sophia, S. R. Wallner, W. B. Bowne, M. D. Hagler, M. E. Zenilman, R. Gross and M. H. Bluth, J. Surg. Res., 2008, 148, 77 CrossRef CAS.
- (a) I. N. A. Van Bogaert, K. Saerens, C. D. Muynck, D. Develter, W. Soetaert and E. J. Vandamme, Appl. Microbiol. Biotechnol., 2007, 76, 23 CrossRef CAS; (b) H. Mager, R. Röthlisberger, F. Wzgner, European patent. 0209783, 1987; (c) H. Isoda, D. Kitamoto, H. Shinmoto, M. Matsumura and T. Nakahara, Biosci., Biotechnol., Biochem., 1997, 61, 609 CrossRef CAS; (d) J. Chen, X. Song, H. Zhang, Y. B. Qu and J. Y. Miao, Appl. Microbiol. Biotechnol., 2006, 72, 52 CrossRef CAS; (e) S. L. Fu, S. R. Wallner, W. B. Bowne, M. D. Hagler, M. E. Zenilman, R. Gross and M. H. Bluth, J. Surg. Res., 2008, 148, 77 CrossRef CAS; (f) M. H. Bluth, E. Kandil, C. M. Mueller, V. Shah, Y. Y. Lin, H. Zhang, L. Dresner, L. Lempert, M. Nowakowski, R. Gross, R. Schulze and M. E. Zenilman, Crit. Care Med., 2006, 34, 188 CAS; (g) H. Vakil, S. Sethi, S. Fu, A. Stanek, S. Wallner, R. Gross and M. H. Bluth, Lab. Invest, 2010, 90, 1750 Search PubMed.
- (a) M. Kasture, S. Singh, P. Patel, P. A. Joy, A. A. Prabhune, C. V. Ramana and B. L. V. Prasad, Langmuir, 2007, 23, 11409 CrossRef CAS; (b) S. Singh, P. Patel, S. Jaiswal, A. A. Prabhune, V. Ramana and B. L. V. Prasad, New J. Chem., 2009, 33, 646 RSC.
- (a) M. C. Daniel and D. Astruc, Chem. Rev., 2004, 104, 293 CrossRef CAS; (b) P. Ghosh, G. Han, M. De, C. K. Kim and V. M. Rotello, Adv. Drug Delivery Rev., 2008, 60, 1307 CrossRef; (c) G. Han, P. Ghosh and V. M. Rotello, Nanomedicine, 2007, 2, 113 CrossRef CAS.
- (a) V. Kattumuri, K. Katti, S. Bhaskaran, E. J. Boote, S. W. Casteel, G. M. Fent, D. J. Robertson, M. Chandrasekhar, R. Kannan and K. V. Katti, Small, 2007, 3, 333 CrossRef CAS; (b) G. Fent, S. Casteel, D. Kim, R. Kannan, K. Katti, N. Chanda and K. V. Katti, Nanomed.: Nanotechnol., Biol. Med., 2009, 5, 128 CrossRef CAS.
- J. D. Gibson, B. P. Khanal and E. R. Zubarev, J. Am. Chem. Soc., 2007, 129, 11653 CrossRef CAS.
- A. Shiras, S. T. Chettiar, V. Shepal, G. Rajendran, G. R. Prasad and P. Shastry, Stem Cells, 2007, 25, 1478 Search PubMed.
- S. Dhar, E. M. Reddy, A. Shiras, V. Pokharkar and B. L. V. Prasad, Chem.–Eur. J., 2008, 14, 10244 CrossRef CAS.
- DIFPACK Ver. 1.0, Gatan Inc., Pleasanton, CA, USA, 1995 Search PubMed.
- P. R. Selvakannan, S. Mandal, S. Phadtare, R. Pasricha and M. Sastry, Langmuir, 2003, 19, 3545 CrossRef CAS.
- G. Zhang, Z. Yang, W. Lu, R. Zhang, Q. Huang, M. Tian, L. Li, D. Liang and C. Li, Biomaterials, 2009, 30, 1928 CrossRef CAS.
- D. Shenoy, W. Fu, J. Li, C. Crasto, G. Jones, C. Dimarzio, S. Sridhar and M. Amiji, Int. J. Nanomed., 2006, 1, 51 CrossRef CAS.
- J. H. Park, L. Gu, G. V. Maltzahn, E. Ruoslahti, S. N. Bhatia and M. J. Sailor, Nat. Mater., 2009, 8, 331 CrossRef CAS.
- P. V. Kamat, S. Barazzouk and S. Hotchandani, Angew. Chem., Int. Ed., 2002, 41, 2764 CrossRef CAS.
- T. Mosmann, J. Immunol. Methods, 1983, 65, 55 CrossRef CAS.
- Y. Liu, W. L. Lu, J. Guo, J. Du, T. Li, J. W. Wu, G. L. Wang, J. C. Wang, X. Zhang and Q. Zhang, J. Controlled Release, 2008, 129, 18 CrossRef CAS.
- L. M. Bareford and P. W. Swaan, Adv. Drug Delivery Rev., 2007, 59, 748 CrossRef CAS.
- M. Song, X. Wang, J. Li, R. Zhang, B. Chen and D. Fu, J. Biomed. Mater. Res., Part A, 2007, 86A, 942.
- A. Skladanowski and J. Konopa, Biochem. Pharmacol., 1993, 46, 375–382 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Confocal Z-stacking images of Texas Red Conjugated SL-GG-Au NPs, thermogravimetic analysis of DOX-SL-GG-Au-NPs and SL-GG-AuNPs, and time-dependent fluorescence spectra of DOX-SL-GG-Au NPs. See DOI: 10.1039/c0nr00598c |
| This journal is © The Royal Society of Chemistry 2011 |
