Magnetically actuated propulsion at low Reynolds numbers: towards nanoscale control
Peer
Fischer
a and
Ambarish
Ghosh
b
aFraunhofer Institute for Physical Measurement Techniques IPM, Heidenhofstrasse 8, 79110, Freiburg, Germany
bCentre for Nano Science and Engineering (CeNSE), Indian Institute of Science, Bangalore, 560012, India
First published on 10th December 2010
Abstract
Significant progress has been made in the fabrication of micron and sub-micron structures whose motion can be controlled in liquids under ambient conditions. The aim of many of these engineering endeavors is to be able to build and propel an artificial micro-structure that rivals the versatility of biological swimmers of similar size, e.g. motile bacterial cells. Applications for such artificial “micro-bots” are envisioned to range from microrheology to targeted drug delivery and microsurgery, and require full motion-control under ambient conditions. In this Mini-Review we discuss the construction, actuation, and operation of several devices that have recently been reported, especially systems that can be controlled by and propelled with homogenous magnetic fields. We describe the fabrication and associated experimental challenges and discuss potential applications.
 Peer Fischer | Peer Fischer received a BSc degree in Physics from Imperial College London and a PhD in Chemistry from the University of Cambridge. He was a visiting scientist at the European Laboratory for Nonlinear Spectroscopy in Florence, and a NATO Postdoctoral Fellow at Cornell University, before joining the Rowland Institute at Harvard as a Rowland Junior Fellow. In 2009 he received a Fraunhofer Attract award and moved his lab from Harvard to the Fraunhofer Institute for Physical Measurement Techniques IPM in Freiburg, Germany. His research interests are linear- and nonlinear spectroscopy, magnetic and electric properties of matter, and the physical chemistry of chirality and chiral nanostructures. Peer Fischer is on the editorial board of the journal Chirality. |
 Ambarish Ghosh | Ambarish Ghosh received a Five year Integrated MSc in Physics from the Indian Institute of Technology, Kharagpur, India. He worked on the optical and acoustic properties of electron bubbles in superfluid helium during his PhD in Physics at Brown University, USA. Subsequently, he moved to Rowland Institute at Harvard University as a post-doctoral fellow, where he worked on various aspects of chirality in the molecular and colloidal length scales. Currently, he is an Assistant Professor in the Center for Nano Science and Engineering at the Indian Institute of Science, Bangalore. His research interests include the study of optical and hydrodynamic properties of nanoscale objects. |
I. Introduction
Considerable progress has been achieved in manipulating nanoscale objects3 on surfaces and in high-vacuum environments. Attaining a similar level of control in fluids would no doubt revolutionize5 the scope of possible applications of nanotechnology. Particularly attractive are applications in biology and medicine, where it has been suggested that microrobots (micro-bots) will one day enable active drug delivery6 or perform micro-surgery,7 perhaps even at the cellular level.Significant advances have been made in manipulating larger scale objects in liquids and tissue, for instance a 1 cm ferromagnetic screw has been propelled through beef with a rotating magnetic field8 and a 1.5 mm ferromagnetic bead has been navigated in a carotid artery by the magnetic gradient fields of an MRI instrument.9 Attaining a high level of control for smaller devices (<millimetre) is, however, far from trivial, since motion at small length scales is dominated by viscous forces. For an object of characteristic dimension L, moving at a speed u, in a fluid of density ρ, and viscosity η, the Reynolds number, given by 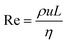 , represents the ratio of inertial to the viscous forces in the flow. The motion of micron-scale objects, including most micro-organisms, such as bacteria, are characterized by very low Reynolds numbers, e.g. 10−4 for E. coli moving at typical speeds of ∼20 μm s−1 in water. (For comparison: the Reynolds number describing a human swimmer is some nine orders of magnitude larger). In low Reynolds number hydrodynamics10 (with Re ≪ 1) the inertial terms in the Navier–Stokes equation can be neglected, leaving a simplified expression, known as the Stokes equation. This is a linear equation resulting in flows proportional to the applied forces, given by ∇p = η∇2
, represents the ratio of inertial to the viscous forces in the flow. The motion of micron-scale objects, including most micro-organisms, such as bacteria, are characterized by very low Reynolds numbers, e.g. 10−4 for E. coli moving at typical speeds of ∼20 μm s−1 in water. (For comparison: the Reynolds number describing a human swimmer is some nine orders of magnitude larger). In low Reynolds number hydrodynamics10 (with Re ≪ 1) the inertial terms in the Navier–Stokes equation can be neglected, leaving a simplified expression, known as the Stokes equation. This is a linear equation resulting in flows proportional to the applied forces, given by ∇p = η∇2![[u with combining right harpoon above (vector)]](https://www.rsc.org/images/entities/i_char_0075_20d1.gif) , where the pressure in the liquid is given by p, and
, where the pressure in the liquid is given by p, and ![[u with combining right harpoon above (vector)]](https://www.rsc.org/images/entities/i_char_0075_20d1.gif) is the flow velocity. The resultant fluid flow is instantaneous and time-reversible, and the flow parameters only depend on the time-dependent boundary conditions with no other explicit time-dependence.
is the flow velocity. The resultant fluid flow is instantaneous and time-reversible, and the flow parameters only depend on the time-dependent boundary conditions with no other explicit time-dependence.
Objects that swim, i.e. move by deforming their body-shape,1,11,12 have to execute non-reciprocal motion at low Reynolds number in order to overcome the viscous drag forces.1,11,12 The shape-changes of the swimmer have to follow an asymmetric time-sequence. Micro-organisms, such as bacteria and spermatozoa achieve this with screw-like and flexible oar-like (non-reciprocal) movements respectively (see Fig. 1).1,13–16 Some of the artificial micro-swimmers we describe in this Mini Review mimic the swimming strategies of biological microorganisms.
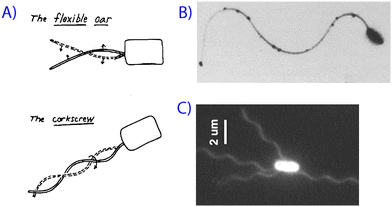 | ||
| Fig. 1 Microorganisms swimming at low Reynolds number. (A) Purcell's schematic of different types of swimming motion,1 along with (B) a scanning electron micrograph of a spermatozoa2 and (C) a fluorescence image of bacterial flagella.4 Reprinted with permission from references 1, 2 and 4. | ||
Small particles or objects that are moved or pulled through solution are in general not subject to the symmetry constraints of “non-reciprocity”. A spherical micro- or nanoparticle can for instance be ‘pulled’ through a fluid with the help of electric or magnetic forces, just as the gravitational force will act on symmetric and asymmetric particles alike and give rise to sedimentation. However, viscous drag still dominates and the motion is described by the Stokes equation. Several of the micro-particles and -bots described in this review are ferromagnetic or paramagnetic and are propelled with the aid of an externally applied magnetic field (gradient).
Apart from the shape and the symmetry it is also important to consider the control-mechanism and the source of power for the locomotion of the micro-bots and in section II we compare control by magnetic fields vs. other methods, especially from the perspective of biological applications. We briefly mention some of the non-magnetic methods in section III, but refer the interested reader to the literature,17–19 as we focus our review on magnetic actuation and control. In section IV we discuss how magnetic control has been implemented in self-propelled devices. In section V we survey several micro-bots which are powered and steered by homogeneous magnetic fields. In section VI, we describe swimming micro-organisms that are used in conjunction with magnetic fields. Finally, we discuss some potential applications and challenges that have to be met.
II. Control by magnetic fields
Magnetic fields are less invasive than other forms of actuation and are — due to the widespread use of magnetic resonance imaging (MRI) — accepted in the medical field and in biological environments. Magnetic tweezers have been used to move and manipulate micro- and nano-particles in cells for micro-rheological studies,20–22 and the gradient forces from magnets are routinely employed in vitro to manipulate paramagnetic and superparamagnetic beads in microfluidic devices23 and biological24–27 applications.Magnetic fields and magnetic field gradients can be produced with strong permanent ferromagnets. However, their use for manipulating and propelling particles entails a number of experimental difficulties in that a change in the field direction or strength invariably requires that the magnets are moved and repositioned in space. This is cumbersome and slow, and we therefore focus on electromagnets. A typical configuration for producing homogeneous or gradient magnetic fields are two stacked current carrying coils that share a common axis and are separated by a gap. In the Helmholtz coil configuration, the current direction in the two coils is the same, giving rise to a homogenous field at the geometrical centre of the coil system. If the direction of the currents in the two coils is opposite, gradient fields can be generated (Maxwell coil configuration). Three orthogonal Helmholtz coil pairs can be used to control the magnetic field in three dimensions. It is also possible to consider a combination of homogenous and gradient magnetic fields using a two-pair coil system.28 More sophisticated control of magnetic fields in space and time can be achieved with more complex geometries.29
A magnetic moment ![[m with combining right harpoon above (vector)]](https://www.rsc.org/images/entities/i_char_006d_20d1.gif) , either permanent as in a ferromagnet, or induced as in a paramagnet, experiences a translational force
, either permanent as in a ferromagnet, or induced as in a paramagnet, experiences a translational force ![[F with combining right harpoon above (vector)]](https://www.rsc.org/images/entities/i_char_0046_20d1.gif) = ∇(
= ∇(![[m with combining right harpoon above (vector)]](https://www.rsc.org/images/entities/i_char_006d_20d1.gif) ·
·![[B with combining right harpoon above (vector)]](https://www.rsc.org/images/entities/i_char_0042_20d1.gif) ) in a magnetic flux density,
) in a magnetic flux density, ![[B with combining right harpoon above (vector)]](https://www.rsc.org/images/entities/i_char_0042_20d1.gif) . For a ferromagnetic particle, it is possible to impart a permanent magnetic moment by applying an external homogenous magnetic field larger than the coercive field of the ferromagnetic material. A magnetic moment may also experience a torque
. For a ferromagnetic particle, it is possible to impart a permanent magnetic moment by applying an external homogenous magnetic field larger than the coercive field of the ferromagnetic material. A magnetic moment may also experience a torque ![[T with combining right harpoon above (vector)]](https://www.rsc.org/images/entities/i_char_0054_20d1.gif) =
= ![[m with combining right harpoon above (vector)]](https://www.rsc.org/images/entities/i_char_006d_20d1.gif) ×
× ![[B with combining right harpoon above (vector)]](https://www.rsc.org/images/entities/i_char_0042_20d1.gif) . The magnetic moment induced by the applied field in a paramagnet is given by
. The magnetic moment induced by the applied field in a paramagnet is given by 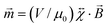 , where
, where 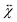 is the dimensionless susceptibility tensor and μ0 the magnetic constant. A ferromagnet has a unique direction associated with its magnetic moment, whereas the magnetic moment in a paramagnet is induced by the magnetic field. The force acting on a ferromagnetic particle depends linearly on the magnetic flux density, whereas for a paramagnetic particle it depends quadratically on the flux density. For a spherical paramagnet the translational force in a gradient is given by
is the dimensionless susceptibility tensor and μ0 the magnetic constant. A ferromagnet has a unique direction associated with its magnetic moment, whereas the magnetic moment in a paramagnet is induced by the magnetic field. The force acting on a ferromagnetic particle depends linearly on the magnetic flux density, whereas for a paramagnetic particle it depends quadratically on the flux density. For a spherical paramagnet the translational force in a gradient is given by ![[F with combining right harpoon above (vector)]](https://www.rsc.org/images/entities/i_char_0046_20d1.gif) = (V/μ0)Δχ(
= (V/μ0)Δχ(![[B with combining right harpoon above (vector)]](https://www.rsc.org/images/entities/i_char_0042_20d1.gif) ·∇)
·∇)![[B with combining right harpoon above (vector)]](https://www.rsc.org/images/entities/i_char_0042_20d1.gif) , where Δχ is the difference in magnetic susceptibility between the particle and the surrounding volume. Most media, including water, are diamagnetic and here the susceptibility is negative. If the susceptibility is positive, then the material is paramagnetic or ferromagnetic and the magnetic field strength is enhanced by the material. The forces and torques respectively in a gradient and homogenous field are schematically shown in Fig. 2. Only if a paramagnet is shape-anisotropic, such as in a rod-shaped particle, may a homogenous magnetic field exert a torque30,31 on the paramagnet, as there then also exists a difference in its susceptibility tensor components. The component along the long axis (χ∥) is larger than that along the short axis (χ⊥) and it follows that the torque will align the paramagnet such that its long axis is parallel to the field.
, where Δχ is the difference in magnetic susceptibility between the particle and the surrounding volume. Most media, including water, are diamagnetic and here the susceptibility is negative. If the susceptibility is positive, then the material is paramagnetic or ferromagnetic and the magnetic field strength is enhanced by the material. The forces and torques respectively in a gradient and homogenous field are schematically shown in Fig. 2. Only if a paramagnet is shape-anisotropic, such as in a rod-shaped particle, may a homogenous magnetic field exert a torque30,31 on the paramagnet, as there then also exists a difference in its susceptibility tensor components. The component along the long axis (χ∥) is larger than that along the short axis (χ⊥) and it follows that the torque will align the paramagnet such that its long axis is parallel to the field.
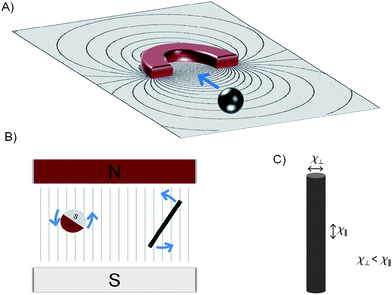 | ||
| Fig. 2 Forces and torques for paramagnetic and ferromagnetic particles in (A) gradient and (B) homogenous magnetic fields. Both a spherical ferromagnetic particle and an anisotropic paramagnetic rod-shaped structure are shown. The latter is depicted in (C) with anisotropy in the magnetic susceptibility. | ||
It is important to consider material properties while designing ferromagnetic or paramagnetic micro-bots, since ferromagnetic materials show superparamagnetic behavior as their size is reduced below a certain critical dimension. The loss of ferromagnetism is essentially due to the absence of the motion of domain walls at sizes approaching a critical size, when the formation of domain walls becomes energetically unfavorable. Single domain magnetic moments are formed below some critical size (see Table 1) and as the sizes are reduced further, the ferromagnetic materials become superparamagnetic. It follows that paramagnetic micro-bots can in principle be smaller than those that rely on a ferromagnetic moment. However, the net magnetic moment will also decrease with size (volume).
| Material | Critical size/nm |
|---|---|
| Co | 70 |
| Fe | 14 |
| Ni | 55 |
| Fe3O4 | 128 |
| γ-Fe2O3 | 166 |
It should be noted that the single domain size shown in Table 1 assumes the absence of shape anisotropy. Although coercivity is higher in a shape-anisotropic ferromagnetic nanoparticle, the critical size to form single domains is larger in shape-anisotropic particles. The coercivity and remanence depend strongly on the material's preparation and are seldom universal and have therefore not been listed.
Both magnetic field gradients and homogenous magnetic fields can be used to propel and control magnetic devices. It is also possible to combine forces due to gradients with torques from homogenous fields.29 However, an important aspect in considering the respective merits of homogenous and gradient fields is the system size. It has been shown that generating gradient forces over larger distances to propel objects, as may be required for in vivo applications, quickly becomes impractical when considering the capabilities of magnetic field sources.33 We therefore place the emphasis of this review on homogenous fields, although a few notable exceptions are mentioned.
III. Control and propulsion by other methods
While the focus of this review are micro- and nanoscale objects, let us mention that several techniques have been used to move or manipulate34,35 components that are in the size range 100 μm or above. The method of propulsion has quite often been magnetic8,9,36–38 fields, although novel control techniques based on thermal,39 electrostatic40,41 and piezoelectric42 properties, and electric fields43,44 – to name but a few – have been used for larger devices.Several “non-magnetic” methods have also been developed that can position and move micro-scale objects in fluidic environments. Optical tweezers45,46 for instance make it possible to trap and independently move a large number of particles.While tweezers provide valuable information in many biophysical problems, especially those involving rheological measurements, the optical traps are typically created very close to a microscope objective in a transparent sample and require a laser beam. This in turn excludes in vivo applications, where either proximity to the biological sample or using a laser beam is undesirable or not possible.
Other methods of moving particles in liquids rely on chemical reactions (for a review, see ref. 47) and we mention those in section IV that include some form of magnetic control.
IV. Magnetic control in self propelled devices
Self propelling devices that do not require an outside source of energy for their locomotion form a fascinating platform for addressing interesting scientific and technological questions. In the last few years a large number of such systems have been developed, with the majority being powered by chemical reactions.47 A large volume of work is centered on bi-metallic rods, which is presently believed to move by self electrophoresis or the ejection of gas bubbles, in a suitable fluidic medium.48–51 The first demonstrations made use of gold–platinum52 and gold–nickel53 rods in hydrogen peroxide. The nanorods typically follow an uncontrolled trajectory.However, an interesting development is to introduce control via magnetic means in the nanostructures. One of the earliest demonstrations54 was achieved in a striped (Pt/Ni/Au/Ni/Au) nanorod, where the ferromagnetic nickel segments were magnetized in a direction perpendicular to the rod axis. The orientation of the rods was perpendicular to the applied magnetic field direction and they moved in a direction such that the platinum pointed in the direction of motion. It was clearly shown that the power was entirely derived from the metal-catalyzed decomposition of H2O2 into oxygen and water. The magnetic field only controlled the direction of motion. The calculated magnetic moment of the rod was about 10−15 A m2, which under the action of an applied magnetic field of 550 Gauss gave rise to a magnetic torque about four orders of magnitude larger than the thermal energy of rotation (∼ 10−21 N m). The level of control was shown by moving the rods along a trajectory (see Fig. 3A), showing the letters “PSU”. A similar device was attached to a polystyrene55 microparticle and was magnetically steered. Recently, a catalytic nanomotor (Au/Ni/Au/Pt-CNT) has been used to carry and deliver56 a micron sized polystyrene bead coated with iron oxide in a microfluidic device (see Fig. 3B). The incorporation of carbon nanotubes (CNT) in the device led to increased speeds. Rapidly turning the device detached the magnetic cargo from the nanorod. Very recently, complex motion has been observed in a catalytic nanomotor, which consists of two components interacting magnetically to form a “helicopter”57 like system. In a different system, powered by microjets of oxygen bubbles, magnetic steering has been demonstrated in a larger conical microtubule, which was formed by self-scrolled thin-film layers.58
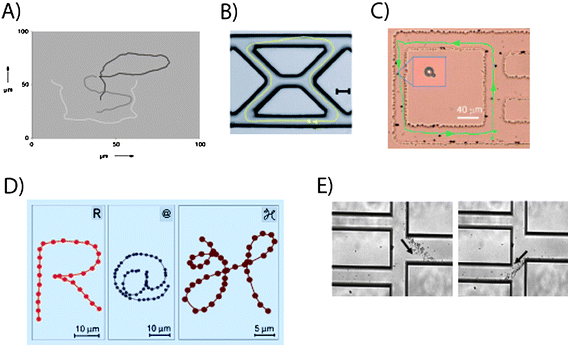 | ||
| Fig. 3 Directionality and control with magnetic fields. (A) Trajectory of a PT/Ni/Au/Ni/Au nanorod54 writing “PSU” in H2O2. (B) Directed Au/Ni/Au/Pt-CNT nanomotor56 motion in a PDMS device (scale bar 25 μm). (C) Controlled motion of a colloidal doublet59 in a glass etched microchannel. (D) Trajectory of nanopropellers63 that ‘write’ “R@H” in solution with micron precision. (E) Swimming path of a swarm of MTB70 controlled in a microfluidic device. Reprinted with permission from references 54, 56, 59, 63 and 70. | ||
V. Magnetically powered and controlled devices
Several systems have also been developed where micron and nano-sized objects are powered and controlled by homogeneous time-varying magnetic fields.One would expect that time-varying homogenous magnetic fields can propel ferromagnetic structures (vide infra), and that paramagnetic structures in general require gradient forces. However, a coupling effect together with symmetry-breaking near a surface has been used to demonstrate propulsion of paramagnetic structures with a homogenous external magnetic field.59 Two paramagnetic beads of differing diameters (2.8 μm and 1.0 μm) were coupled by mixing polystyrene paramagnetic microparticles coated with streptavidin and linked to each other by biotin-terminated cDNA strands. The polystyrene was doped with iron oxide powder which made the bead-doublets paramagnetic. An externally applied (gradient-free) magnetic field was made to precess (say around the y-axis) such that it induced precession of the bead-doublet around the same axis, which in turn caused the doublet to move along the x-axis. The beads were placed very close to a glass surface (surface normal along the z-axis) and it is important to note that the presence of the glass plate was crucial for the net linear motion60 in this system. In effect, the viscous friction due to the proximity of the glass plate is higher when the smaller particle is closer to the plate, which in turn breaks the time reciprocity of the system. The motion was controllable (see Fig. 3C) and it was possible to steer the doublets along shallow channels in a microfluidic device.
Although counterintuitive due to symmetry constraints, unrestricted 3D motion with paramagnetic beads has been achieved by time varying homogenous magnetic fields.61 In this strategy, through cooperative and anisotropic magnetic effects of the paramagnetic beads, it was possible to induce magnetic dipole moments, which were not in the direction of the applied magnetic field. The resultant torque induced motion in this novel artificial swimmer61 mimicked the motion of spermatozoa. The swimmer was built by linking several superparamagnetic colloidal beads and attaching the microstructure to a red blood cell. The short links of the flexible filament were made of ∼100 nm long strands of DNA. The external magnetic field caused dipole–dipole interactions between the beads. Also, the magnetic susceptibility of the beads was anisotropic, i.e. the beads contained a preferred direction of magnetization. During assembly, the minimum energy configuration was achieved by aligning the preferred direction of magnetization of the beads along the length of the filament. A constant axial field along the length of the filament superimposed onto a sinusoidally varying transverse field caused the microstructure to undergo an undulating motion, where a bending wave propagated from the free end towards the red blood cell, causing a net motion in the direction towards the free end. This is exactly opposite to spermatozoa where the bending waves propagate from head to tail. The applied fields had amplitudes around a hundred Gauss and frequencies around tens of Hertz.
Ferromagnetic structures naturally couple to homogenous magnetic fields and the direction in their motion can further be ensured if the structures themselves are non-centrosymmetric. This is for instance the case for chiral structures that are naturally distinct from their mirror image, and that similar to bacterial flagella, rely on cork-screw motion. Helical and screw-shaped artificial structures (See Fig. 4) have recently been developed in length scales ranging from a few tens62 of microns down to one micron.63
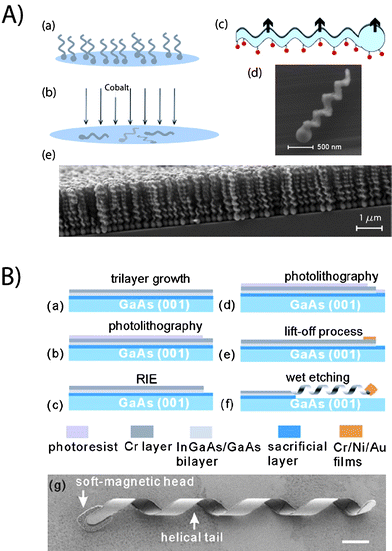 | ||
| Fig. 4 Fabrication details for screw-like and helical structures. (A) Micron sized propellers63 are made by the evaporation scheme “GLAD”. The steps consist of (a) glancing angle deposition and (b) evaporation of a ferromagnetic material. (c) Schematic of the nanopropeller, where the fluorescent molecules attached to the silica surface have been indicated. SEM pictures of the (d) individual propeller and (e) nanostructured thin film have been shown (B) Helices of approximate 50 μm length are fabricated with a self-scrolling technique62 based on (a) trilayer growth, (b) photolithography, (c) reactive ion etch, (d) photolithography, (e) lift-off and (f) wet etching processes. (g) SEM picture of the self-scrolled helix (scale bar is 4 μm). Reprinted with permission from references 62 and 63. | ||
The principle of operation of the helical and screw-like structures is that the magnetic moment is permanent and orthogonal to the long axis. The externally applied homogenous magnetic field exerts a torque by coupling to the magnetic moment of the micro-bot. A magnetic field rotating (in a plane orthogonal to the axis of the helix) will rotate the micro-bot which in turn will – due to its rotation-translation coupling – propel like a screw forwards or backwards, depending on the sense of the rotation. The viscous drag force ensures that the micro-bots move along the direction of the long axis.
A system of magnetic helices has been developed where the typical lengths of the devices62,64 were around 50 microns, with the method of propulsion also based on rotating magnetic fields. This device consisted of a helical tail resembling that of a flagellum and a soft-magnetic head in the shape of a thin square plate. The fabrication is primarily based on a self-scrolling technique. First, an AlGaAs sacrificial layer and an InGaAs/GaAs bilayer are epigrown on a GaAs (001) wafer by molecular beam epitaxy. After the deposition of a 15 nm Cr layer, the InGaAs/GaAs/Cr trilayer is patterned to a ribbon-like mesa for the helical tail by reactive ion etching. Then, the soft-magnetic head is made by depositing thin films of Cr/Ni/Au (thickness of 10/180/10 nm) and a subsequent lift-off process, whose dimensions were 4.5 μm × 4.5 μm × 200 nm. After the “top-down” fabrication processes, the entire patterned mesa is released from the substrate using a 2% HF aqueous solution that etches the AlGaAs sacrificial layer. The released trilayer automatically scrolls into a helix in order to minimize the internal stress. In the experiments two types of devices have been prepared, one with an InGaAs/GaAs semiconductor bilayer tail and the other with an InGaAs/GaAs/Cr-hybrid semiconductor–metal trilayer tail. The InGaAs/GaAs/Cr helical tail was more robust than the bilayer helical tail for placing the helical micro-bots in liquid. The InGaAs/GaAs/Cr trilayer has thicknesses of 11/16/15 nm, respectively, the ribbon width is 1.8 μm, and the diameter of the InGaAs/GaAs/Cr structure is 2.8 μm. It is possible to precisely control the geometrical shape of the helical tail, e.g., chirality, helicity angle, diameter etc. The devices have been driven at speeds ∼ 14 μm s−1 by applying magnetic fields with amplitude around 20 Gauss rotating at around 20 Hz.
A different fabrication scheme permits large numbers of micron sized colloidal screw-propellers63 to be made in a single evaporation run. The method is based on “glancing angle deposition” (GLAD), a physical vapor deposition technique where the incident vapor flux is incident at extreme grazing angles on a substrate. By manipulating the substrate during the deposition and in combination with geometric shadowing, a wide variety of nano-structured surface morphologies can be obtained with a number of materials, including oxides such as silicon dioxide (glass). The general method (for a review, see M. M. Hawkeye and M. J. Brett, J. Vac. Sci. Technol., A, 2007, 25, 1317) of fabricating chiral screw-propellers (with sizes between 1 to 2 μm) is to rotate the substrate azimuthally with a fixed tilt at an extreme grazing angles on a substrate, typically around 85 degree angle with a modest angular speed. The observed pitch is typically given by the ratio of the deposition rate (few Ångstrom s−1) to the angular speed (∼0.1 rpm). In order to obtain large numbers of identical nano-propellers, the substrate is seeded prior to the deposition. This involves the fabrication of arrays of relatively ordered seeds on the substrate prior to the GLAD process. The size and shape of the seeds is critical in determining the morphology of the resultant nano-structured surface. The typical thickness of the screws is a few hundred nm, which requires arrays of billions of highly uniform sub-micron seeds on a typical wafer substrate. The subsequent step in the fabrication of the nano-propellers is to render them ferromagnetic. A material with a high remnant magnetization ensures the propellers can be rotated in small magnetic field strengths (∼50 Gauss). At the same time, the coercivity needs to be high enough that the magnetization is not rewritten by the driving field. The propellers are actuated by a tri-axial Helmholtz coil. A very high level of control can be achieved on the micron-scale, as shown in Fig. 3D. Under magnetic field amplitudes of around 60 Gauss and frequencies around 140 Hz, the propellers moved at speeds more than 20 body lengths per second (>40 μm s−1), which is comparable to the speed of motile bacterial cells.
VI. Magnetotactic bacteria
Several micro-organisms have evolved swimming strategies and therefore possess self-propelled micro-engines. It is natural to wonder whether the sophisticated ‘machinery’ of these swimmers can be coupled to artificial structures or whether they can be steered externally. Indeed, bacterial manipulation for transporting polystyrene beads or PDMS structures of several microns has been achieved by attaching the bacteria (bacterial carpets65) to the artificial structures. In many of these studies, moderate motion control was achieved by influencing the chemical environment of chemotactic bacteria66 or by affecting phototactic bacteria with UV light.67Magnetic control has been demonstrated with magnetotactic bacteria (MTB), which have also been used for transporting micron sized cargo. Magnetospirillum gryphiswaldense bacteria, which are about 1–3 μm long, were moved at speeds 40–80 μm s−1.68 Each MTB contains a chain of magnetosomes, which are ferromagnetic69nanoparticles containing iron oxide. The chain orients along the direction of magnetic field, thus allowing for external control of the MTB. 3-μm diameter microbeads were attached to the MTB and transported with speeds up to 20 μm s−1. The field strengths were typically very small, less than a few Gauss. Very recently,70 commercial MRI systems have been used to track and control the MTB. The high contrast in MRI imaging is automatically provided by the iron oxide in the magnetosomes, and the steering control (see Fig. 3E) was achieved by placing three magnetic coils located outside the MRI bore. The small size of the MTBs means that they can enter the smallest blood vessels (micro-vasculature) and swim at high speeds (some MTBs have been reported70 to move at speeds of 300 μm s−1)
VII. Conclusions and outlook
The main focus of this Mini Review is the controlled manipulation of micro- and nano-sized objects using homogenous magnetic fields. Magnetic fields are an attractive means to actuate ferro- and paramagnetic micro- and nano-structures in fluidic and biological environments. We have surveyed the main coupling and actuation mechanisms and have shown that – while this field of research is still in its nascent stage – several devices have already been fabricated that can be controlled in or with magnetic fields in low Reynolds number environments.Some of the smallest objects that have been moved in solutions to date are catalytically powered ‘self-propelled’ magnetic nanoparticles. The propulsion forces derive from chemical reactions and easily overcome thermal forces and often exceed those that can be obtained in magnetically powered systems. The addition of magnetic fields to steer these ‘nano-rockets’ offers some control. The primary ‘steering’ mechanism is via ferromagnetic coupling to a homogenous magnetic field which in principle allows for full directional control. The challenge for biological and biomedical applications is to increase the level of control in some catalytic systems and to effectively embrace ‘benign’ reactions that are biocompatible, such as glucose–oxygen reactions.71 An exciting prospect is to maintain or even replenish the fuel supply, which would allow continued operation for long periods of time.
An interesting micro-robotic concept is the hybrid system, where a biological micro-organism is moved subject to external control, or where a sophisticated biological motor is coupled to artificial structures. Taming and effectively controlling these organic robots remains an active area of research.
Homogenous magnetic fields offer control from a distance and are fully biocompatible. In general only modest magnetic field strengths are required. The first examples of magnetically controlled and powered micro-bots have been demonstrated that suggest applications in micro-surgery and drug delivery. The systems can be navigated with a high degree of control. Some fabrication schemes permit large numbers (billions) of micro-bots to be fabricated. This will be important for many applications, as the payload that micro-bots can carry is limited. Their use in applications like drug delivery will require the simultaneous action and control of large numbers (swarms) of micro-bots.
For many applications, including in vivo usage, it is essential to visualize the micro-bots. Non-optical methods, such MRI imaging is useful – and paramagnetic and ferromagnetic microparticles enhance the MRI contrast and can thus be observed, except where the high magnetic field typically associated with MRI instrumentation influences the magnetization72 of micro-bots, particularly in ferromagnetic systems actuated with homogenous magnetic fields. In this case, there is a need for alternative imaging modalities that offer the required resolution to visualize the micro-bots in situ in 3D.
In summary, the field of propulsion at the nanoscale using magnetic methods is a truly inter-disciplinary effort that extends the reach of nanomanipulation into fluids and soft or porous systems. It will be particularly exciting to see the first biological applications of actively propelled and controlled micro- and nano-particles. Active nanoparticles are a fascinating development with numerous potential applications. We expect that this exciting area of research will make important contributions to diverse fields, ranging from microrheological studies in soft matter physics to micro-surgery and targeted drug delivery in nano-medicine.
References
- E. M. Purcell, Am. J. Phys., 1977, 45, 3.
- C. J. Brokaw, J. Cell Biol., 1991, 114, 1201 CrossRef CAS.
- A. A. G. Requicha, Proc. IEEE, 2003, 9, 1922 CrossRef.
- L. Turner, W. S. Ryu and H. C. Berg, J. Bacteriol., 2000, 182, 2793 CrossRef CAS.
- G. A. Ozin, I. Manners, S. Fournier-Bidoz and A. Arsenault, Adv. Mater., 2005, 17, 3011 CrossRef CAS.
- K. K. Coti, M. E. Belowich, M. Liong, M. W. Ambrogio, Y. A. Lau, H. A. Khatib, J. I. Zink, N. M. Khashab and J. F. Stoddart, Nanoscale, 2009, 1, 16 RSC.
- J. R. A. Freitas, Int. J. Surg., 2005, 3, 243 Search PubMed.
- K. Ishiyama, M. Sendoh, A. Yamazaki and K. I. Arai, Sens. Actuators, A, 2001, 91, 141 CrossRef.
- S. Martel, J.-B. Mathieu, O. Felfoul, A. Chanu, E. Aboussouan, S. Tamaz, P. Pouponneau, L. H. Yahia, G. Beaudoin, G. Soulez and M. Mankiewicz, Appl. Phys. Lett., 2007, 90, 114105 CrossRef.
- J. Happel, H. Brenner, Low Reynolds Number Hydrodynamics, Prentice-Hall, Englewood Cliffs, NJ, 1965 Search PubMed.
- W. Ludwig, J. Comparative Physiol. A, 1930, 13, 397–504 Search PubMed.
- G. I. Taylor, National Committee for Fluid Mechanics Films (NCFMF), “Low Reynolds Number Flow”, http://web.mit.edu/hml/ncfmf.html, 1967.
- H. C. Berg, E. coli in Motion, New York: AIP Press/Springer-Verlag., 2004 Search PubMed.
- C. Brennen and H. Winet, Annu. Rev. Fluid Mech., 1977, 9, 339 CrossRef.
- E. Lauga and T. R. Powers, Rep. Prog. Phys., 2009, 72, 096601 CrossRef.
- G. T. Yates, Am. Sci., 1986, 74, 358–365.
- J. Wang and K. M. Manesh, Small, 2010, 6, 338 CrossRef CAS.
- S. J. Ebbens and J. R. Howse, Soft Matter, 2010, 6, 726 RSC.
- T. Mirkovic, N. S. Zacharia, G. D. Scholes and G. A. Ozin, Small, 2010, 6, 159 CrossRef CAS.
- A. R. Bausch, W. Möller and E. Sackmann, Biophys. J., 1999, 76, 573 CrossRef CAS.
- F. Amblard, B. Yurke, A. Pargellis and S. Leibler, Rev. Sci. Instrum., 1996, 67, 818 CrossRef CAS.
- A. H. B. de Vries, B. E. Krenn, R. van Driel and J. S. Kanger, Biophys. J., 2005, 88, 2137 CAS.
- N. Pamme, Lab Chip, 2006, 6, 24 RSC.
- J.-W. Choi, K. W. Oh, J. H. Thomas, W. R. Heineman, H. B. Halsall, J. H. Nevin, A. J. Helmicki, H. T. Henderson and C. H. Ahn, Lab Chip, 2002, 2, 27 RSC.
- M. A. M. Gijs, Microfluidics Nanofluidics, 2004, 1, 22 CAS.
- J. Pipper, M. Inoue, L. F. P. Ng, P. Neuzil, Y. Zhang and L. Novak, Nat. Med., 2007, 13, 1259 CrossRef CAS.
- I. M. Hsing, X. Ying and Z. Wenting, Electroanalysis, 2007, 19, 755 CrossRef CAS.
- H. Choi, et al, Smart Mater. Struct., 2009, 18, 055007 CrossRef.
- M. P. Kummer, J. J. Abbott, B. E. Kratochvil, R. Borer, A. Sengul and B. J. Nelson, 2010 IEEE International Conference on Robotics and Automation, 2010 DOI:10.1109/ROBOT.2010.5509241.
- P. Tierno, J. Claret, F. Sagués and A. Cēbers, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2009, 79, 021501 CrossRef.
- J. A. Stratton, Electromagnetic Theory, McGraw-Hill, New York, 1941 Search PubMed.
- D. L. Leslie-Pelecky and R. D. Rieke, Chem. Mater., 1996, 8, 1770 CrossRef CAS.
- J. J. Abbott, K. E. Peyer, M. C. Lagomarsino, L. Zhang, L. Dong, I. K. Kaliakatsos and B. J. Nelson, Int. J. Rob. Res., 2009, 28, 1434 CrossRef.
- E. W. H. Jager, O. Inganas and I. Lundstrom, Science, 2000, 288, 2335 CrossRef CAS.
- T. G. Leong, C. L. Randall, B. R. Benson, N. Bassik, G. M. Stern and D. H. Gracias, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 703 CrossRef CAS.
- M. S. Sakar, E. B. Steager, D. H. Kim, M. J. Kim, G. J. Pappas and V. Kumar, Appl. Phys. Lett., 2010, 96, 043705 CrossRef.
- K. Vollmers, D. R. Frutiger, B. E. Kratochvil and B. J. Nelson, Appl. Phys. Lett., 2008, 92, 144103 CrossRef.
- M. T. Hou, H.-M. Shen, G.-L. Jiang, C.-N. Lu, I. J. Hsu and J. A. Yeh, Appl. Phys. Lett., 2010, 96, 024102 CrossRef.
- O. J. Sul, M. R. Falvo, R. M. Taylor Ii, S. Washburn and R. Superfine, Appl. Phys. Lett., 2006, 89, 203512 CrossRef.
- B. R. Donald, C. G. Levey, C. D. McGray, I. Paprotny and D. Rus, J. Microelectromech. Syst., 2006, 15, 1 CrossRef.
- C. Pawashe, S. Floyd and M. Sitti, Appl. Phys. Lett., 2009, 94, 164108 CrossRef.
- B. Watson, J. Friend and L. Yeo, J. Micromech. Microeng., 2009, 19, 022001 CrossRef.
- S. T. Chang, V. N. Paunov, D. N. Petsev and O. D. Velev, Nat. Mater., 2007, 6, 235 CrossRef CAS.
- Y. Osada, H. Okuzaki and H. Hori, Nature, 1992, 355, 242 CrossRef CAS.
- A. Ashkin, J. M. Dziedzic, J. E. Bjorkholm and S. Chu, Opt. Lett., 1986, 11, 288 Search PubMed.
- D. G. Grier, Nature, 2003, 424, 810 CrossRef CAS.
- M. Pumera, Nanoscale, 2010, 2, 1643 RSC.
- H. Ke, S. Ye, R. L. Carroll and K. Showalter, J. Phys. Chem. A, 2010, 114, 5462 CrossRef CAS.
- J. G. Gibbs and Y. P. Zhao, Appl. Phys. Lett., 2009, 94, 163104 CrossRef.
- J. R. Howse, R.A. L. Jones, A. J. Ryan, T. Gough, R. Vafabakhsh and R. Golestanian, Phys. Rev. Lett., 2007, 99, 048102 CrossRef.
- G. Rückner and R. Kapral, Phys. Rev. Lett., 2007, 98, 150603 CrossRef.
- W. F. Paxton, K. C. Kistler, C. C. Olmeda, A. Sen, S. K. St. Angelo, Y. Cao, T. E. Mallouk, P. E. Lammert and V. H. Crespi, J. Am. Chem. Soc., 2004, 126, 13424 CrossRef CAS.
- S. Fournier-Bidoz, A. C. Arsenault, I. Manners and G. A. Ozin, Chem. Commun., 2005, 441 RSC.
- T. R. Kline, W. F. Paxton, T. E. Mallouk and A. Sen, Angew. Chem., Int. Ed., 2005, 44, 744 CrossRef CAS.
- S. Sundararajan, P. E. Lammert, A. W. Zudans, V. H. Crespi and A. Sen, Nano Lett., 2008, 8, 1271 CrossRef CAS.
- J. Burdick, R. Laocharoensuk, P. M. Wheat, J. D. Posner and J. Wang, J. Am. Chem. Soc., 2008, 130, 8164 CrossRef CAS.
- J. G. Gibbs and Y. Zhao, Small, 1656, 6 Search PubMed.
- A. S. Alexander, M. Yongfeng, U. Esteban Bermúdez, H. Gaoshan and G. S. Oliver, Small, 2009, 5, 1688 CrossRef CAS.
- P. Tierno, R. Golestanian, I. Pagonabarraga and F. Sagués, J. Phys. Chem. B, 2008, 112, 16525 CrossRef CAS.
- P. Tierno, R. Golestanian, I. Pagonabarraga and F. Sagués, Phys. Rev. Lett., 2008, 101, 218304 CrossRef.
- R. Dreyfus, J. Baudry, M. L. Roper, M. Fermigier, H. A. Stone and J. Bibette, Nature, 2005, 437, 862 CrossRef CAS.
- L. Zhang, J. J. Abbott, L. Dong, B. E. Kratochvil, D. Bell and B. J. Nelson, Appl. Phys. Lett., 2009, 94, 064107 CrossRef.
- A. Ghosh and P. Fischer, Nano Lett., 2009, 9, 2243 CrossRef CAS.
- L. Zhang, J. J. Abbott, L. Dong, K. E. Peyer, B. E. Kratochvil, H. Zhang, C. Bergeles and B. J. Nelson, Nano Lett., 2009, 9, 3663 CrossRef CAS.
- N. Darnton, L. Turner, K. Breuer and H. C. Berg, Biophys. J., 2004, 86, 1863 CrossRef CAS.
- B. Behkam and M. Sitti, Appl. Phys. Lett., 2007, 90, 023902 CrossRef.
- E. Steager, C.-B. Kim, J. Patel, S. Bith, C. Naik, L. Reber and M. J. Kim, Appl. Phys. Lett., 2007, 90, 263901 CrossRef.
- S. Martel, C. C. Tremblay, S. Ngakeng and G. Langlois, Appl. Phys. Lett., 2006, 89, 233904 CrossRef.
- E. Alphandéry, A. T. Ngo, C. Lefévre, I. Lisiecki, L. F. Wu and M. P. Pileni, J. Phys. Chem. C, 2008, 112, 12304 CrossRef CAS.
- S. Martel, M. Mohammadi, O. Felfoul, L. Zhao and P. Pouponneau, Int. J. Rob. Res., 2009, 28, 571 CrossRef.
- N. Mano and A. Heller, J. Am. Chem. Soc., 2005, 127, 11574 CrossRef CAS.
- S. Martel, O. Felfoul, M. Mohammadi, in Biomedical Robotics and Biomechatronics, 2008. BioRob 2008. 2nd IEEE RAS & EMBS International Conference on, 2008, pp. 264 Search PubMed.
| This journal is © The Royal Society of Chemistry 2011 |
