The role of nanostructured mesoporous silicon in discriminating in vitro calcification for electrospun composite tissue engineering scaffolds
Dongmei
Fan
a,
Giridhar R.
Akkaraju
b,
Ernest F.
Couch
b,
Leigh T.
Canham†
c and
Jeffery L.
Coffer
*a
aDepartment of Chemistry, Texas Christian University, Fort Worth, TX 76129, USA. E-mail: j.coffer@tcu.edu
bDepartment of Biology, Texas Christian University, Fort Worth, TX 76129, USA
cpSiMedica Ltd., Malvern Hills Science Park, Malvern, WR14 3SZ, UK
First published on 25th November 2010
Abstract
The impact of mesoporous silicon (PSi) particles—embedded either on the surface, or totally encapsulated within electrospun poly (ε-caprolactone) (PCL) fibers—on its properties as a tissue engineering scaffold is assessed. Our findings suggest that the resorbable porous silicon component can sensitively accelerate the necessary calcification process in such composites. Calcium phosphate deposition on the scaffolds was measured via in vitro calcification assays both at acellular and cellular levels. Extensive attachment of fibroblasts, human adult mesenchymal stem cells, and mouse stromal cells to the scaffold were observed. Complementary cell differentiation assays and ultrastructural measurements were also carried out; the levels of alkaline phosphatase expression, a specific biomarker for mesenchymal stem cell differentiation, show that the scaffolds have the ability to mediate such processes, and that the location of the Si plays a key role in levels of expression.
 Dongmei Fan | Dr Dongmei Fan received her Ph.D. degree in 2008 at the Department of Chemistry, College of Science and Engineering, Texas Christian University under the supervision of Prof. Jeffery L. Coffer. After completion of her degree, she joined the Department of Nanomedicine at The University of Texas Health Science Center at Houston as a postdoctoral fellow. Her research focuses on fabrication of porous silicon/biopolymer composites for drug delivery and orthopedic tissue engineering applications. |
 Giridhar R. Akkaraju | Dr Giridhar Akkaraju, is an Associate Professor in the Department of Biology, Texas Christian University, Fort Worth where he has been since 2002. His research interests include the pathogenesis of Hepatitis C virus infection, anti-cancer drug discovery and tissue engineering. He received his PhD from the University of Pittsburgh School of Medicine in 1997. Prior to his appointment at Texas Christian University, he was a post-doctoral fellow in the laboratory of Dr Zhijian Chen at the University of Texas Southwestern Medical Center, Dallas. |
 Ernest F. Couch | Dr Ernest F. Couch is Emeritus Professor of Biology at Texas Christian University, Fort Worth, Texas. He received his Ph.D. at Tulane University in New Orleans and is a member of the corporation at the Marine Biological Laboratory in Woods Hole, Massachusetts. He spent a yearlong sabbatical at the Endocrine Institute in Maebashi Japan supported by the US-Japan Cooperative Science Program of the National Science Foundation and has frequently returned to Japan as a visiting scientist at Saitama University. He is also a past president of the Texas Society for Microscopy. |
 Leigh T. Canham | Dr Leigh Canham is currently the CSO of Intrinsiq Materials Ltd at Malvern, UK and an Honorary Professor of Physics at the University of Birmingham, UK. He has over 25 years of research and product development experience of widely differing aspects of semiconducting silicon technology. Two personal discoveries, that silicon can emit light efficiently (1990) and be rendered medically biodegradable (1995) have received significant academic and industrial attention. He co-founded pSiMedica (pSivida) Ltd in 2000 to develop BioSiliconTM - based drug delivery systems, a NASDAQ-listed company since 2005. His R&D interests are now the use of silicon materials science in nutrition, consumer care and cosmetic products. |
 Jeffery L. Coffer | Dr Jeffery L. Coffer is a Professor of Chemistry at Texas Christian University in Fort Worth, Texas. One major focus of his research is an examination of the fundamental properties of nanostructured silicon materials of potential relevance to therapy. He has published more than 110 papers, three patents, and directed the research of more than 17 doctoral students and 28 undergraduates. Among his most recent awards is the 2009 Chancellor’s Award for Distinguished Achievement as a Creative Teacher and Scholar at TCU and the 2010 Wilfred T. Doherty Award of the Dallas-Fort Worth Section of the American Chemical Society. |
Introduction
Given the great number of bone transplantations annually1 and an ever-increasing need for bone substitutes due to aging and injuries, scientists are eager to investigate and fabricate various biomaterials—ceramics, metals, natural and synthetic biopolymers—to meet the demand.2–6 The shortcomings of existing bone regeneration materials are well-known: the osteoconductivity of ceramics are offset by their brittleness and challenges in fabrication and degradation rate in vivo;7 while metals demonstrate superior mechanical strength, their poor osteoconductivity and nonoptimal biodegradability limit their use for orthopedics; the facile processing of biopolymers is offset by limited osteoinductivity and an associated poor mechanical strength.8 One general strategy to overcome such limitations is to introduce an inorganic component whose intrinsic benefit as a bone regeneration material can be tailored by control of its structure or concentration.9In this study, new fibrous platforms based on nanostructured mesoporous silicon (PSi) are described in order to explore their potential for orthopedic tissue engineering. PSi's advantages as a biomaterial are many fold, but one of the most critical is that it can be resorbed in soft tissue with a negligible inflammatory responsein vivo.10 PSi's degradation product – silicic acid, Si(OH)4 is the natural form of silicon in the human body, which can be predominantly and rapidly excreted in the urine.11 The release of silicic acid due to the corrosion of this elemental semiconductor in plasma stimulates calcification, which could ideally accelerate new bone regeneration.12
In previous studies, mesoporous PSi particles have been introduced into the biopolymer poly (ε-caprolactone) (PCL) by salt-leaching and microemulsion/freeze-drying methods in order to fabricate PSi/PCL sponges.13,14 In such systems, PCL is selected due to its biocompatible and biodegradable character that is widely exploited in guided bone regeneration and drug delivery.15 The results from this earlier work demonstrated that porous Si-containing composites may calcify in an acellular environment and support bone precursor cell proliferation and differentiation;14 however, given the relatively low Si loadings and lack of facile accessibility of the porous Si particles to the surroundings, it was difficult to sensitively assess directly the role of the siliconin vitro. Electrically conductive composite materials composed of PCL, polyaniline (PANi), and PSi have also been fabricated, which demonstrated accelerated calcification when cathodic bias was applied to the scaffolds.16
In this paper, we utilize electrospinning methods to produce a three dimensional fibrous structure of PSi/PCL of potential merit for orthopedic purposes. Electrospinning is an attractive way to fabricate biomaterials for tissue regeneration.17,18 This method offers polymer fibers with high porous surface areas and the possibility of surface modification to improve their cytocompatibility. Electrospun fibers, with tunable diameters ranging from few nanometres to a few micrometers,19,20 have been widely studied as scaffold biomaterials for vascular grafts, wound dressings, nerve regeneration and bone tissue engineering.21 We demonstrate here that the highly porous and slowly degrading PSi/PCL micro-fibrous scaffolds, with the resorbable Si at a location that more rapidly impact cellular processes, can support human mesenchymal stem cells (hMSCs) and bone-marrow-derived mouse stromal cells in their ability to proliferate, migrate, and differentiate. It is also discovered that the degradation products of PSi can stimulate the formation of calcium phosphate in mouse stromal cell overlayers.
Materials and methods
Mesoporous Si/poly-(ε-caprolactone) electrospun fiber fabrication
Mesoporous silicon particles were prepared by a three-stage process of silicon wafer anodisation, mesoporous membrane detachment and mesoporous microparticle classification. Heavily boron doped (0.01 ohm cm) 150 mm diameter wafers were anodized in methanoic HF electrolyte in custom-built equipment to create 150 μm thick membranes of 65% porosity. These were then mechanically milled and classified into porous microparticles of specific size distributions. Most PSi/PCL composites were prepared using PSi particles sieved at a cutoff value less than 45 μm; the corresponding mean surface area of such material is 248 mm2/g, and associated pore volume of 0.425 ml/g. PSi/PCL composites with PSi fully encapsulated within PCL fibers with the concentration of PSi ranging from 1% to 20% were fabricated by an electrospinning technique. To fabricate these composites, 0.5 g of PCL pellets (Aldrich, MW∼65,000) were dissolved in 2 ml of chloroform (Pharmco) under gentle shaking to obtain a 25 wt. % solution. Different amounts of PSi (65% porosity) were added to this PCL solution to achieve the target percentage loading of the PSi. The PSi/PCL mixture was stirred via vortex mixing to form a homogeneous suspension and then placed into a 5 ml glass syringe fitted with a 21 gauge needle. A high voltage of 20 kV was applied to the needle, with a tip-to-grounded Al collector distance of approximately 20 cm. The as-spun fibers were dried under vacuum at room temperature for 2 days prior to further studies.PSi/PCL composites with PSi embedded on the surface of PCL fibers with the concentration of PSi ranging from 1% to 10% were fabricated using a slightly modified version of the above method. A 25 wt. % PCL solution was used to first prepare pure PCL electrospun fibers. PSi was then embedded on the surface of PCL fibers evenly by physical pressing, followed by wetting the surface with DI water. This physical pressing process can be repeated multiple times until satisfactory coverage is obtained, with physical inspection by optical microscopy or SEM. The wet composites were placed in an oven at 110 °C for a few seconds and then dried under vacuum at room temperature for 2 days prior to further studies. This brief heating treatment softens the PCL fiber surface but does not distort the cylindrical fiber morphology. After vacuum drying, weakly bound PSi particles were readily removed from the scaffold by brief (minutes) immersion in water.
Acellular calcification assays
All acellular calcification assays were performed by soaking 1.0 cm × 1.0 cm pieces in simulated body fluid (SBF), which consisted of salt concentrations very similar to that of human blood plasma.12,22 SBF was changed every other week. Pieces were removed on weeks 1, 2, 3, 4, rinsed with DI water, and dried in low vacuum for at least two days before SEM observation.General cell culture
Transformed human embryonic kidney (293HEK) fibroblast cells and mouse stromal cells (D1-ORL-UVA) were obtained from American Type Culture Collection (ATCC). Human adult mesenchymal stem cells (hMSCs) were from the Tulane Center for Gene Therapy (New Orleans, LA). Cells were cultured in 25 cm3 tissue culture polystyrene flasks (VWR) containing growth medium and maintained at 37 °C in a humidified, 5% CO2 atmosphere. The complete medium is Modified Eagle's Medium (DMEM) (500 ml) supplemented with 10% fetal bovine serum (FBS) (Sigma-Aldrich), L-Glutamine (2 mM, VWR), 1% antibiotics (100 U/ml penicillin, 100 U/ml streptomycin) (Sigma-Aldrich). For differentation experiments, the hMSC and mouse stromal cells were exposed to differentiation medium composed of DMEM supplemented with 10% FBS, L-Glutamine (2 mM), sodium pyruvate (1.0 mM), β-glycerophosphate (10 mM), ascorbic acid–2–phosphate (0.05 mM), and dexamethasone (100 nM). Prior to exposure to a given cell type, samples with a dimension of 1.0 cm × 1.0 cm were soaked in 70% ethanol for 24 h, and then immersed in sterilized DI water for 5 min followed by UV exposure for 2 h.Cell attachment
After removal from cell culture, all samples were washed by phosphate buffered saline (PBS) to detach the dead cells; cells were fixed on the scaffold according to a modified version of a known standard protocol:23 while removing the medium incrementally, a fixative composed of a buffered mixture of 8% glutaraldhyde and 6% paraformaldhyde was added. After exposure for an hour, the cells were washed with buffer, followed by a series of careful dehydration steps using 30%, 50%,70%, 85%, 95%, 100% ethanol solution, followed by two prolonged exposures to HMDS (30 min, then overnight). The dehydrated samples were placed on aluminium thimbles with a double-sided carbon tape and sputter coated with a 15 nm thick gold layer. Observation was done using the JEOL SEM 6100 with an accelerating voltage of 20 kV.Alkaline Phosphatase (ALP) assays
Cell differentiation ability was measured by cell alkaline phosphatase activity.24 The two types of PSi/PCL scaffolds (PSi was fully encapsulated in PCL fibers and PSi embedded on the surface of PCL fibers) were used for ALP studies and their ability to direct cells to differentiate was compared. PCL only scaffolds were used as control for both types of studies. The scaffolds were sterilized by the same procedure described above. Mouse stromal cells then were plated onto the scaffolds in a 12 well cell culture plate at a density of 50,000 cells per well. The mouse stromal cells cultured in the presence of all scaffolds were fed with complete medium on day 1 and seeding in each type of scaffold was performed in six of the wells. Half of the wells for each type of scaffolds were switched to differentiation medium on day 2 and the cells in the other half of the wells were continued to be fed by complete medium. The cells cultured in the presence of PCL fibers in complete medium or differentiation medium were used as control. The cells were fed every three days. At days 3, 6, 9, and 14 in culture, the medium was aspirated and 1 ml PBS was added into each well to wash the cells as well as the scaffolds. The remainder of the assay was carried out according to a previously published spectrophotometric procedure for other scaffold types using P-nitrophenyl phosphate (pNPP) substrates.14 The experiments were performed in triplicate.Ultrastructural studies
Mouse stromal cells (10,000 cells per well) seeded in the presence of the PSi/PCL fiber scaffolds were cultured in complete medium and differentiation medium respectively. The mouse stromal cells were cultured in the presence of PCL fibers in the complete medium and differentiation medium respectively as control. Scaffolds were removed from the cell culture wells at weeks 1, 2, 3, and 4. The cells and scaffolds were washed by 1 ml of phosphate buffer per well to remove the dead cells followed by aspiration, and then fixed in a fixative for 60 min followed by washed in phosphate buffer and post-fixed in 1% osmium tetraoxide in phosphate buffer for 30 min as described previously. Cells and scaffolds were then dehydrated through increasing concentrations of alcohol followed by three immersions of propylene oxide washings. The cells were flat embedded in plastic which was made according to a previously reported procedure,25 followed by sectioning by ultramicrotone with a diamond knife. Sections were collected immediately on carbon-coated copper grids (Structure Probe. Inc) and then stained with a combination of uranyl acetate and lead citrate, and dried at room temperature. The samples were observed using a Philips EM 300 transmission electron microscope (TEM).Results and discussion
Fibrous scaffolds were prepared by electrospinning solutions containing 25 wt% PCL in chloroform and loaded with 1% to 20% concentrations of mesoporous Si (PSi) by mass. To ensure optimal dispersion of the PSi particles (Fig. 1a) in the PCL/chloroform solution, the mixture was stirred via vortex mixing until homogeneous. SEM images in Fig. 1b and 1c demonstrate that the composite fibers are randomly arrayed in a nonwoven mat. The average diameters of these composite fibers are found to depend on the concentration of PSi originally in the solution to be electrospun. For the pure PCL fibers obtained from 25 wt. % solutions, the average fiber diameter is around 8.3 μm (Table 1), while the average fiber diameter ranges from 10.4 μm to 16.2 μm as the concentration of PSi is increased from 1% to 20%. It should be noted that the composite fibers are composed of wider areas and thinner sections. PSi is expected to be located in the wider portion of a given fiber because of the possible heterogeneous dispersion of PSi in the PCL/chloroform solution. Energy dispersive X-ray (EDX) analysis further confirms the presence of PSi in the wider regions of fibers as seen in Fig. 1d. The location of the PSi particles in these wider regions of the fiber is confirmed by inspection of the composite morphology by optical microscopy, where the dark brown PSi particles are readily visualized (Fig. 1e). While the size of the mesoporous Si particles is sieved to a value less than 45 μm (Fig. 1a), analysis of the PSi/PCL composite fiber images suggest that only particles less than 25 μm are present in the encapsulated fibers.| PSi % | 0% | 1% | 5% | 10% | 20% |
|---|---|---|---|---|---|
| Diameter | 9.75 μm | 10.59 μm | 11.72 μm | 13.80 μm | 16.33 μm |
| σ | 2.12 μm | 2.25 μm | 3.06 μm | 3.29 μm | 3.89 μm |
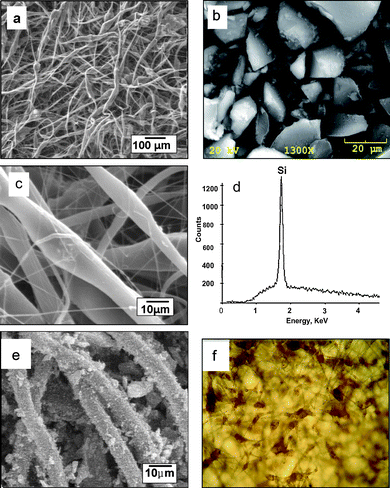 | ||
| Fig. 1 (a) SEM image of typical mesoporous silicon microparticles used in these triclosan loading experiments; SEM images of PSi/PCL fibers at (b) lower and (c) higher magnification; (d) EDX analysis confirms the PSi presents in the wider portions of the fibers; (e) PSi/PCL fibers with PSi embedded on the surface of PCL. (f) transmission optical microscopy image (400 X) of 5% PSi/PCL scaffolds (5% wt. % Si) with PSi encapsulated within the PCL fibers. PSi appears as brownish regions in the image. | ||
As indicated in previous studies,18 the fiber diameter produced by the electrospinning technique can be controlled in principle by many physical parameters including applied voltage, solvent composition, polymer solution viscosity, along with the distance between the tip of the needle and the collecting drum. To achieve the proper balance between PSi loading percentage and the viscosity of PCL solution, the most optimum experimental conditions were found to be a 25 wt. % PCL solution containing 20% PSi, a 20 kV applied voltage, and 20 cm electrode to grounding plate distance.
In order to more sensitively study the influence of PSi spatial location on calcification and osteogenic differentiation, we also fabricated PSi/PCL composites with the PSi embedded on the outer surface of PCL fibers. PCL microfibers containing the PSi particles embedded at the surface can easily be detected along a given fiber (Fig. 1d), in contrast to those structures where the presence of the PSi particles present during the electrospinning event results in a larger diameter fiber due to the presence of the encapsulated PSi particle increasing the width of the fiber where it is located. In the surface embedded material, the PSi particles and the PCL electrospun fibers both retained their original morphology. The adhesion of PSi on the surface of PCL fibers did not increase the PCL fiber diameter to a measurable level, as diameter of the fibers remained at a similar value to that of the pure PCL electrospun fibers.
One initial means to screen the relevance of a given material for orthopedic applications is to estimate the extent of calcium phosphate formation in simulated body fluid (SBF) at 37 °C. Such fluid has ion concentrations similar to those present in human plasma. Previous studies have shown that the degradation product of PSi is monomeric Si(OH)4, which can accelerate the deposition of calcium phosphate.12
In the acellular calcification studies of these new materials (done by immersion in SBF solution at physiological temperature), samples containing PSi particles (at 1, 5, and 10% by mass) totally encapsulated within the PCL fibers were contrasted with those materials whereby the PSi was embedded on the outer surfaces of the fiber network at similar Si concentrations, along with a third group consisting of PCL-only fiber controls. After one week immersion in SBF, the samples were analyzed by EDX. It is significant to note that for the fibers containing PSi totally encapsulated in the PCL, only the 10% Si demonstrated the presence of detectable spherulites composed of Ca and P; for the fibers containing PSi embedded at the surface, calcification was observed for all three Si concentrations by week 1. For the PCL-only control group, no calcium phosphate deposition was observed. At subsequent weekly time intervals, all PSi-containing samples were observed to calcify, with an increasing extent of calcification observed for a given sample type with increasing SBF exposure (as reflected in the Ca/Si atomic % ratios—data not shown). According to an analysis of the calcification of 1% to 10% PSi/PCL fibers in SBF over 4 weeks, no apparent relationship between the concentration of PSi and the amount of calcification were observed, however. Typical morphologies of calcium phosphate nodules formed on PCL fibers containing 5% PSi (totally encapsulated in the fiber) after a SBF exposure period of 3 weeks are shown in Fig. 2a; the associated EDX spectrum in Fig. 2b confirms the presence of calcium and phosphorous. For a given sample, the ratio of calcium to phosphorous values (corrected EDX integrated intensities) range from 1.5 to 1.7 for a total four-week exposure period in SBF; this suggests a mixture of phases including octacalcium phosphate and hydroxyapatite.
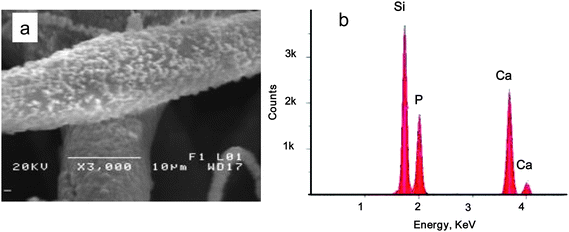 | ||
| Fig. 2 (a) 20% PSi/PCL fibers soaked in SBF for 3 weeks. The fiber surface was deposited with calcium phosphate nanocrystals upon SBF exposure. (b) EDX analysis confirms the composition of the nanoparticles are calcium and phosphorous. | ||
There are two main points to emphasize from these specific assays; first, the location of the PSi particles, and the associated kinetic influence on Si(OH)4 release, plays a key role in the observation of acellular calcium phosphate growth. Calcification is observed in the lower concentrations of Si (1, 5%) that is surface embedded in the PCL fiber within the first 7 days, presumably because the PSi degradation products are more quickly exposed to the SBF electrolyte. Second, the presence of the porous Si is essential to calcification in these composites. For the entire experimental period, the control samples consisting PCL-only electrospun fibers did not induce calcification under our conditions.
In terms of in vitro assays, we began an examination of the mesoporous Si-containing scaffolds with respect to attachment by three cell types—Fibroblast cells (HEK 293), human mesenchymal stem cells (hMSCs) and bone-marrow-derived mouse stromal cells (D1-ORL-UVA). Fibroblast cells, widely distributed in connective tissue are used here initially for preliminary screening experiments for biocompatibility. The highly porous surface area of the PSi/PCL electrospun fibers provides channels to transfer oxygen and nutrients necessary for cell growth. At the very beginning, most of the cells tended to form small spherical shapes and did not attach very well to the scaffold surface as seen (Fig. 3a). This problem is likely a result of the hydrophobicity of the PCL surface. By day 3, a few elongated spindle-shaped HEK 293 cells were observed on the scaffolds (Fig. 3a). With time, more cells spread out and elongate on the scaffolds, as shown after 10 days of culture in Fig. 3b. All cells eventually became enlarged and spindle-shaped. HEK 293 cells cultivated on day 18 were observed not only on the surface of the scaffolds, but also into the void regions because of the relatively high surface areas of the PSi/PCL scaffolds. The cells crosslinked the fibers, grew over the open regions, and formed a morphology resembling a three dimensional extra cellular matrix (ECM) by integrating the surrounding microfibers (Fig. 3c). Due to the similarity between the fibrous scaffolds and the naturally-occurring ECM, the scaffolds provide an environment for the cells to attach, migrate, and proliferate. Even though the PSi/PCL scaffold is biodegradable, it is mechanically stable enough to hold the cells and maintain structural integrity in the media during the culture period.
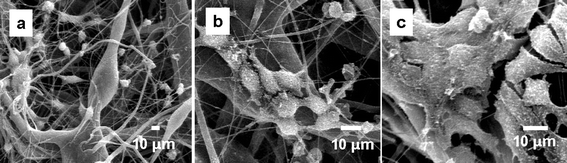 | ||
| Fig. 3 SEM Images of HEK 293 cells growing on PSi/PCL scaffolds as a function of time. (a) At day three, cells attached on the surface of the fibers with spherical or spindle morphology at a low density. (b) At day 10, cells elongated to a typical spindle-like morphology and began to interact with one another by contacting other cells. (c) At day 18, cells proliferated, migrated, and became cell layers on the surface of the scaffolds. ECM has been formed. | ||
As an important primary cell line, we next turned our attention to hMSCs—bone marrow-derived cells that have the potential to differentiate in vitro into bone, fat, and cartilage under appropriate culture conditions.26 On day 3 of culture, hMSCs were found to be scattered sparsely over the PSi-containing scaffolds at a relatively low density. Cells were separated from one another and retained a flat fibroblastic morphology with a thin layer of cell body as shown in Fig. 4a. Cells crosslinked the fibers through the elongated filopodia with actin fibers in the cell body. By day 10, cells proliferated and began to contact to one another in some areas (Fig. 4b). By 18 days of culture, some areas of the scaffolds were covered with dense multilayers of cells (Fig. 4c).
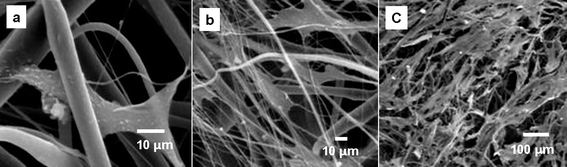 | ||
| Fig. 4 Human mensenchymal cells growing on the surface of the PSi/PCL scaffolds during 18 days of cell culture. (a) hMSCs scattered on the scaffolds at a very low density with a typical flat fibroblast morphology. (b) hMSCs proliferated to a higher density and crosslinked to the fibers by their filopodia to form cell-scaffold composite. (c) hMSCs proliferated to a high density. Some parts of the scaffolds were covered with thick cell layers. | ||
In a similar manner, mouse stromal cells were cultured in complete medium up to 4 weeks. Mouse stromal cells attached to the PSi-containing PCL scaffolds presented fusiform or asteroidal in shape and had an elongated, thin cell body on day 11 as shown in Fig. 5a. There were many star-shaped, triangle and polygon cells on three dimensional scaffolds by day 14 (Fig. 5b). After 21 days, the matrix showed extensive calcification. Numerous calcium phosphate particles were found sitting on the cell multilayer film (Fig. 5c), consistent with the extensive mineralization found inside and around the cells cultured on the PSi/PCL scaffolds with PSi fully encapsulated in PCL fibers by TEM vide supra. EDX confirms the formation of calcium phosphate in these particles formed by the culturing of mouse stromal cells with PSi/PCL scaffolds (Fig. 5d). The control experiment with mouse stromal cells cultured with complete medium did not show calcification after the same period of time. The PSi/PCL fibers retained their initial dimensions during this time period.
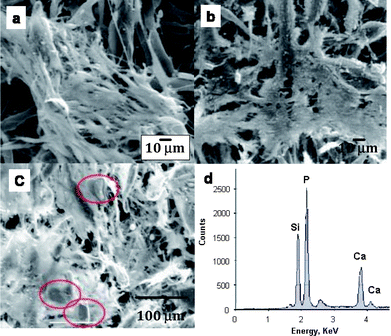 | ||
| Fig. 5 Mouse stromal cells growing on the PSi/PCL scaffolds. (a) Day 11: Mouse stromal cells began to form aggregates on some areas of the scaffolds. (b) Day 14: Most of the scaffolds were covered by multi-cell layers. (c) On day 21, the whole scaffold was covered with mouse stromal cells. Calcium phosphate particles are shown in the red circles. (d) EDX analysis further confirms the presence of calcium and phosphorous. | ||
Stromal cells are capable of differentiating into osteoblasts, chondrocytes, and adipocytes in the presence of appropriate stimuli.27–29Alkaline phosphatase is an enzyme marker for stromal cell osteogenic differentiation. To test the hypothesis that the degradation product of PSi, Si(OH)4, has the ability to accelerate the differentiation process of mouse stromal cells, three sets of experiments were performed. Mouse stromal cells cultured with PCL fibers (no silicon) prepared from 25 wt. % PCL solution in both the presence and absence of differentiation medium were used as controls. The other two sets of experiments were (1) mouse stromal cells cultured with fibers containing PSi (20%) fully encapsulated in PCL in both the presence and absence of differentiation medium, and (2) along with mouse stromal cells cultured with fibers with PSi (2%) embedded at the outer surface of PCL fibers in both presence and absence of differentiation medium.
For the cells cultured with PSi fully encapsulated in PCL fibers, the initial ALP activities of all samples cultured in all conditions were relatively low at day 3 (Fig. 6a) and there was no significant difference among the samples; the percentage of the differentiated cells was low. As time progressed, the ALP activities steadily increased in all wells. By day 14, the ALP activities from the cells cultured in different environments were statistically indistinguishable.
![Alkaline phosphatase assay results for (a) mouse stromal cells cultured with PSi fully encapsulated in PCL fibers; (b) mouse stromal cells cultured with PSi embedded on the surface of PCL electrospun fibers. [Dex here refers to dexamethasone.]](/image/article/2011/NR/c0nr00550a/c0nr00550a-f6.gif) | ||
| Fig. 6 Alkaline phosphatase assay results for (a) mouse stromal cells cultured with PSi fully encapsulated in PCL fibers; (b) mouse stromal cells cultured with PSi embedded on the surface of PCL electrospun fibers. [Dex here refers to dexamethasone.] | ||
For the mouse stromal cells cultured with PSi embedded at the surface of PCL fibers, the initial ALP activities showed the same trend as the ALP activities of the mouse stromal cells cultured with PSi fully encapsulated in PCL fibers, which remained at a very low level at day 3. (Fig. 6b) There was not a significant difference of the ALP activities among samples cultured in different growth media at this stage. By day 6, however, the ALP activities of mouse stromal cells cultured with PSi embedded at the surface of PCL fibers in differentiation medium begins to show a significantly higher value than the other three samples. At day 14, this difference had grown four fold in value between it and the non-PSi containing analog. More importantly, it should be noted that the PSi-containing fiber sample without differentiation medium shows a two fold greater level of ALP expression by the fourteenth day relative to its PCL-only analog. Thus it is clear that the average location of the PSi component has a sensitive influence on the subsequent differentiation of the stromal cells that attach and proliferate in its presence.
To understand the detailed structural changes that can take place inside a single cell during mouse stromal cell differentiation induced by these composites, two types of scaffolds, PSi fully encapsulated in PCL fibers and pure PCL fibers were used for this experiment. All the samples were fixed and dehydrated, embedded in plastic, sectioned by microtome, stained by heavy metal salt solutions as described in the materials and methods section, and observed by TEM at weeks 1, 2, 3, and 4 of culture (Table 2). By week 1, cell mineralization was only found in mouse stromal cells cultured with 20% PSi/PCL scaffolds in differentiation medium (Fig. 7a,b), but not in any other samples, including the other controls (Fig. 7c). Fig. 7a shows that many tiny needle – like crystals were found in the cell nucleus at week 1. The average length of the crystals was about 200 nm. The earliest cellular mineralization thus requires the presence of two factors: differentiation medium and PSi, thereby suggesting that the PSi degradation product facilitates cellular mineralization. By week 2, extensive mineralization was also observed outside the cells (Fig. 7d). Compared to the randomly ordered crystals found inside the cells, these mineral structures found the outside of the cells were relatively well oriented, possibly due to more mature stages. Some mineralization also appeared in the mouse stromal cells cultured with PCL fibers in differentiation medium by week 2 in culture (data not shown). Significantly, the cells cultured with PSi/PCL fibers did not require differentiation medium to show mineralization by the third week. This is likely due to the requisite corrosion of both PCL (to expose enough PSi) and importantly, adequate Si(OH)4. Thus, on the third week, PSi/PCL had eroded to some extent, so that more PSi triggered the mineralization. This result confirms prior observations of the inductive or enhancing effect of PSi on in vitromineralization.30 It is also worthwhile to emphasize that the stromal cell-only control cultured in the absence of dexamethasone (differentiation medium) failed to show any evidence of mineralization within the observed 4 week period. Thus, PSi is not only able to induce acellular calcification, but also shows the ability to trigger cellular calcification as well.
| Scaffold | PCL – Dex | PCL + Dex | PSi/PCL-Dex | PSi/PCL + Dex |
|---|---|---|---|---|
| Time | ||||
| a × no mineralization √ mineralization. | ||||
| Week 1 | × | × | × | √ |
| Week 2 | × | √ | × | √ |
| Week 3 | × | √ | √ | √ |
| Week 4 | × | √ | √ | √ |
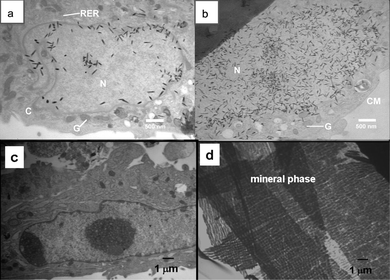 | ||
| Fig. 7 Morphology of mouse stromal cells at the ultrastructural level. (a) Cells cultured with PSi/PCL fibers in differentiation medium at week 1. Needle-like crystals were found in the cell nucleus. N: nucleus, C: cytoplasm, G: Golgi apparatus, RER: rough endoplasmic reticulum. (b) Cells cultured with PSi/PCL fibers in differentiation medium at week 1. Needle-like structures were also found in the cell cytoplasm. N: nucleus, G: Golgi apparatus, CM: cell membrane. (c) Mouse stromal cells cultured with PCL scaffold in presence of differentiation medium at week 1 as control. no crystals were observed inside or outside of the cells. (d) Mouse stromal cells cultured with PSi/PCL scaffold in presence of differentiation medium at week 2. Well organized crystals were found outside of the cells. | ||
Conclusions
Taken in concert, all of these results suggest that the presence of the degradable porous Si structures in these composites plays a significant role in both the kinetics of mineralization for the scaffold and for the corresponding differentiation of stromal cells into more mature osteogenic moieties of relevance to orthopedic therapies. Given the favorable in vitro results shown here, the highly porous structure of these PSi/PCL scaffolds with a controllable and tunable porosity also makes it worthwhile to pursue more complex formulations, including those involving use as a drug delivery carrier for related therapeutic agents such as VEGF and BMP-2. Additional refinements of PSi surface modification can be exploited to not only control the degradation rate, but also promote cell adhesion.31Acknowledgements
J.L.C. and D.F. gratefully acknowledge financial support by the Robert A. Welch Foundation (Grant P-1212). The authors also thank Mengjia Wang for assistance with selected SEM measurements.References
- B. O. Palsson and S. N. Bhatia. “Tissue Engineering”, New York: Pearson, 2004, p. 3 Search PubMed.
- J. Keating and M. M. McQueen, Substitutes for autologous bone graft in orthopaedic trauma, J. Bone Joint Surg. Br., 2001, 82B, 3–8 Search PubMed.
- W. R. Moore, S. E. Graves and G. I. Bain, Synthetic bone graft substitutes, ANZ J. Surg., 2001, 71, 354–361 CrossRef CAS.
- S. N. Parikh, Bone graft substitutes in modern orthopedics, Orthopedics, 2002, 25, 1301–1309 Search PubMed.
- K. J. Burg, L. S. Porter and J. F. Kellam, Biomaterial developments for bone tissue engineering, Biomaterials, 2000, 21, 2347–2359 CrossRef CAS.
- J. M. Orban, K. G. Marra and J. O. Hollinger, Composition options for tissue-engineered bone, Tissue Engineering, 2002, 8, 529–539 CrossRef CAS.
- T. Kokubo, H.-M. Kim and M. Kawashita, Novel bioactive materials with different mechanical properties, Biomaterials, 2003, 24, 2161–2175 CrossRef CAS.
- D. W. Hutmacher, Polymeric Scaffolds in Tissue Engineering Bone and Cartilage, Biomaterials, 2000, 21, 2529–2543 CrossRef CAS.
- W. L. Murphy, D. H. Kohn and D. J. Mooney, Growth of continuous bonelike mineral within porous poly(lactide-co-glycolide) scaffolds in vitro, J. Biomed. Mater. Res., 2000, 50, 50–58 CrossRef CAS.
- P. K. Bowditch, H. Waters, P. Gale, E. Rice, A. M. Scott, L. T. Canham, C. L. Reeves, A. Loni and T. I. Cox, In vivo assessment of tissue compatibility and calcification of bulk and porous silicon., Mat. Res. Soc. Symp. Proc., 1999, 536, 149–154.
- D. M. Reffit, R. Jugdaohsingh, R. P. Thompson and J. J. Powell, Silicic acid: its gastrointestinal uptake and urinary excretion in man and effects on aluminium excretion, J. Inorg. Biochem., 1999, 76, 141–147 CrossRef CAS.
- L. T. Canham, Bioactive silicon structure fabrication through nanoetching techniques, Adv. Mater., 1995, 7, 1033–1037 CAS.
- J. L. Coffer, M. A. Whitehead, D. K. Nagesha, P. Mukherjee, G. Akkaraju, M. Totolici, R. S. Saffie and L. T. Canham, Porous Silicon-Based Scaffolds for Tissue Engineering and Other Biomedical Applications, Phys. Stat. Sol. A., 2005, 202, 1451–1455 CrossRef CAS.
- M. A. Whitehead, D. Fan, P. Mukherjee, G. R. Akkaraju, L. T. Canham and J. L. Coffer, High-Porosity Poly(ε-Caprolactone)/Mesoporous Silicon Scaffolds: Calcium Phosphate Deposition and Biological Response to Bone Precursor Cells, Tissue Engineering, 2008, 14, 195–206 CrossRef CAS.
- X. P. Ma, Scaffolds for tissue fabrication, Mater. Today, 2004, 7, 30–40 CrossRef CAS.
- M. A. Whitehead, D. Fan, G. R. Akkaraju, L. T. Canham and J. L. Coffer, Accelerated calcification in electrically conductive polymer composites comprised of poly(ε-caprolactone), polyaniline, and bioactive mesoporous silicon, J. Biomed. Mater. Res., Part A, 2007, 83A, 225–234 CrossRef CAS.
- H. Yosimoto, Y. M. Shin, H. Terai and J. P. Vacanti, A biodegradable nanofiber scaffold by electrospinning and its potential for bone tissue engineering, Biomaterials, 2003, 24, 2077–2082 CrossRef CAS.
- X. Zong, K. Kim, D. Fang, S. Ran, B. Hsiao and B. Chu, Structure and process relationship of electrospun bioabsorbable nanofiber membranes, Polymer, 2002, 42, 4402–4412.
- X. Fong, I. Chun and D. H. Reneker, Beaded nanofibers formed during electrospinning, Polymer, 1999, 40, 4585–4592 CrossRef CAS.
- Y. Dzenis, Spinning continuous fibers for nanotechnology, Science, 2004, 304, 1917–1919 CrossRef CAS.
- T. B. Bini, S. Gan, T. C. Tan, S. Wang, A. Lim, B. H. Lim and S. Ramakrisshna, Electrospun poly(L-lactide-co-glycoside) biodegradable polymer nanofiber tubes for peripheral nerve regeneration, Nanotechnology, 2004, 15, 1459–1464 CrossRef CAS.
- T. Kokubo, H. Kushitrani, S. Sakka, T. Kitsigi and T. Yamamuro, Solutions able to reproduce in vivo surface structure changes in bioactive glass ceramic A-W, J. Biomed. Mater. Res., 1990, 24, 721 CrossRef CAS.
- C. M. Helmick, F. Bailey and S. S. Ristow, Effects of ethanol dehydration and critical point drying on fish tissue culture cell membrane elemental composition by scanning electron microscopy/X-ray microanalysis, Microsc. Res. Tech., 1994, 28, 259–260 CrossRef CAS.
- V. Souvannavong, C. Lemaire, D. De Nay, S. Brown and A. Adam, Expression of alkaline phosphatase by a B-cell hybridoma and its modulation during cell growth and apoptosis, Immunol. Lett., 1995, 47, 163–167 CrossRef CAS.
- M. A. Hayat. in Principles and Techniques of Electron Microscopy: Biological Applications, Cambridge:Cambridge University Press, 2000, p 101 Search PubMed.
- S. Taed, S. Lee, J. Park and G. Im, Mesenchymal stem cells for tissue engineering and regenerative medicine, Biomed. Mater., 2006, 1, 63–71 Search PubMed.
- G. Ferrari, A. G. Cusella-De and M. Coletta, Muscle regeneration by bone marrow-derived myogenic progenitors, Science, 1998, 279, 1528–1530 CrossRef CAS.
- B. E. Petersen, W. C. Bowen and K. D. Patrene, Bone marrow as a potential source of hepatic oval cells, Science, 1999, 284, 1168–1170 CrossRef CAS.
- G. C. Kopen, D. J. Prockop and D. G. Phinney, Marrow stromal cells migrate throughout forebrain and cerebellum, and they differentiate into astrocytes after injection into neonatal mouse brains, Proc. Natl. Acad. Sci. U. S. A., 1999, 96, 10711–10716 CrossRef CAS.
- W. Sun, J. E. Puzas, T.-J. Sheu, X. Liu and P. M. Fauchet, Nano to microscale porous silicon as a cell interface for bone-tissue engineering, Adv. Mater., 2007, 19, 921–924 CrossRef CAS.
- S. P. Low, K. A. Williams, L. T. Canham and N. H. Voelcker, Evaluation of mammalian cell adhesion on surface-modified porous silicon, Biomaterials, 2006, 27, 4538–46 CrossRef CAS.
Footnote |
| † Current address, Intrinsiq Materials Ltd, Geraldine Road, Malvern WR14 3SZ UK |
| This journal is © The Royal Society of Chemistry 2011 |
