Nanoscale surface modifications of medically relevant metals: state-of-the art and perspectives
Fabio
Variola
ab,
John B.
Brunski
c,
Giovanna
Orsini
d,
Paulo
Tambasco de Oliveira
e,
Rima
Wazen
b and
Antonio
Nanci
*b
aFaculty of Engineering, Department of Mechanical Engineering, University of Ottawa, Ottawa, ON K1N 6N5, Canada
bLaboratory for the Study of Calcified Tissues and Biomaterials, Faculté de Médecine Dentaire, Université de Montréal, Montréal, QC H3C 3J7, Canada. E-mail: antonio.nanci@umontreal.ca
cDivision of Plastic & Reconstructive Surgery, Department of Surgery PSRL, School of Medicine, Stanford University, 257 Campus Drive, Stanford, CA 94305, USA
dDepartment of Clinical Sciences and Stomatology, University of Marche, Via Tronto 10, 66026, Ancona, Italy
eDepartment of Morphology, Stomatology and Physiology, University of São Paulo, Ribeirão Preto, SP 14040-904, Brazil
First published on 26th October 2010
Abstract
Evidence that nanoscale surface properties stimulate and guide various molecular and biological processes at the implant/tissue interface is fostering a new trend in designing implantable metals. Cutting-edge expertise and techniques drawn from widely separated fields, such as nanotechnology, materials engineering and biology, have been advantageously exploited to nanoengineer surfaces in ways that control and direct these processes in predictable manners. In this review, we present and discuss the state-of-the-art of nanotechnology-based approaches currently adopted to modify the surface of metals used for orthopedic and dental applications, and also briefly consider their use in the cardiovascular field. The effects of nanoengineered surfaces on various in vitro molecular and cellular events are firstly discussed. This review also provides an overview of in vivo and clinical studies with nanostructured metallic implants, and addresses the potential influence of nanotopography on biomechanical events at interfaces. Ultimately, the objective of this work is to give the readership a comprehensive picture of the current advances, future developments and challenges in the application of the infinitesimally small to biomedical surface science. We believe that an integrated understanding of the in vitro and particularly of the in vivo behavior is mandatory for the proper exploitation of nanostructured implantable metals and, indeed, of all biomaterials.
 Fabio Variola | Dr Fabio Variola received his B. Eng. and M. Eng. in Materials Engineering from the University of Trieste (Italy) in 2004. In 2009, he completed a joint doctoral program in Materials Science at Institut National de la Recherche Scientifique-Énergie, Matériaux et Télécommunications (INRS-ÉMT) and Université de Montréal. He then carried out postdoctoral training at the Laboratory for the Study of Calcified Tissues and Biomaterials (Université de Montréal). In July 2010, he joined the Department of Mechanical Engineering at the University of Ottawa as Assistant Professor. His research interests focus on surface nanoengineering of biomaterials. |
 John B. Brunski | Dr John B. Brunski has a Ph.D. in Metallurgy & Materials Science (1977) from the University of Pennsylvania. He is currently Senior Research Engineer, Division of Plastic & Reconstructive Surgery, School of Medicine, Stanford University. Previously he was Professor in the Department of Biomedical Engineering, Rensselaer Polytechnic Institute (Troy NY). Among other awards, he received the 2006 Jerome M. and Dorothy Schweitzer Research Award (Greater New York Academy of Prosthodontics) and the 2008 Astra Tech Scientific Award for Applied Research in Osseointegration. His research focuses on dental implant design and interfacial bone biology. |
 Giovanna Orsini | Dr Giovanna Orsini obtained her degree in dentistry at the University of L'Aquila (Italy) in 1994. After a Certification in Esthetic Dentistry (UCLA, USA), in 2000 she received a M.Sc. in Biomedical Sciences from the Université de Montréal (Canada). In 2005, Dr Orsini completed a Ph.D. in Stomatology and Oral Sciences from the University of Chieti-Pescara (Italy). She is now Lecturer at the Polytechnic University of Marche (Italy). Her research interests focus on clinical trials, oral calcified tissues, dental biomaterials, and tissue engineering. |
 Paulo Tambasco de Oliveira | Dr Paulo Tambasco de Oliveira graduated from the Faculty of Dentistry of the University of São Paulo at Ribeirão Preto (Brazil) in 1987 and received his Ph.D. in Oral Pathology in 1997. In 2001 he joined the Laboratory for the Study of Calcified Tissues and Biomaterials (Université de Montréal) for postdoctoral studies. Since 1991 he is Professor of Oral Histology at the University of São Paulo. His current research interest focuses on the study of osteogenic differentiation on metallic and glass-based materials with nanostructured and functionalized surfaces for dental and orthopaedic applications. |
 Rima Wazen | Dr Rima Wazen received her Ph.D. (2006) in Biomedical Sciences from the Université de Montréal (Canada). She is now working as a research assistant at the Laboratory for the Study of Calcified Tissues and Biomaterials at Faculty of Dentistry (Université de Montréal). Her research focuses on the study of cell biology of calcified tissues and tissue integration of biomaterials. |
 Antonio Nanci | Dr Antonio Nanci received his Ph.D. in Anatomy and Cell biology from McGill University in 1982 and was a postdoctoral fellow in Craniofacial Molecular Biology at University of Southern California. He is now Professor of Cell Biology and Director of the Department of Stomatology of Université de Montréal. He is also Director of the Laboratory for the Study of Calcified Tissues and Biomaterials, and member of the Scientific Board of the Rizzoli Orthopedic Hospital in Bologna (Italy). His research focuses on the study of basic cell biological processes in normal and pathological calcified tissues and on surface nanoengineering and nanomedicine. |
1. Introduction
Current generations of dental and orthopedic implants are relatively effective but they still need significant improvement, particularly in their capacity to selectively influence and guide cell and tissue events at the implantation site. Their longevity is limited, and their success depends on the patient's overall health. The benefit of better mastication offered by implant-supported prostheses not only improves the quality of life but also has an impact on the overall health of the patient. Dental implants are generally limited to patients with “good bone quality”, leaving out a large segment of the population that has lost supporting jaw bone with age. In orthopedics, there is an increasing number of cases of total hip replacement to treat advanced degenerative changes (such as those caused by arthritis and osteonecrosis), as well as fractures in patients with osteoporosis. Here, also overall bone status plays a major role in securing both short- and long-term implant stability. The growing frequency of joint replacements, not only in a population that lives longer but also in younger people, has led to an increasing number of patients requiring revision surgery, with the average implant needing replacement approximately every 10 years.1 The need for revision surgery typically arises from loss of bone supporting the prosthesis (osteolysis). Because of the additional bone loss and restructuring at the surgical site, revisions are more difficult to perform and generally result in less support for the prosthesis and poorer overall outcomes than the original surgery. Most importantly for the patient, they are associated with higher morbidity. In addition, they are extremely costly for health providers and a significant drain on healthcare resources in terms of surgeon time and post-surgery care of patients. Hip replacements are familiar examples, but there are similar needs for improved procedures for knee and spine implants.Similarly, each year millions of individuals undergo balloon angioplasty to unblock arteries. In the U.S., about 80% of them also receive a self-expanding, metal mesh tube, called a stent, intended to prevent renarrowing of blood vessels after they have been reopened.2 Balloon angioplasty has been performed since the late 1970's, but coronary stents were only introduced in the early 1990's. Major problems associated with stents are thrombosis and closure of the blood vessel after surgery (restenosis), the latter occurring in up to 30% of patients.2 A promising development in stent technology is the advent of drug-eluting stents (DESs), whose surface is coated with polymers containing agents that regulate cell division and prevent clot formation. So far, clinical results with DESs are encouraging; however, there are still problems with this technology. For instance, the polymer coating may weakly adhere, cause inflammation and increase the propensity for thrombosis.3
Different classes of materials (metals, ceramics, polymers and composites) are currently used to manufacture prosthetic implants and biomedical devices.4 However, metals still represent the gold standard in implantology because of their mechanical properties and biocompatibility,4,5 that is the ability of a material to accomplish specific biomedical functions without causing adverse immune and tissue reactions. Stainless steels (such as 316L) as well as Ti and its alloys (such as TiAl and shape-memory NiTi alloys) are widely encountered in orthopedics, dentistry and cardiology.5–7 In addition, because of their low friction coefficient and high wear resistance, CrCo alloys are also currently used to manufacture components of knee and hip joints.8,9 For implantation in the spine, Ta has been exploited to create three-dimensional porous structures that favor bone ingrowth for a more effective osseointegration.10
Degradable metallic biomaterials, such as Mg and iron-based alloys, are increasingly gaining interest for skeletal and cardiovascular applications (e.g. wires, fixation plates, stents) which do not require the permanent presence of an implanted device.11 They essentially have the capacity to provide specific functions (e.g. structural support, favoring the healing process) and dissolve afterwards through corrosion, without generating toxic byproducts.12,13
Progress in nanotechnology now makes it possible to precisely design and modulate at the nanoscale the surface properties of materials used for various applications in medicine, offering new prospects for the patient.14,15 Nanoengineered surfaces possess the unique capacity of directly affecting the molecular and cellular events that ultimately determine the overall biological response to an implanted material, such as protein adsorption, cell adhesion and proliferation, among others.16–20 As a result of this exceptional ability, various nanotechnology-based techniques have been developed to generate nanoscale surface features on existing biocompatible materials (reviewed in ref. 14, 15).
In this review paper, we bring attention to methods currently used for nanostructuring the surfaces of implantable metals, highlighting in particular those approaches that offer a clinical applicability or that have already resulted in commercial devices in the orthopedic and dental fields. We then provide evidence of the enhanced biological activity achieved with nanotechnology-based surface modifications by presenting an overview of in vitro studies on how cells respond to nanostructured metallic surfaces. In this context, unlocking the precise mechanisms which govern cell-substrate interactions will ultimately permit to endow implantable metals with the exact physicochemical properties needed to elicit a specific biological outcome (e.g. osseointegration, anticoagulation, etc). However, although essential in the progress of biological surface science and in the development of the future generation of implantable materials, in vitro experiments, as it is well recognized in biomedical sciences, only reflect part of the multifactorial and dynamic biological environment of living organisms. This translates into the need to ultimately complete the study and evaluate the performance of a biomaterial in vivo, an aspect that is often forgotten or just briefly discussed in review articles on nanostructured materials. Such oversight must be addressed, and this work is meant to take a step in this direction by illustrating not only the in vitro outcomes, but by also integrating the in vivo biological and biomechanical performances of nanoengineered implants. We ultimately bring attention to some important considerations we believe need to be made to correlate in vitro conditions with the in vivo reality, concluding with our perspectives for the future development of improved metals for biomedical applications.
2. Approaches for nanoscale surface modification
The surface properties of implantable metals can be modified on a range of scales by different techniques.22,23 Various approaches have been used so far to create micron scale topographies on the surface of biocompatible metals.24–26 Although the resulting surface features are effective in enhancing in vitro27–29 and in vivo30–33 biological events, it is now recognized that material-host tissue interactions are principally governed by nanometric surface cues.14,16,17,34,35 As a consequence, micron-scale features can only at best have an indirect influence on cellular activity and thereby can inherently only have limited activity and success. Therefore, there is a need for surface features that can have a more direct and rapid outcome. To this end, various strategies have been devised and implemented to nanoengineer surfaces that can directly influence biological functionalities.14,21 Only some of these methods, however, can at this time be easily exported to a large-scale production for medical implant manufacturing. In this section, we therefore present the approaches that have the potential for industrial exploitation. These techniques, divided into chemical and physical methods, have been selectively chosen according to the following parameters, necessary for large-scale manufacturing: (1) ability to simultaneously reach all surfaces in devices with complex geometries (e.g. femoral stems, dental screws and cardiovascular stents); (2) possibility to modify at the nanoscale commercially available biocompatible metals and implants; and (3) simple integration in the industrial process line.2.1 Chemical methods
Electrochemical modification is one of the most common and flexible ways to modify metallic surfaces on the nanoscale.36 Anodic oxidation has been successfully used to transform smooth Ti surfaces into nanotubular structures with diameters inferior to 100 nm (Fig. 1).37–40 By adjusting parameters such as the chemistry of the electrolyte, voltage and current density, one can precisely modulate physicochemical properties of surfaces,41,42 and the diameter and the spacing between nanotubes.43,44 In addition, by adjusting the applied potential, it is also possible to transform the protective amorphous oxide layer into one of its crystalline forms.45 On Ti surfaces, anodization also permits to create, through a porous alumina mask, pillar-like nanostructures with tunable sizes as well as to deposit an array of 10 μm-long titania nanotubes.46,47 Nanostructured layers on various metallic surfaces have been similarly created using electrophoretic deposition.36 For example, nanocrystalline hydroxyapatite (nano-HA) coatings (crystals size in the 15–25 nm range) and multi-walled carbon nanotubes have been deposited on titanium-based metals, resulting in an improved bioactivity.48,49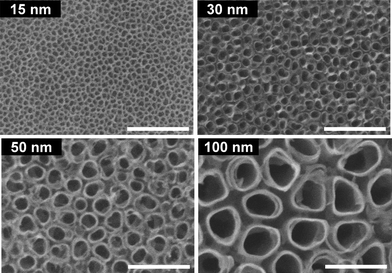 | ||
| Fig. 1 SEM images of vertically oriented TiO2 nanotubes of different diameters. Scale bars: 200 nm. Adapted from ref. 41. Copyright 2009, Wiley. | ||
A simple chemical patterning approach using combinations of strong acids (or bases) and oxidants has been shown to efficiently generate networks of nanopits (pit diameter ranging from 20 to 100 nm) on Ti, Ti6Al4V and CrCoMo alloys, and Ta (Fig. 2).50,51 Surface topography, wettability, micro and nanoroughness, as well as the thickness of the protective oxide layer, can be precisely controlled by adjusting the length of exposure, the temperature and the composition of the etching solutions.51–53 In addition, varying the nature of the etching solution makes it possible to incorporate selected elements (e.g. fluorine, which has antibacterial effects54 and contributes to bone formation55) in nanotopographic surfaces created by oxidative treatment.50
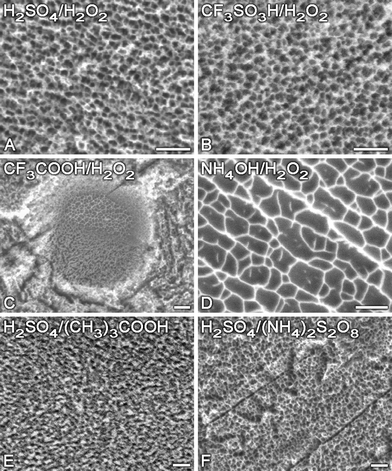 | ||
| Fig. 2 Characteristic SEM images of Ti surfaces nanostructured by oxidative etching with different solutions (scale bars 100 nm). Reproduced with permission from ref. 50. Copyright 2008, ACS. | ||
Anodic oxidation and chemical etching have been combined to create metal/polymer composites with enhanced biological properties. Anodized nanotubular Ti has been coated with NaOH-treated nanoporous poly-lactic-co-glycolic acid (PLGA). The generated nanostructured Ti/PLGA composite stimulated cell activity, but compared to anodized Ti, no significant difference was seen.56 A one-step chemical method based on a combination of NaOH and hydrothermal treatments has also been applied to Ti to create a wide variety of bioactive nanostructures, such as nanoleaves, nanoneedles, nanorods, nanotubes and multiscale octahedral whiskers (Fig. 3).57
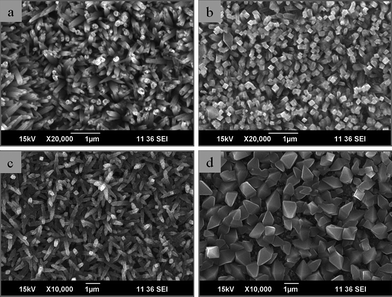 | ||
| Fig. 3 SEM images of the structures formed on Ti metal at 250 °C, 5 h by varying the hexamine to NaOH molar ratio. Transition from nanoneedles to nanorods. Reproduced with permission from ref. 57. Copyright 2010, IOP. | ||
The properties of metallic surfaces can also be modified at the nanometric level by sol–gel chemistry and chemical vapor deposition (CVD), among others.58,59 Niobium oxide and diamond-like carbon presenting characteristic nanotopographies have been deposited by these techniques on Ti and other substrates, providing additional avenues for improving the bioactivity of implantable metals.60–63
A different strategy to improve commonly used implantable metals is grafting bioactive molecules by simple adsorption or covalent linkage, usually peptides or proteins, to reproduce the biochemical environment that naturally sustains biological processes in the body (reviewed in ref. 64–66). These are usually peptides which regulate cellular adhesion (e.g. Arginine-Glycine-Aspartate (RGD)-containing peptides) or extracellular matrix (ECM) proteins (e.g. collagen type I, fibronectin (FN), vitronectin, bone sialoprotein (BSP)) that coordinate the mineralization process.67–70 Growth factors such as bone morphogenic proteins (BMPs) and transforming growth factor beta (TGFβ) participate in the recruitment and final osteogenic differentiation of undifferentiated mesenchymal cells.66,71–73 In addition, pectic polysaccharides, a group of molecules normally present in plants, have demonstrated the capacity to activate cell proliferation.74 Chitosan, a molecule generally extracted from crustaceans, has also been used to coat titanium and other metals.75,76 Coatings with such molecules thus represent additional strategies for improving the biocompatibility of medical devices. The same complexing approach can be exploited to graft antibacterial agents (e.g. lysozyme and/or poly(ethylene glycol)77 and antibiotics,78–82 as well as more complex structures such as self-assembled organic helical rosette nanotubes.83,84
Most works so far have dealt with the simple adsorption of molecules. However, silanes and phosphonates have shown to be very effective spacers to covalently link bioactive proteins and peptides to Ti surfaces.79,85–87 Noteworthy, silanes and phosphonates have the additional capacity to directly affect cellular functions.88,89
Molecular coatings have not been developed solely to favor and enhance cellular functions. In fact, in addition to antibacterial surfaces, there are other applications where the capacity to prevent adhesion is strongly required. For example, in angioplasty, the adhesion of platelets and uncontrolled cell growth onto the inner surface of cardiovascular stents may potentially result in the obstruction of the vessel. For this reason, biochemical surface coatings composed of non-fouling90,91 and anticoagulant92 agents have been created to suppress interactions between the metallic surface and the surrounding biological environment. Interestingly, a common protein such as bovine serum albumin (BSA) can be used to control the subsequent adsorption cascade by exploiting its capacity to efficiently block non-specific interactions.93 In the case of cardiovascular stents, biochemical functionalization has been applied to deposit plasmid DNA and self-assembled monolayers (SAMs) that provide drug-eluting capacities.94,95 Finally, electrodeposition of nanoparticles and their slow-release has recently been reported as an alternative for targeting cardiovascular malfunctions.96
2.2 Physical methods
A variety of physical methods has been used to generate bioactive nanotopographies on metal surfaces. These include plasma97 and physical vapor deposition (PVD).98,99 In addition, self-assembled nanonodules and nanorough Ta layers with well-controlled nanoroughness have been deposited on Ti by e-beam PVD technology.99,100A particular category of physical methods includes technologies which provoke atomic rearrangements, such as ion implantation and thermal oxidation. Approaches based on ion implantation (reviewed in ref. 101) offer the possibility to insert selected biologically effective ions (i.e. Ca2+, F−, Na+, etc.).102,103 This technique allows the fine control of the concentration and depth distribution of the implanted elements. However, the potential creation of superficial stresses (removable with a post-annealing)101 and/or modification of preexisting surface nanometric features21 must be carefully considered when using this highly energetic process. Annealing and/or thermal oxidation have been explored on Ti-based metals to enhance their bioactivity by changing the crystalline structure of the nanometric native oxide layer. Previous studies have in fact compared the different crystalline forms of TiO2, and assessed that rutile enhances cellular response.104,105 However, the anatase crystalline phase with a superimposed nanometric topography, generated by direct current (DC) reactive magnetron sputtering, yielded better biological results than amorphous TiO2 and rutile.45
3. In vitro protein and cellular studies
3.1 Biological surface science
The scientific rationale behind the application of nanotechnology to biomedical surface science correlates with the capacity of cells to sense and recognize specially designed substrate features. Living cells are composed of various structural and molecular elements, synergistically interconnected in a hierarchical system capable of relating events occurring at different levels (i.e. from the molecular to the micrometric scale) and reacting accordingly. In fact, initial cell-substrate interactions take place at the molecular level, but their effects propagate efficiently throughout the entire structure of the cell, ultimately generating a concerted multiscale cellular response.106 For this reason, the capacity of initiating and controlling cellular reactions at the nanometric level through nanoscale physicochemical cueing translates into the ability to affect and direct the global behavior of the cell.When a surface is in contact with the biological environment, the first event which takes place prior to protein adsorption and cell colonization is water adsorption (time scale of order of nanoseconds).107 The properties of the surface water shell dictate the following events, i.e. the adsorption of plasma and ECM proteins, determining their orientation, coverage and potential denaturation.107–109 Ultimately, the resulting protein adlayer will act as a framework on which cells can adhere, spread, migrate and proliferate. However, it is still not clear whether the observed cellular effects are solely mediated by the protein adlayer or whether the physical surface can also provide cues.
3.2 In vitro protein-substrate interactions
In vitro studies have revealed that the adsorbed protein layer is sensitive to specific physicochemical properties of the implant surface. Nanometric features are more effective in dictating protein adsorption and ultimately determining the biochemical characteristics of the adlayer.110 It is believed that only topographical features with dimensions similar to those of surface-bound proteins (∼10 nm) can significantly affect their morphology and activity.110 Surface structuring at the nanoscale may result in changes in surface area/energy, distribution of functional groups, hydrophilicity and oxide composition/thickness, which have been shown to be critical factors to control protein adsorption.111–114 In particular, surface topography is also known to affect protein orientation and denaturation, which play a fundamental role in determining the outcome of the subsequent cell colonization.115–117 There have been several studies to determine how proteins respond to nanometric features of diverse sizes and morphologies.115,118–122 These have concluded that the adsorption of proteins such as fibrinogen, albumin, and FN is generally enhanced by nanorough surfaces, although the effect is not always significant.123 In the case of FN, the vertical dimension of nanometric surface features seems to be critical in determining its adsorption profile. This protein is differentially responsive to the depth of nanometric cavities and to the height of spherical ordered nanostructures, ultimately determining the size of the focal adhesions (FAs) (see section 3.3.3).124,1253.3 In vitro cell-substrate interactions
Cells used for in vitro experiments generally derive from transformed cell lines. Although these cells in large part reflect the activity of the cell type from which they derive, they may not necessarily yield similar bioactivity outcomes. On the other hand, primary cultures with cells isolated from tissues are more difficult to grow, generally loose phenotypic specificity as they are passaged, and exhibit greater biological and differentiation variability. The latter, however, reflects more closely the in vivo healing situation where cells at various stages of differentiation are found.
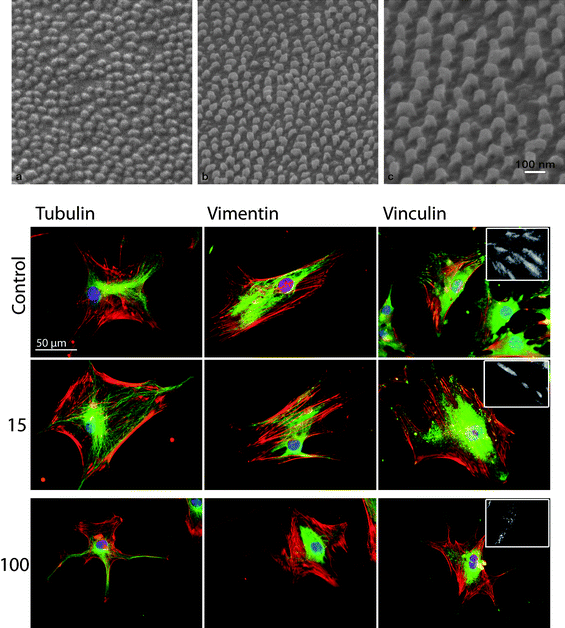 | ||
| Fig. 4 Side-view SEM images of Ti surfaces after anodization at three different voltages and chemical removal of the alumina masks (above). Cytoskeletal and focal adhesions (FA) staining in human skeletal mesenchymal stem cells (hMSCs) cultured on planar control and nanostructured Ti surfaces (below). Red, actin microfilaments; green, either tubulin microtubules; vimentin intermediate filaments or vinculin; blue, cell nucleus. Cells cultured on 15 nm high structured surfaces display a well-defined cytoskeleton and large FA sites, whereas on the higher structured surfaces (100 nm) cells have a less organized cytoskeleton and fewer, smaller FAs. Picture adapted from ref. 47. Copyright 2009, Elsevier. | ||
Oxidative nanopatterning confers Ti-based metals the exciting capacity to selectively influence cellular behavior by favoring the growth of osteoblastic cells while limiting that of fibroblastic ones.21,52 Very interestingly, adhesion of the two cell types after 1 and 4 h of treatment was similar on smooth and nanoporous surfaces, suggesting that the effects of the nanotexture do not significantly relate to cell adhesion but rather to cell growth and differentiation. Selective cell growth and differentiation also takes place on carbon nanofiber compacts (surface roughness of about 0.2 nm as determined by SEM stereoimaging), which have been demonstrated to enhance the osteoblastic activity and limit that of smooth muscle cells, fibroblasts and chondrocytes.134 The physicochemical cueing resulting from oxidative nanopatterning also impacts on gene and protein expression, leading to enhanced osteogenic cell adhesion, spreading and proliferation.50–52,135,136 The generated nanoporous surface induces an early upregulation of BSP and osteopontin (OPN) expression and promotes extracellular FN assembly.50,135,136 Early enhanced secretion of proteins with cell adhesion capacity and their assembly onto the surface with which cells interact will affect cell dynamics at an early crucial stage when key parameters of tissue healing are decided.137–140 In addition, osteogenic cells grown on nanostructured Ti surfaces created by either H2SO4/H2O2 or NH4OH/H2O2 etching exhibit a significant upregulation of genes associated with cell adhesion and migration,50 such as integrin alpha-5 and hyaluronan, as well as an enhanced extracellular FN assembly (Fig. 5).50,136 Similarly to anodization, oxidative nanopatterning can harness the power of stem cells by promoting their growth.50 Importantly, a differential gene expression profile has been detected for hMSCs and osteogenic cells grown on nanopatterned Ti surfaces.50,141 Also, expression of key bone markers was upregulated, including that of alkaline phosphatase and Runx2.141 These results indicate that anodization and oxidative nanopatterning are likely to have a key impact on regenerative medicine, as a strategy to achieve predictable tissue healing around the implant.
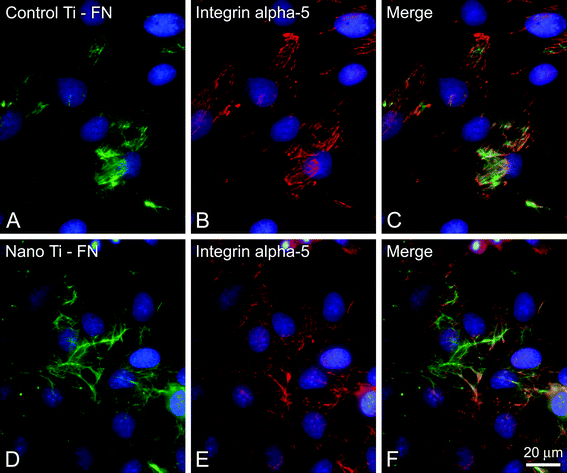 | ||
| Fig. 5 Epifluorescence of calvarial osteogenic cells grown on control (A–C) and nano Ti (chemically oxidated with H2SO4/H2O2) (D–F) at 3 days, labeled with anti-fibronectin (FN) (A,D, green fluorescence) and anti-integrin alfa 5 (B,E, red fluorescence) antibodies. (C,F) Merged pictures of A,B and D,E, respectively, indicating that integrin alfa 5 expression colocalizes with extracellular FN (yellowish). Integrin alfa-5 expression is up-regulated in osteogenic cells grown on nano Ti.50 Blue fluorescence indicates cell nuclei (DAPI DNA stain). Scale bar = 20 μm, (unpublished picture). | ||
Nanostructured bioactive coatings generated by sol–gel and chemical vapor deposition enhance osteoblastic cell adhesion and proliferation.61,63,142 Surfaces modified by covalently bonded bioeffector molecules have the capacity to positively influence in vitro osteogenic functions, mainly cell attachment, adhesion, proliferation and gene expression.64,143–147
Concomitant with these chemical methods, physical approaches confer an enhanced biocompatibility to implantable materials by creating on their surfaces coatings presenting nanoscale features. For example, PVD was applied to create different nanotopographical coatings on titanium surfaces for in vitro studies aimed at evaluating the effects of surface chemistry and topography on the cellular and/or tissue response.98,148
The results of in vitro studies are summarized in Table 1.
| Metal | Method | Nanoscale surface features | Tunable surface properties | Biological outcome | Ref. |
|---|---|---|---|---|---|
| Titanium, Ti6Al4V | Anodization | Nanotubes (diameter < 100 nm) | Pore size, nanotube length, oxide thickness, crystallinity | Enhanced osteogenic activity (adhesion, proliferation, bone matrix secretion and mineralization), promotion of FAs assembly, stimulation of hMSC cell activity (growth and differentiation), enhanced adhesion of chondrocytes and keratinocytes, limitation of bacterial growth | 36,38–41,62,127,128,131–133,239 |
| Titanium, Ti6Al4V, CrCoMo, Tantalum | Oxidative nanopatterning | Nanoporous structures (20–100 nm diameter) | Micro and nanotopographies, oxide thickness, chemical inclusions (F, S) | Differential activity (enhancement of osteoblastic activity and limitation of fibroblast growth), gene and protein (BSP, OPN, Alkaline phosphatase, Runx2) upregulation, stimulation of HUC stem cells, antibacterial effect | 50–53,135,136,141 |
| Titanium | Hydrothermal + NaOH treatment | Nanoscaffolds, nanoflowers, nanoleaves, nanoneedles, nanorods, nanotubes and multi-scale octahedral whiskers | Dimensions of nanostructures | Enhanced protein adsorption | 57 |
| Titanium | Sol–gel and Chemical vapor deposition (CVD) | Nanotopograhical niobium oxide and diamond-like carbon | Nanoscale roughness, layer thickness, crystallinity | Enhanced osteoblast adhesion and proliferation | 58,59,61,63,142 |
| Titanium, Ti6Al4V | Biochemical functionalization | Molecular coatings (molecules with RGD motifs and ECM proteins, antibacterial agents, antibiotics, nanorosette, non-fouling and anticoagulant agents) | Molecular functional groups, coating thickness | Enhanced osteoblast attachment, adhesion, proliferation, gene expression, antifouling and anticoagulant properties | 38,64,67,68,77–84,90–92,143,145,146 |
| Titanium, Ti6Al4V | Plasma deposition, physical and e-beam vapor deposition (PVD), electron-gun evaporation | Nanostructured Ti, TiN, ZrN, Ta coatings | Nanoscale roughness, coating thickness | Enhanced osteoblast proliferation and Ca deposition, enhanced fibroblast attachment | 97–100 |
| Titanium | Ion implantation | Implanted Ca2+, F−, Na+, P+, etc | Concentration and depth distribution of implanted elements | Improved osteoconductivity | 101–103 |
| Titanium, Ti6Al4V | Thermal oxidation/Annealing and DC reactive magnetron sputtering | Crystalline TiO2 layers | Nanotopography, crystalline structure | Improved osteoblast activity, enhanced osteoblast adhesion, spreading, proliferation and differentiation (on anatase) | 45,104,105 |
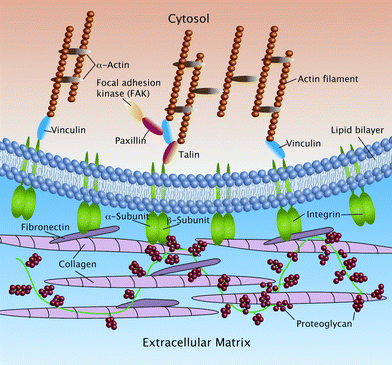 | ||
| Fig. 6 Schematic drawing of focal adhesions (FAs) showing the interactions of integrin molecules with other proteins on both sides of the lipid bilayer (unpublished picture). | ||
Threshold dimensions of nanostructures relating to the assembly of FAs have been established (reviewed in ref. 152). The observed cellular effects on nanostructured substrates may in part relate to the propensity of the cell membrane to ‘stretch towards points of stability’ and in so doing trigger nanoscale cytoskeletal reorganization. The total sum of these nanoscale deformations at various points along the cell membrane will trigger typical signaling pathways that regulate cell behavior. The mechanisms by which integrins signal into the cell interior are complex and involve several different pathways, such as the cytoplasmic protein tyrosine kinase, called focal adhesion kinase (FAK).150 Nanoscale pits upregulate FAK signaling in osteoblastic cells.152 Similarly, nanoporous surfaces are believed to significantly affect the activation of GTPase Rho at FAs,152,153 an enzyme known to play a part in the maturation of the adhesive complex by promoting recruitment of actin filaments and integrins to the contact site.150,154
During interaction with bi-dimensional substrates the cell changes its shape and dynamically develops extensions called lamellipodia (membrane veils rich in actin) and filopodia (finger-like protrusions) that act as sensing and traction elements to allow cells to crawl and perceive topographical cues.151,153 Nanotopography promotes the formation of these membrane specializations (Fig. 7). Changes in cell shape are as influential as signaling molecules in the differentiation process.155–159 Indeed, it has been shown that cell shape regulates the switch in commitment of stem cells towards an osteoblastic phenotype by modulating endogenous RhoA activity.160
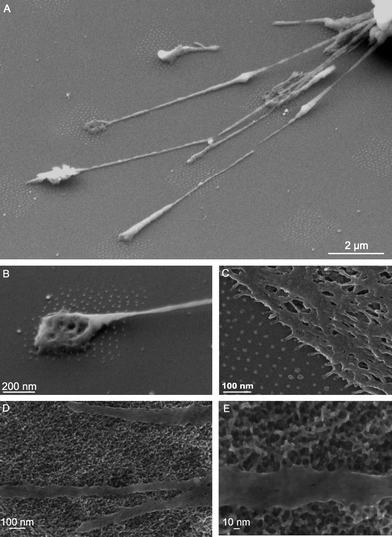 | ||
| Fig. 7 SEM micrographs displaying 3T3-fibroblast adhering to micro-nanopatterns (A and B) and to an extended nanopattern (C). The dot spacing was 58 nm in all samples. Reproduced with permission from ref. 153. Copyright 2007, RSC. Filopodia of primary calvarian cells sensing the nanotexture generated on Ti by oxidative nanopatterning (D–E)135 (unpublished picture). | ||
4. In vivo and clinical applications
4.1 In vivo response of nanostructured implants
Significant efforts have been made so far to integrate surface modification approaches with implant manufacturing in order to ensure superior clinical performance of current prostheses.161–163 In this context, while the mechanical performance of a device is mainly defined by its macroscopic shape, tissue integration and long-term stability result from the interactions between the implant surface and the surrounding biological environment at the cellular and molecular level.164–167 Based on the in vitro results, nanostructured metals undoubtedly have the potential to yield a faster and more stable integration of biomedical implants.34,56 To validate this hypothesis, several nanoscale surface modification approaches are currently under investigation to determine their effects in vivo and their potential applicability to commercial implants.34,168–170 Here, we present an overview of methods that have been successfully adapted to nanomodify metallic implants and the resulting animal and clinical outcomes. The results of the in vivo studies are summarized in Table 2.| Metal/Implant | Method | Nanoscale surface features | Surface chemistry (Doping) | Surgical protocol | Biological outcome | Ref. |
|---|---|---|---|---|---|---|
| Titanium rod | Anodization | TiO2 nanotubes (30 nm diameter) | N/A | Pig frontal skull – 3, 7, 14, 30, 90 days | Stimulation of collagen-I expression | 168 |
| Titanium screw, rod | Electrochemical deposition, Promimic HANano© | HA nanocrystals | Ca, P | Rabbit proximal tibia – 4, 6, 12 weeks; | Improved bone-to-implant contact (BIC), early bone formation | 172,173 |
| Titanium screw | Micro-arc oxidation (MAO) | N/A | Mg | Rabbit Tibia – 3, 6 weeks | Improved bone-integration and removal torque | 175–178 |
| Titanium screw | Oxidative nanopatterning | Nanopores (20–25 nm diameter) | N/A | Dog mandible – 3, 8 weeks | Improved BIC | 179 |
| Titanium rod, screw | TiO2 blasting + HF acid treatment | Uncharacterized features (50–200 nm in size) | F | Rat tibia – 21 days; Dog mandible – 2 weeks | Stimulation of osteoblastic gene expression, enhanced bone formation and bone-implant fixation, good osteointegration and stability after 1 year | 55,180–183,185 |
| Titanium rectangular plates, rod | Dual acid etching (DAE) + Discrete crystalline deposition (DCD) | CaP nanocrystals (20–100 nm in size) and nanoparticles | N/A | Rat femur – 9 days; Human posterior maxilla – 4, 8 weeks; Dog tooth socket – 4 h, 1, 2, 4 and 8 weeks | Improved BIC and bone formation, enhanced tissue mineralization, less bone desorption | 186–189 |
| Titanium rod | Ion beam assisted deposition (IBAD) | Nano-thick (20–500 nm) bioactive (Ca- and P-based) ceramic layers | Ca, P | Dog proximal tibia – 2, 4 weeks | Improved fixation | 190,191 |
| Titanium screw | Hydrothermal alkaline treatment | Uncharacterized features (about 100 nm in size) | Ca | Rabbit femur – 6 weeks; | Improved BIC and removal torque, enhanced osteoconductivity | 171,192–194 |
| Titanium screw | Bioceramic grit-blasting (BGB) + Acid Etching (AE) | Nanorough topography | Ca, P | Dog tibia – 2, 4, 8 weeks | Early biomechanical fixation and improved BIC | 195,196 |
A recent study has exploited controlled anodization to endow the surface of Ti implants with self-organized and highly ordered TiO2 nanotubes (30 nm diameter). Implanted in the frontal skull of pigs, these nanotubular surfaces stimulated collagen type-I expression by osteoblasts.168 However, no significant difference in the amount of peri-implant bone formation was observed, suggesting that these nanotubular surfaces ultimately have no impact on osseointegration. These results differ from previous in vitro40 and in vivo171 studies with similarly treated surfaces but different nanotube diameters (i.e. 70–80 nm and 250–800 nm diameter nanotubes, respectively). The authors concluded that anodized Ti does not have an impact on the total collagen content but enhances peri-implant bone formation.40,171 Such differences may result from various factors related to the material properties, such as the different surface topography (i.e. nanotube diameter), degree of crystallinity of the TiO2 overlayer, or to other factors unique to in vivo studies, such as surgical considerations (see discussion). Consistent with the results previously described (see section 2.1), anodic oxidation also allows deposition of nano-HA coatings (Fig. 8).172 Histomorphometric evaluation showed that nano-HA modified implants have significantly greater bone-to-implant contact (BIC) values compared to acid-treated implants. The enhancement of BIC by HA nanocrystals was confirmed in a different study in which it was also shown that early bone formation depends on the size and diameter of the nanosized HA crystals.173 The same research group exploited a sol–gel technique to deposit nanotopographic TiO2 coatings that also enhanced BIC.174
 | ||
| Fig. 8 SEM micrograph of the CaP rodlike crystals. Reproduced with permission from ref. 172. Copyright 2009, Elsevier. | ||
Micro-arc oxidation (MAO) has been used to dope the implant surface with Mg.175,176 Although this technique does not result in topographical modifications at the nanoscale, the presence of Mg ions yielded a more rapid and stronger bone-integration, as well as higher removal torques than commercial microrough highly crystalline and phosphate-enriched Ti implants.177,178
Oxidative nanopatterning with H2SO4/H2O2 creates nanotopography on bulk metals,50 such as Ti screw-shaped implants (Fig. 9). Oxidative treatment of commercial screw-shaped Ti implants placed in dog mandibles resulted in enhanced BIC values and osteogenesis and this even though no nanotexture was obtained.179 The authors suggested that the implant handling (e.g. passivation) during manufacture may have generated chemical conditions that affected the dynamics of the deoxidation/reoxidation processes. This study, however, shows that existing commercial implants can be significantly improved using a simple treatment that does not require elaborate changes in manufacturing.
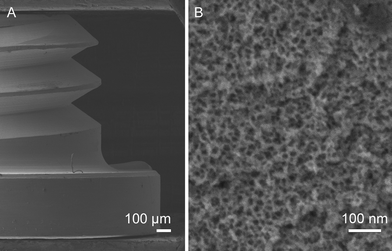 | ||
| Fig. 9 Nanoporous surface (A) generated on miniature (1.0 mm inner diameter; 1.5 mm outer diameter; 2.4 mm head diameter; 0.4 mm head thickness; 1.6 mm implant length) Ti6Al4V screw-shaped implants (B) (Micro Med Machining Inc, Miami Lakes, FL) by oxidative nanopatterning (unpublished picture). | ||
The combination of TiO2 blasting and HF acid treatment has been used to create a commercial endosseous Ti implant with microrough surfaces with superimposed uncharacterized features ranging between 50 and 200 nm.180 These implants stimulated osteoblastic gene expression, as well as enhanced bone formation, osseointegration and bone-implant fixation.181–183 While some inflammatory response was reported,184 the overall success rate was satisfactory, with the majority of implants yielding good osseointegration and stability after one year post-surgery. Noteworthy, high success rate was also reported under more challenging clinical conditions such as early loading.185
Dual-acid-etching (DAE) followed by discrete-crystalline-deposition (DCD) has been used to create calcium phosphate (CaP) nanocrystals (20–100 nm in size) coated Ti implants.186 These implants were clinically evaluated in a randomized controlled trial, which concluded that BIC in the posterior maxilla increased by about 12%.187 In addition, bone formation around CaP nanocrystals-coated implants was enhanced.188 The observed capacity to accelerate bone formation is expected to result in a faster healing response which ultimately may permit earlier loadings. In contrast, a recent study in dogs comparing bone formation around DAE and CaP nanocrystals-coated implants showed that there was no statistical difference in BIC in the early healing phase (1–4 weeks).189 However, test sites containing implants coated with nanoparticles exhibited less bone resorption.
Another commercial implant, which was however discontinued, was produced by ion-beam-assisted-deposition (IBAD) of thin (20–500 nm) bioactive Ca- and P-based ceramic layers.190 With a 300–500 nm thick coating, the BIC value was not significantly affected, but the implants presented higher torque-to-interface fracture levels compared to controls, indicating an improved fixation.191 However, the biomechanical properties degraded when the bioactive layer thickness was reduced to 20–50 nm and there was no improvement of BIC values and bone fixation at early implantation times (2 and 4 weeks).190 This outcome was partially attributed to the reduced thickness and to the amorphous microstructure of the deposited material, which may have caused its rapid dissolution.
Nanostructured surfaces presenting uncharacterized features of approximately 100 nm have been obtained on microrough Ti implants by hydrothermal alkaline treatment.192 This method also caused incorporation of calcium as CaTiO3 in the protective surface oxide layer. Titanium and Ti6Al4V implants with treated surfaces significantly increased BIC and removal torque forces in rabbit tibiae.171,193,194 In addition, this treatment improved bone formation around implants placed in trabecular bone.192
Bioceramic grit-blasting and acid etching (BGB/AE) technologies have been integrated to produce submicrometric topographies on Ti implants.195 Histological evaluation after 2 months post-surgery showed significantly higher BIC and osteocyte density around modified implants when compared to DAE implants.196
4.2 Antibacterial and cardiovascular implants
Infection at the implantation site represents an important clinical complication. In the U.S., approximately 4.3% of the orthopedic implants developed bacterial infection.197 In the case of cardiovascular devices, the annual infection rate is even higher (7.4%).197 Coatings deposited by chemical (e.g. wet-chemistry approaches) and physical (e.g. PVD) processes (reviewed in ref. 198) have been successfully applied to control bacterial colonization of implanted prostheses. For example, biodegradable gentamicin-loaded poly(d,l-lactide) coatings prevented implant-related osteomyelitis (i.e. infection of bone) around Ti wires.199 Degradable Mg- and iron-based alloys are also now being considered in cases when the implant is only temporarily needed, such as for stents.12,13While Ti-based metals are the most common material for dental and orthopedic applications, their use in cardiology is still limited.200 Stainless steels, NiTi and Co–Cr alloys, as well as Ta, are in fact more often encountered in cardiovascular applications.200 Stents capable of slowly releasing drugs (i.e. immunosuppressant, chemotherapeutic, etc), for example to block cell proliferation or prevent the formation of scar tissue at the site of coronary intervention, are already approved and commercially available (DESs).201 However, the polymeric coating used in DESs may be cause short-term inflammation as well as long-term thrombotic events.202 Nanostructuring may represent an alternative to polymer-based DESs. In this context, nanoporous surfaces generated on coronary stents by various techniques yielded promising results in terms of drug-eluting capacities (reviewed in ref. 202) (Fig. 10). For example, a nanostructured stent was developed by coating a 316L stainless steel with electrochemically generated nanoporous Al2O3.203 However, the clinical performance of this system was not satisfactory.204 In addition, nanoporous metallic coatings were created through sputtering techniques, which allow deposition of a variety of materials, including Co–Cr, NiTi, Pt, and stainless steel.202 Oxidative nanopatterning has also been efficiently applied to sputtered Ti to create a network of nanopores.122 Therefore, deposition of thin films of Ti on substrates that are not readily amenable to chemical oxidation could be advantageously exploited to create nanotextures offering antibacterial and antiadhesive properties,50 thus providing a valuable strategy to improve the integration of stents made of different metals.
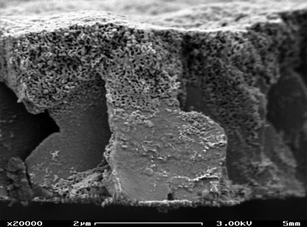 | ||
| Fig. 10 A nanoporous stent surface: a SEM view of a Setagon stent (x 20,000). Stent struts are gilded with this nanoporous layer (estimated porosity of 30%), which functions as a reservoir for prolonged release of antirestenotic drugs. Reproduced with permission from ref. 202. Copyright 2007, Informa Healthcare. | ||
4.3 Micromotion and nanotopography
The primary stability of an implant results from the biomechanical interlocking of the implant and the surrounding bone. In addition to implant-related factors (material, design, topography and surface chemistry), surgical procedure and patient variable (bone quantity and quality, health condition),205 the local mechanical environment has been recognized as a critical factor for bone repair and regeneration around an implant.206 However, the exact biomechanical parameters involved in the initial healing process at the tissue-implant interface remain elusive. Several studies have demonstrated that micromotion during implant loading, can arise from various factors including: (1) the physical properties of the bone tissue (e.g. elastic moduli of adjacent bone and strength of attachment between implant and tissue), (2) the mechanical interaction between the implant and tissue, (3) the initial fit of the implant in the site, (4) the geometry of the implant,207–209 and of course (5) the characteristics of the loading.206,208 Depending on the magnitude and direction of resulting micromotion at the interface, tissue differentiation around immediately loaded implants can respond differently; excessive micromotion may be a compromising factor that leads to interfacial fibrous tissue formation and prevention of osseointegration, while at the lower range of micromotion, there is evidence that there may be positive effects on bone repair and regeneration.210–215 Overall, the exact dynamics of the interfacial events that lead to osseointegration when healing occurs under loading and the limits of relative motion as well as the specific aspects of micromotion that actually cause problems at the bone-implant interface still have to be established.Implant micromotion per se may not be the decisive factor in interfacial healing; instead, there is now evidence that the motion-associated interfacial principal strain magnitude – along with its spatial distribution, duty cycle, waveform, etc. – is the factor that most influences osseointegration.216,217 This is a significant finding because of the current debates surrounding ‘immediate’ vs. ‘delayed’ loading of implants.218 Recall that implant loading inevitably creates strain fields at the interface, whether the loading starts immediately after implantation, or later, after the bone has had a chance to heal to some degree. Therefore, as one contemplates strategies for the design and use of dental and orthopedic implants, it is not the timing of loading that is the primary issue, but rather the propensity of the implant to undergo micromotion and thereby creating local interfacial strain. Although previous studies have tried to derive a single value of limiting strain and stress levels beyond which bone regeneration is compromised,206,219 it may not be possible to define such a value. It is well to remember that continuum-based models of mechanotransduction will always have limitations when it comes to unraveling detailed molecular-level mechanisms at the cell level, as elegantly discussed by Humphrey.220 However, based on ongoing studies on a mouse tibial model, it has been estimated that ∼30% principal strain (tensile or compressive) represented an upper limit to allowable principal strain magnitude during interfacial bone healing.217,221 There are also orthopedic studies from the fracture healing and dynamization literature that also support this view, although again, exact thresholds remain elusive.222–224 Since an appreciable number of implant losses have been considered to possibly be the result of excessive strains and stresses at the bone-implant interface, a better knowledge of the interfacial stress-strain conditions that are tolerable as opposed to deleterious interfacial stress-strain conditions is essential, and would lead to understanding the mechanically-controlled tissue differentiation process, that is the “mechanobiology” at the interface.
One question that has received relatively less attention is whether surface characteristics have an impact on the interfacial mechanobiology. This aspect should be considered at multiscale levels, including the macro, micro and even the nano levels. Macro- and micron-scale surface modifications have been exploited for some years now to create biomechanical environments that can significantly modulate tissue healing around bone implants. For example, there is a long history of exploiting “optimal” porous implant designs for bone ingrowth as well as attempts to understand the role of surface roughness at the micron scale in terms of implant accommodation in bone.225 The more recent recognition that interactions between biomaterials and host tissues also take place on the nanoscale has focused attention of both researchers and industry on a new generation of implantable metals with nanoengineered surfaces. This has in part been driven by intriguing work suggesting that an implant's surface texture or roughness may alter cell shape and cytoskeleton, which in turn may influence gene expression.226–229
As a consequence, there is a growing body of literature exploring the role of nano-level mechanics on cell-substrate interactions as well as cell-cell interactions. For example, recent reviews indicate that substrate elasticity as well as mechanical deformations (strain) of cells play a role in determining stem cell fate decisions.106,230 This latter perspective naturally leads to the idea that the nano-level mechanical properties of an implanted biomaterial could be influential in determining cell fate decisions adjacent to a biomaterial. As stated by Discher et al. “… not only physical contributions to differentiation” but also that “carefully made materials can help prime the expansion of specific progenitors.”230 Finally, this last perspective also leads to the notion that finite element analyses and similar mechanical modeling methods could help take account of the truly multiscale character of the biomechanics problem at bone-implant interfaces; such models could be helpful to fully scope out the possible interplay of cell deformations in relation to implant surface topography.106
Given the body of work indicating that physical factors at the tissue and cell levels, including roughness, mechanical properties, as well as related stresses and strains, are ultimately transferred to single cells in some manner, local strain fields at the macroscale may then affect cellular events at the micron- and nano- (molecular) scale.231 Yet from both mechanistic and implant-design viewpoints, there is little to bridge the gap between implant design at macro- and micron-levels vs. biological mechanisms at the nanoscale. Hence it can be hypothesized that local deformation in healing tissue (e.g. by micromotion) generates changes in the biomechanical relationships between tissue and implant at multiple size scales (macro, micro and nano) and therefore influences local cellular (e.g., cell morphology) and molecular (e.g. gene expression) events. Surface features on the implant may also participate in creating strain fields that regulate cell fate decisions at the bone-implant interface. A series of nanoscale cell distortions might cumulatively create sufficient strain to trigger changes in cytoskeletal organization and activation of different signaling pathways in diverse cell types.232 Since nanoscale topography and chemistry have been demonstrated in vitro to influence the mechanical properties of cells,233,234 it seems likely that this could be advantageously exploited in vivo to allow the design of an implant that might counterbalance negative effects of ‘excessive’ micromotion. For example, nanoscale cell distortions induced by micromotion could be exploited to selectively trigger cell fate decisions (e.g. osteogenic vs. fibroblastic pathway)232 thereby minimizing fibrous tissue formation and promoting bone formation around loaded implants.
4.4 Considerations on the biocompatibility and cytotoxicity of implantable metals
The cytotoxicity of implanted metals is a major concern in the biomedical field and still object of debate.235 Ions and/or debris released from an implanted material can in fact provoke adverse immune and inflammatory reactions that can ultimately defeat the purpose of the biomedical device. It is commonly accepted that Ti and its alloys are biocompatible. However, some Ti alloys show a certain degree of cytotoxicity due to the alloying elements.236 For example, Al and V were associated with neurological disorders.237 In addition, high levels of Cr, Ni and Co raise the risk of carcinogenicity.236 While these effects are known, it is important to point out that the links between a precise concentration of metallic ions and adverse phenomena such as DNA damage, DNA mutation, carcinogenicity and metal sensitivity have not yet been clearly established,235 and further studies are thus required.5. Outlook and perspectives
In this paper, we have presented an overview of current nanotechnology-based strategies (chemical, biochemical and physical) which have been used to improve the in vitro biological response to implantable metals, and in some cases have also shown enhanced in vivo experimental outcomes and found commercial applications. In general, while it can be concluded that nanoscale surface modification introduces novel bioactive capacity into the arena of metallic biomaterials, many of the modification approaches described in this review still remain to be tested in vivo and few have actually been exported to the medical device industry. There are in fact comparatively very few structured animal and clinical studies that investigated the short and especially long-term effects of such surfaces, evaluating whether the enhanced biological activities demonstrated in vitro actually translate to the complex in vivo environment. However, even though it is well known in biology that in vitro conditions do not fully reflect the in vivo reality, such studies are nonetheless essential to screen potentially successful treatments for subsequent animal validation.From the material point of view, it should be noted that most in vitro studies aimed at probing in detail how cells respond to a given nanostructured substrate have been carried out on non-metallic materials, generally characterized by nanoscale protrusions created by techniques such as colloidal and electron beam lithography, and polymer phase separation.152 There are comparatively few studies that have investigated pits which fit the commonly-accepted nanometric definition (i.e. with dimension smaller than 100 nm),125,238 on implantable metals. While studies carried out with laboratory materials are undoubtedly important to understand the dynamics involved in cell adhesion to substrates, the resulting cell behaviors may ultimately differ from those on medically relevant metals which exhibit different surface physicochemical parameters. It should be noted that anodic oxidation and chemical nanopatterning have been successfully applied to create networks of nanometric pits on implantable metals to probe cell adhesion.47,50,135,239
Since in most cases cell cultures on nanopatterned materials and, as a matter of fact even on microstructured surfaces, are carried out in the presence of proteins, one important question that remains to be addressed is whether there is a direct contribution by surface modification. It is now well established that proteins rapidly form an adsorbed layer on biomaterial surfaces.107–109 It is therefore not clear whether the resulting in vitro effects are due to direct signaling by the nanometric surfaces cues, to the protein adlayer or the synergistic combination of these two factors. It can, in any case, ultimately be argued that the cellular outcomes result from the nanometric surface features since these significantly affect protein morphology and activity.107,118–121 Irrespectively, there is another important consideration that needs to be made when translating in vivo protein adsorption profiles to the body. The identity of the first proteins to adsorb onto the surface and subsequent protein-protein interactions may not be precisely predicted due to the complexity, variability and dynamism of the biological environment. In multi-component solutions such as bodily fluids, competitive adsorption and protein displacement concur in determining the final adsorption profile.240,241 For instance, with time some proteins will likely be displaced from the surface. Therefore, one would expect that overall a multi-component mixture will interact in a semi-stochastic manner with the substrate, inducing a certain degree of variability in the overall adsorption profile. In addition, in the body at sites where there is bleeding, a fibrinogen coagulate will form rapidly and may partially or completely mask the surface thereby complicating further the formation of an adlayer on an implant.242 One must be therefore extremely careful in extrapolating data obtained in vitro to the reality of the biological environment where the behavior of a protein will not only depend on its physicochemical properties and those of the biomaterial surface, but will also reflect modulation of their behavior by the various tissue and body fluid components it is exposed to.243 Predictable adsorption may ultimately require more selective approaches such as nanopatterning of surfaces with molecular arrays that will determine interactions of the substrate with selected proteins.
There are additional variables determining the final outcome of an implant, such as the implantation site and the overall health condition of the patient. Longitudinal and descriptive human studies, as well as histological data, have indicated that early osseointegration and clinical success rates for endosseous implants vary according to the anatomic location.244 A high success rate has been reported for implants placed in regions with dense bone such as the mandible,245,246 whereas placement in the maxilla can yield insufficient osseointegration, partially related to poorer bone quality at this site.247 Difficulty in achieving short- and long- term integration and stability of dental implants has also been observed in selected patient populations, such as smokers and diabetics.248,249 Due to the complexity of the clinical scenario, new nanotextured implants may not necessarily yield the expected outcome when used clinically and longer evaluation periods may be required.250 As stated by Albrektsson, “novel implant systems or moderate/major changes of existing implants need to be clinically tested preferably before clinical introduction, not afterwards”.251 While clearly caution is needed, the animal and clinical studies carried out so far suggest that nanoscale surface modification of metallic implants has a promising clinical future.
A particular problem is that implant studies seldom provide a detailed characterization of surfaces at the nanoscale.252 As stated by Wennerberg, “measurements and evaluation techniques need to be standardized”.253 For example, roughness measurements as well as protocols to determine surface wettability should be more uniform in order to provide the consistent data that is required for reliable comparison of different implants.254 In this context, contact angle measurements have been extensively used to determine the wettability and free energy of nanostructured substrates53,255 and characterize biomedical implants, since these parameters affect the interactions between a material and the surrounding biological environment.256–259 Experimental conditions (e.g. drop size and symmetry, vibration of the substrate), as well as the physicochemical properties of the substrate (e.g. surface roughness, porosity, chemical homogeneity and presence of adsorbates) and the presence of surface nanostructures, are all factors that influence the overall wettability of a material, and should therefore be carefully taken into consideration during data analysis.258–260 In addition, some prosthetic devices commercially-advertised as nanostructured are characterized by inhomogeneous nanofeatures which lack statistical distribution and/or precise reproducibility. In this context, even smooth implant surfaces could be considered nanostructured since they could actually exhibit a nanoscale roughness or some kind of nanometric features. These are, most of the times, the direct consequence of the fact that materials are not naturally atomically flat and characterized by defects. Therefore, the need for a distinction between engineered nanostructures and the native topography of materials at the nanoscale, is legitimate. All these factors may introduce additional variables which, in addition to biological variability, may ultimately confound assessment of the precise role of nanometric surfaces in controlling biological events in living systems. It will be therefore important to optimize current and future nanotechnology approaches to generate surfaces with controlled and uniform surface nanofeatures in order to achieve a better understanding of material/host tissue interactions.
While the majority of studies with nanostructured metals has focused on dental and orthopedic implants, the benefits of nanoscale surface modifications for cardiovascular applications have just started to be assessed.130,261,262 A problem associated with current DESs is the potential cracking/detachment of the polymeric coating during balloon expansion.263 In addition, delayed healing and hypersensitivity reaction to DES polymeric components have been reported.264 Direct nanoscale modification of surfaces without deposition of polymeric overlayers could represent an effective strategy to limit adverse reactions to metallic stents. As an example, porous surfaces capable of selectively influencing cell growth (e.g. chemically-generated nanoporous surfaces50) could improve the performance of existing stents by hampering undesired cell adhesion and proliferation, and also improve tissue healing by synergistic drug release (see below).
Nanostructured materials are also used as biologically active molecule-delivering systems.265 Compared to surfaces with nano-protrusions, nanoporous surfaces are very attractive because they 1) offer an increased surface area which translates into a greater surface reactivity, and 2) provide nanoconfined volumes which are expected to modulate dissolution rates. The fact that higher area-to-volume ratios translate into a higher amount of surface reactive sites is well known in physical chemistry and biology, since it determines, for instance, catalytic reaction rates and extracellular substance exchange, among others. On the other hand, nanoconfined volumes influence physicochemical events such as fluid mobility.266 Taken together, these factors indicate that nanoporous surfaces have the potential for enhanced loading ability and the capacity to provide controlled dissolution rates over time.
The greater surface area provided by nanopores can be synergistically exploited to link more efficiently bioactive molecules to metals, with or without the use of intermediate linking agents such as silanes and phosphonates.267 To this end, it will be interesting to compare the outcome of smooth and nanostructured surfaces with covalently-linked synthetic peptides known to promote cell adhesion and osteoblastic differentiation such as (1) the noncollagen-derived RGD,268 and (2) the collagen-derived P-15,269 DGEA (Aspartate-Glycine-Glutamate-Alanine)270 and GFOGER (Glycine-Phenylalanine-Hydroxyproline-Glycine-Glutamate-Arginine)271 collagen-related cell binding peptides. However, the best linking agents for bioeffector molecules are likely to be more complex molecules, capable of forming multiple stable covalent links (e.g. multidentate molecules) onto metallic surfaces while creating lateral self-assembly. This is also expected to result in the capacity of generating various molecular patterns, with tunable properties (e.g. patterned geometry, nature and orientation of active groups), even on surfaces which are not flat at the atomic scale (such as those of commercially-available implantable metals). In this way, it will be possible to extend the panoply of tailored nanostructures by designing organic patterns otherwise non-easily achievable by direct physicochemical methods. In addition, we can envisage molecular arrays capable of responding to specific variations of the surrounding biological environment, such as local changes in the pH or temperature at the material-host tissue interface, paving the way for novel biomaterials.
We believe that future generations of implants should aim at “intelligent” surfaces, capable not only of providing cues to the surrounding tissues but also of receiving and responding to events occurring in them. Here, we anticipate that synergistic approaches will be critical to achieve the challenging goal of endowing implantable materials with the capacity to “dialogue” with the surrounding environment. For example, nanoporous surfaces could be further functionalized with molecular arrays or multi-layered coatings that would in a first step provide signaling to cells and that could in-time respond to local changes in the environment, exposing underlying nanopatterns capable of physical cueing or drug release. It may be conceived that molecular reorganization could also be initiated by external stimuli, such as infrared light, ultrasounds and magnetic fields, for instance. Similarly, functionalized nanoparticles could be embedded in molecular arrays sensitive to local chemical variations.
There is also limited information on whether the release of toxic metallic ions can be reduced by nanoengineered surfaces. For example, chemical treatments that, besides generating nanoscale cues, yield an increase in the protective oxide layer could be a possible solution to better ‘insulate’ metals from the surrounding biological environment. In addition, it will be interesting to determine whether surface nanostructures can by themselves alter the dynamics of dissolution of metals and thus reduce cytotoxic effects. This could be the case for nanoconfined volumes such as nanotubes and nanopororosity, coatings with HA nanocrystals, and functionalization with molecular arrays.
A major challenge in regenerative medicine is the capacity to attract local stem cells and to induce their proliferation and differentiation. Physicochemical surface characteristics may offer an interesting strategy to achieve such a capacity around implantable metals and other biomaterials. In addition, biochemical functionalization could be an efficient complement to physical cueing. In this context, in vitro studies have already shown that nanoscale surface cueing can stimulate stem cell growth and guide their differentiation without exposure to molecular signals.50,272 Although this aspect is just beginning to be explored and still lacks in vivo validation, current results are certainly promising for skeletal applications. An exciting new development in biology is the finding that stem cells can also be obtained from skin, thus rendering them more accessible.273 Bone reconstruction around an implant could thus be induced by exploiting the ability of nanostructured surfaces to activate stem cells and to accelerate bone formation while avoiding tissue encapsulation.51,52,274 This will translate into faster healing and improved stability of dental and orthopedic prostheses, critical aspects especially in aging patients with poor bone support.
In conclusion, nanoscale surface modification approaches are likely to foster profound changes in the ways implants and other biomaterials are designed and manufactured. The application of medical prostheses to improve health and even save lives will continue to grow until tissue engineering reaches a mature level and regeneration of complete tissues can be achieved. Therefore, any procedure that can improve the performance of implantable biomaterials will have a major impact on the quality of life and result in a major economic benefits to society. Advances in metallurgical and surface-engineering techniques, and nanotechnology promise a new generation of improved prosthetic devices with selective bioactive surfaces, and eventually with “intelligent surfaces” capable of responding to the implantation site environment.
Acknowledgements
A.N. acknowledges funding from the Canada Foundation for Innovation and a Collaborative Health Research Project grant funded jointly by the Canadian Institutes of Health Research (CIHR) and the Natural Sciences and Engineering Research Council of Canada (NSERC), and separate funding from the CIHR. AN and JB are also recipients of National Institute of Health (NIH) funding 7R01 EB000504-07. PTO acknowledges support from the State of São Paulo Research Foundation (FAPESP) and the National Council of Scientific and Technological Development (CNPq). Caption figure was produced by Lightbox Visual Communication Inc.References
- S. Kurtz, K. Ong, E. Lau, F. Mowat and M. Halpern, J. Bone Joint Surg. Am., 2007, 89, 780 CrossRef.
- J. M. Hodgson, R. K. Bottner, L. W. Klein, H. T. Walpole, D. J. Cohen, D. E. Cutlip, R. B. Fenninger, B. G. Firth, D. Greenberg, I. Kalisky, T. Meskan, W. Powell, G. W. Stone, J. P. Zito and M. A. Clark, Catheter. Cardiovasc. Interventions, 2004, 62, 1 Search PubMed.
- D. J. Kereiakes, J. K. Choo, J. J. Young and T. M. Broderick, Rev. Cardiovasc. Med., 2004, 5, 9 Search PubMed.
- J. Y. Wong and J. D. Bronzino, Biomaterials, CRC Press, Boca Raton USA, 2007 Search PubMed.
- B. D. Ratner, A. S. Hoffman, F. J. Schoen and J. E. Lemons, Biomaterials Science: An Introduction to Materials in Medicine, Elsevier Academic Press, San Diego USA, 2004 Search PubMed.
- S. A. Brown and J. E. Lemons, Medical Applications of Titanium and Its Alloys: The material and biological issues, West Conshohocken USA, 1996 Search PubMed.
- G. Mani, M. D. Feldman, D. Patel and C. M. Agrawal, Biomaterials, 2007, 28, 1689 CrossRef CAS.
- S. V. Bhat, Biomaterials, London UK, 2002 Search PubMed.
- J. Black, Corrosion, degradation. Orthopedic biomaterials in research, practise, Livingstone, New York, 1998 Search PubMed.
- J. D. Bobyn, G. J. Stackpool, S. A. Hacking, M. Tanzer and J. J. Krygier, J. Bone Joint. Surg. B, 1999, 81, 907 Search PubMed.
- F. Witte, Acta Biomater., 2010, 6, 1680 CrossRef CAS.
- A. Purnama, H. Hermawan, J. Couet and D. Mantovani, Acta Biomater., 2010, 6, 1800 CrossRef CAS.
- H. Hermawan, D. Dubé and D. Mantovani, Acta Biomater., 2010, 6, 1693 CrossRef CAS.
- H. Liu and T. J. Webster, Biomaterials, 2006, 28, 354.
- K. v. d. Mark, S. Bauer and P. Schmuki, Cell Tissue Res., 2010, 339, 131 CrossRef.
- G. A. Horley, Small, 2006, 2, 3 CrossRef CAS.
- G. M. Whitesides, Nat. Biotechnol., 2003, 21, 1161 CrossRef CAS.
- J. Y. Rho, L. K. Spearing and P. Zioupos, Med. Eng. Phys., 1998, 20, 92 CrossRef CAS.
- N. J. Sniadecki, R. A. Desai, S. A. Ruiz and C. S. Chen, Ann. Biomed. Eng., 2006, 34, 59 CrossRef.
- M. M. Stevens and J. H. George, Science, 2005, 310, 1135 CrossRef CAS.
- F. Variola, F. Vetrone, L. Richert, P. Jedrzejowski, J.-H. Yi, S. Zalzal, S. Clair, A. Sarkissian, D. F. Perepichka, J. D. Wuest, F. Rosei and A. Nanci, Small, 2009, 5, 996 CrossRef CAS.
- R. Barbucci, D. Pasqui, A. Wirsen, S. Affrossman, A. Curtis and C. Tetta, J. Mater. Sci.: Mater. Med., 2003, 14, 721 CrossRef CAS.
- X. Liu, P. K. Chu and C. Ding, Mater. Sci. Eng., R, 2004, 47, 49 CrossRef.
- S. Guizzardi, C. Galli, D. Martini, S. Belletti, A. Tinti, M. Raspanti, P. Taddei, A. Ruggeri and R. Scandroglio, J. Periodontol., 2004, 75, 273 CrossRef.
- K. Anselme and M. Bigerelle, Acta Biomater., 2005, 1, 211 CrossRef CAS.
- M. Bigerelle, K. Anselme, B. Noel, I. Ruderman, P. Hardouin and A. Iost, Biomaterials, 2002, 23, 1563 CrossRef CAS.
- K. Anselme and M. Bigerelle, J. Mater. Sci.: Mater. Med., 2006, 17, 471 CrossRef CAS.
- M.-J. Kim, M.-U. Choi and C.-W. Kim, Biomaterials, 2006, 27, 5502 CrossRef CAS.
- C. Aparicio, F. J. Gil, J. A. Planell and E. Engel, J. Mater. Sci.: Mater. Med., 2002, 13, 1105 CrossRef CAS.
- J.-W. Park, I.-S. Jang and J.-Y. Suh, J. Biomed. Mater. Res., Part B, 2008, 84b, 400 CrossRef CAS.
- H. Daugaard, B. Elmengaard, J. E. Bechtold and K. Soballe, J. Biomed. Mater. Res., Part A, 2008, 87a, 434 CrossRef CAS.
- A. Piattelli, R. Celletti, V. C. Marinho, T. Traini, G. Orsini, G. Bracchetti and V. Perrotti, J. Long Term Eff. Med. Implants, 2006, 16, 131 Search PubMed.
- M. Piattelli, A. Scarano, M. Paolantonio, G. Iezzi, G. Petrone and A. Piattelli, J. Oral Implantol., 2002, 28, 2 Search PubMed.
- D. Khang, J. Lu, C. Yao, K. M. Haberstroh and T. J. Webster, Biomaterials, 2008, 29, 970 CrossRef CAS.
- G. Mendonça, D. B. S. Mendonça, F. J. L. Aragao and L. F. Cooper, Biomaterials, 2008, 29, 3822 CrossRef CAS.
- K.-H. Kim and N. Ramaswamy, Dent. Mater. J., 2009, 28, 20 CrossRef CAS.
- B. Ercan and T. J. Webster, Biomaterials, 2010, 31, 3684 CrossRef CAS.
- G. Balasundaram, C. Yao and T. J. Webster, J. Biomed. Mater. Res., Part A, 2008, 84a, 447 CrossRef CAS.
- J. M. Macak, H. Tsuchiya, L. Taveira, A. Ghicov and P. Schmuki, J. Biomed. Mater. Res., Part A, 2005, 75a, 928 CrossRef CAS.
- C. Yao, E. B. Slamovich and T. J. Webster, J. Biomed. Mater. Res., Part A, 2008, 85a, 157 CrossRef CAS.
- J. Park, S. Bauer, K. A. Schlegel, F. W. Neukam, K. v. d. Mark and P. Schmuki, Small, 2009, 5, 666 CrossRef.
- S.-H. Oh, R. R. Finones, C. Daraio, L.-H. Chen and S. Jin, Biomaterials, 2005, 26, 4938 CrossRef CAS.
- S. Bauer, S. Kleber and P. Schmuki, Electrochem. Commun., 2006, 8, 1321 CrossRef CAS.
- J. Park, S. Bauer, K. v. d. Mark and P. Schmuki, Nano Lett., 2007, 7, 1686 CrossRef CAS.
- J. He, W. Zhou, X. Zhou, X. Zhong, X. Zhang, P. Wan, B. Zhu and W. Chen, J. Mater. Sci.: Mater. Med., 2008, 19, 3465 CrossRef CAS.
- S. C. Roy, M. Paulose and C. A. Grimes, Biomaterials, 2007, 28, 4667 CrossRef CAS.
- T. Sjostrom, M. J. Dalby, A. Hart, R. Tare, R. O. C. Oreffo and B. Su, Acta Biomater., 2009, 5, 1433 CrossRef CAS.
- R. Narayanan, S.-Y. Kim, T.-Y. Kwon and K.-H. Kim, J. Biomed. Mater. Res., Part A, 2008, 87a, 1053 CrossRef CAS.
- C. Lin, H. Han, F. Zhang and A. Li, J. Mater. Sci.: Mater. Med., 2008, 19, 2569 CrossRef CAS.
- F. Vetrone, F. Variola, P. T. Oliveira, S. F. Zalzal, J.-H. Yi, J. Sam, K. F. Bombonato-Prado, A. Sarkissian, D. F. Perepichka, J. D. Wuest, F. Rosei and A. Nanci, Nano Lett., 2009, 9, 659 CrossRef CAS.
- F. Variola, J.-H. Yi, L. Richert, J. D. Wuest, F. Rosei and A. Nanci, Biomaterials, 2008, 29, 1285 CrossRef CAS.
- L. Richert, F. Vetrone, J.-H. Yi, S. F. Zalzal, J. D. Wuest, F. Rosei and A. Nanci, Adv. Mater., 2008, 20, 1488 CrossRef CAS.
- F. Variola, A. Lauria, A. Nanci and F. Rosei, Adv. Eng. Mater., 2009, 11, B227 CrossRef.
- M. Yoshinari, Y. Oda, T. Kato and K. Okuda, Biomaterials, 2001, 22, 2043 CrossRef CAS.
- L. F. Cooper, Y. Zhou, J. Takebe, J. Guo, A. Abron, A. Holmén and J. E. Ellingsen, Biomaterials, 2006, 27, 926 CrossRef CAS.
- L. J. Smith, J. S. Swaim, C. Yao, K. M. Haberstroh, E. A. Nauman and T. J. Webster, Int. J. Nanomedicine, 2007, 2, 493 Search PubMed.
- V. V. D. Rani, K. Manzoor, D. Menon, N. Selvamurugan and S. V. Nair, Nanotechnology, 2009, 20, 195101 CrossRef.
- E. Eisenbarth, D. Velten and J. Breme, Biomol. Eng., 2007, 24, 27 CrossRef CAS.
- S. Popescu, I. Demetrescu, C. Sarantopoulos, A. N. Gleizes and D. Iordachescu, J. Mater. Sci.: Mater. Med., 2007, 18, 2075 CrossRef CAS.
- E. Eisenbarth, D. Velten, M. Muller, R. Thull and J. Breme, J. Biomed. Mater. Res., Part A, 2006, 79a, 166 CrossRef CAS.
- M. Amaral, A. G. Dias, P. S. Gomes, M. A. Lopes, R. F. Silva, J. D. Santos and M. H. Fernandes, J. Biomed. Mater. Res., Part A, 2008, 87a, 91 CrossRef CAS.
- T. Das, D. Ghosh, T. K. Bhattacharyya and T. K. Maiti, J. Mater. Sci.: Mater. Med., 2007, 18, 493 CrossRef CAS.
- R. K. Roy and K.-R. Lee, J. Biomed. Mater. Res., Part B, 2007, 83b, 72 CrossRef CAS.
- M. Morra, Eur. Cell. Mater., 2006, 12, 1 CAS.
- R. Beutner, J. Michael, B. Schwenzer and D. Scharnweber, J. R. Soc. Interface, 2010, 7, S93 CrossRef CAS.
- H. Schliephake and D. Scharnweber, J. Mater. Chem., 2008, 18, 2404 RSC.
- S. Bierbaum, et al. , J. Biomed. Mat. Res. A, 2003, 67, 421 Search PubMed.
- Y. Ku, C.-P. Chung and J.-H. Jang, Biomaterials, 2005, 26, 5153 CrossRef CAS.
- R. Junker, A. Dimakis, M. Thoneick and J. A. Jansen, Clin. Oral Implants Res., 2009, 20, 185 CrossRef.
- G. C. O'Toole, E. Salih, C. Gallagher, D. FitzPatrick, N. O'Higgins and S. K. O'Rourke, J. Orthop. Res., 2004, 22, 641 CrossRef CAS.
- U. M. Wikesjö, Y. H. Huang, A. V. Xiropaidis, R. G. Sorensen, M. D. Rohrer, H. S. Prasad, J. M. Wozney and J. Hall, J. Clin. Periodontol., 2008, 35, 992 CrossRef CAS.
- C. Schouten, G. J. Meijer, J. J. v. d. Beucken, P. H. Spauwen and J. A. Jansen, Clin. Oral Implants Res., 2009, 20, 421 CrossRef CAS.
- C. Susin, M. Qahash, G. Polimeni, P. H. Lu, H. S. Prasad, M. D. Rohrer, J. Hall and U. M. Wikesjö, J. Clin. Periodontol., 2010, 37, 574 CrossRef CAS.
- C. Bussy, R. Verhoef, A. Haeger, M. Morra, J. L. Duval, P. Vigneron, A. Bensoussan, E. Velzenberger, G. Cascardo, C. Cassinelli, H. Schols, J. P. Knox and M. D. Nagel, J. Biomed. Mater. Res. A, 2008, 86, 597 CrossRef.
- J. D. Bumgardner, B. M. Chesnutt, Y. Yuan, Y. Yang, M. Appleford, S. Oh, R. McLaughlin, S. H. Elder and J. L. Ong, Implant Dent., 2007, 16, 66 CrossRef.
- A. H. Greene, J. D. Bumgardner, Y. Yang, J. Moseley and W. O. Haggard, Clin. Orthop. Relat. Res., 2008, 466, 1699 Search PubMed.
- A. Caro, V. Humblot, C. Méthivier, M. Minier, M. Salmain and C. M. Pradier, J. Phys. Chem. B, 2009, 113, 2101 CrossRef CAS.
- G. Cardenas, P. Anaya, C. v. Plessing, C. Rojas and J. Sepulveda, J. Mater. Sci.: Mater. Med., 2008, 19, 2397 CrossRef CAS.
- H. J. Martin, K. H. Schulz, J. D. Bumgardner and K. B. Walters, Langmuir, 2007, 23, 6645 CrossRef CAS.
- V. Antoci, C. S. Adams, J. Parvizi, P. Ducheyne, I. M. Shapiro and N. J. Hickok, Clin. Orthop. Relat. Res., 2007, 461, 81 Search PubMed.
- V. Antoci, S. B. King, B. Jose, J. Parvizi, A. R. Zeiger, E. Wickstrom, T. A. Freeman, R. J. Composto, P. Ducheyne, I. M. Shapiro, N. J. Hickok and C. S. Adams, J. Orthop. Res., 2007, 25, 858 CrossRef CAS.
- R. O. Darouiche, M. D. Mansouri, D. Zakarevicz, A. AlSharif and G. C. Landon, J. Bone Joint. Surg. Am., 2007, 89, 792 CrossRef.
- A. L. Chun, J. G. Moralez, T. J. Webster and H. Fenniri, Biomaterials, 2005, 26, 7304 CrossRef CAS.
- A. L. Chun, J. G. Moralez, H. Fenniri and T. J. Webster, Nanotechnology, 2004, 15, S234 CrossRef CAS.
- E. Jansson and P. Tengvall, Colloids Surf., B, 2004, 35, 45 CrossRef CAS.
- R. Muller, J. Abke, E. Schnell, D. Scharnweber, R. Kujat, C. Englert, D. Taheri, M. Nerlich and P. Angele, Biomaterials, 2006, 27, 4059 CrossRef.
- A. Nanci, et al. , J. Biomed. Mater. Res., 1998, 40, 324 CrossRef CAS.
- G. K. Toworfe, S. Bhattacharyya, R. J. Composto, C. S. Adams, I. M. Shapiro and P. Ducheyne, J. Tissue Eng. Regener. Med., 2009, 3, 26 Search PubMed.
- C. Viornery, et al. , J. Biomed. Mater. Res., 2002, 62, 149 CrossRef CAS.
- D. Falconnet, G. Csucs, H. M. Grandin and M. Textor, Biomaterials, 2006, 27, 3044 CrossRef CAS.
- E. Monchaux and P. Vermette, J. Biomed. Mater. Res. A, 2008, 85, 1052 CrossRef.
- K. N. Sask, I. Zhitomirsky, L. R. Berry, A. K. Chan and J. L. Brash, Acta Biomater., 2010, 6, 2911 CrossRef CAS.
- Y. L. Jeyachandran, J. A. Mielczarski, E. Mielczarski and B. Rai, J. Colloid Interface Sci., 2010, 341, 136 CrossRef CAS.
- T. G. Kim, Y. Lee and T. G. Park, Int. J. Pharm., 2010, 384, 181 CrossRef CAS.
- A. Mahapatro, D. M. Johnson, D. N. Patel, M. D. Feldman, A. A. Ayon and C. M. Agrawal, Curr. Top. Med. Chem., 2008, 8, 281 CrossRef CAS.
- K. Nakano, K. Egashira, S. Masuda, K. Funakoshi, G. Zhao, S. Kimura, T. Matoba, K. Sueishi, Y. Endo, Y. Kawashima, K. Hara, H. Tsujimoto, R. Tominaga and K. Sunagawa, JACC Cardiovasc. Interv., 2009, 2, 277 Search PubMed.
- A. Reising, C. Yao, D. Storey and T. J. Webster, J. Biomed. Mater. Res., Part A, 2008, 87a, 78 CrossRef CAS.
- B. Großner-Schreiber, M. Herzog, J. Hedderich, A. Duck, M. Hannig and M. Griepentrog, Clin. Oral Implants Res., 2006, 17, 736 CrossRef.
- T. Ogawa, L. Saruwatari, K. Takeuchi, H. Aita and N. Ohno, J. Dent. Res., 2008, 87, 751 CrossRef CAS.
- M. Hovgaard, J. Chevallier, M. Foss and F. Besenbacher, Appl. Phys. Lett., 2005, 87, 073105 CrossRef.
- T. R. Rautray, R. Narayanan, T.-Y. Kwon and K.-H. Kim, J. Biomed. Mater. Res. Part B: Applied Biomater., 2010, 93b, 181 Search PubMed.
- T. Hanawa, Mater. Sci. Eng., A, 1999, 267, 260 CrossRef.
- T. Hanawa, Y. Kamiura, S. Yamamoto, T. Kohgo, A. Amemiya, H. Ukai, K. Murakami and K. Asaoka, J. Biomed. Mater. Res., 1997, 36, 131 CrossRef CAS.
- L. Saldana, V. Barranco, J. L. Gonzalez-Carrasco, M. Rodrıguez, L. Munuera and N. Vilaboa, J. Biomed. Mater. Res. A, 2007, 81, 334 CrossRef CAS.
- L. Saldana, N. Vilaboa, G. Valles, J. Gonzalez-Cabrero and L. Munuera, J. Biomed. Mater. Res. A, 2005, 73, 97 CrossRef CAS.
- A. J. Engler, P. O. Humbert, B. Wehrle-Haller and V. M. Weaver, Science, 2009, 324, 208 CrossRef.
- B. Kasemo, Surf. Sci., 2002, 500, 656 CrossRef CAS.
- K. Anselme, Biomaterials, 2000, 21, 667 CrossRef CAS.
- D. A. Puleo and A. Nanci, Biomaterials, 1999, 20, 2311 CrossRef CAS.
- B. Kasemo and J. Gold, Adv. Dent. Res., 1999, 13, 8 Search PubMed.
- N. Hori, W. Att, T. Ueno, N. Sato, M. Yamada, L. Saruwatari, T. Suzuki and T. Ogawa, J. Dent. Res., 2009, 88, 663 CrossRef CAS.
- M. E. Nagassa, A. E. Daw, W. G. Rowe, A. Carley, D. W. Thomas and R. Moseley, Clin. Oral Implants Res., 2008, 19, 1317 CrossRef CAS.
- C. J. Wilson, R. E. Clegg, D. I. Leavesley and M. J. Pearcy, Tissue Eng., 2005, 11, 1 CrossRef CAS.
- D. Gugutkov, G. Altankov, J. C. R. Hernández, M. M. Pradas and M. S. Sánchez, J. Biomed. Mater. Res. A, 2010, 92, 322 CrossRef.
- M. N. Sela, L. Badihi, G. Rosen, D. Steinberg and D. Kohavi, Clin. Oral Implants Res., 2007, 18, 630 CrossRef.
- P. Roach, D. Farrar and C. C. Perry, J. Am. Chem. Soc., 2006, 128, 3939 CrossRef CAS.
- A. G. Hemmersam, M. Foss, J. Chevallier and F. Besenbacher, Colloids Surf., B, 2005, 43, 208 CrossRef CAS.
- F. A. Denis, P. Hanarp, D. S. Sutherland, J. Gold, C. Mustin, P. G. Rouxhet and Y. F. Dufrene, Langmuir, 2002, 18, 819 CrossRef CAS.
- A. Dolatshahi-Pirouz, K. Rechendorff, M. B. Hovgaard, M. Foss, J. Chevallier and F. Besenbacher, Colloids Surf., B, 2008, 66, 53 CrossRef CAS.
- M. B. Hovgaard, K. Rechendorff, J. Chevallier, M. Foss and F. Besenbacher, J. Phys. Chem. B, 2008, 112, 8241 CrossRef CAS.
- K. Rechendorff, M. B. Hovgaard, M. Foss, V. P. Zhdanov and F. Besenbacher, Langmuir, 2006, 22, 10885 CrossRef CAS.
- L. Richert, F. Variola, F. Rosei, J. D. Wuest and A. Nanci, Surf. Sci., 2010, 604, 1445 CrossRef CAS.
- K. Cai, J. Bossert and K.D.J, Colloids Surf., B, 2006, 49, 136 CrossRef CAS.
- J. Carpenter, D. Khang and T. J. Webster, Nanotechnology, 2008, 19, 505103 CrossRef.
- C. González-García, S. R. Sousa, D. Moratal, P. Rico and M. Salmerón-Sánchez, Colloids Surf B Biointerfaces, 2010, 77, 181 CrossRef CAS.
- T. D. Pollard and W. C. Earnshaw, Cell Biology, 2002 Search PubMed.
- B. Ercan and T. J. Webster, Int. J. Nanomed., 2008, 3, 477 Search PubMed.
- C.-Y. Chiang, S.-H. Chiou, W.-E. Yang, M.-L. Hsu, M.-C. Yung, M.-L. Tsai, L.-K. Chen and H.-H. Huang, Dent. Mater., 2009, 25, 1022 CrossRef CAS.
- S. Bauer, J. Park, J. Faltenbacher, S. Berger, K. v. d. Mark and P. Schmuki, Integr. Biol., 2009, 1, 525 RSC.
- J. Park, S. Bauer, P. Schmuki and K. v. d. Mark, Nano Lett., 2009, 9, 3157 CrossRef CAS.
- K. Burns, C. Yao and T. J. Webster, J. Biomed. Mater. Res., Part A, 2009, 88a, 561 CrossRef CAS.
- S. D. Puckett, P. P. Lee, D. M. Ciombor, R. K. Aaron and T. J. Webster, Acta Biomater., 2010, 6, 2352 CrossRef CAS.
- S. D. Puckett, E. Taylor, T. Raimondo and T. J. Webster, Biomaterials, 2010, 31, 706 CrossRef CAS.
- R. L. Price, K. Ellison, K. M. Haberstroh and T. J. Webster, J. Biomed. Mater. Res., 2004, 70a, 129 Search PubMed.
- P. T. Oliveira and A. Nanci, Biomaterials, 2004, 25, 403 CrossRef.
- P. T. Oliveira, S. F. Zalzal, M. M. Beloti, A. L. Rosa and A. Nanci, J. Biomed. Mater. Res., Part A, 2007, 80a, 554 CrossRef.
- D. Gingell, Symp. Soc. Exp. Biol., 1993, 47, 1 Search PubMed.
- J. L. Sechler, S. A. Corbett, M. B. Wenk and J. E. Schwarzbauer, Ann. N. Y. Acad. Sci., 1998, 857, 143 CrossRef CAS.
- J. L. Sepulveda, V. Gkretsi and C. Wu, Curr. Top. Dev. Biol., 2005, 68, 183 CrossRef CAS.
- Y. Mao and J. E. Schwarzbauer, Matrix Biol., 2005, 24, 389 CrossRef CAS.
- G. Mendonça, D. B. Mendonça, F. J. Aragão and L. F. Cooper, J. Biomed. Mater. Res. A, 2010, 94, 169.
- M. P. Bajgai, D. C. Parajuli, S.-J. Park, K. H. Chu, H.-S. Kang and H. Y. Kim, J. Mater. Sci.: Mater. Med., 2010, 21, 685 CrossRef CAS.
- S. Tosatti, et al. , J. Biomed. Mat. Res. A, 2004, 68, 458 Search PubMed.
- G. Balasundaram and T. J. Webster, J. Biomed. Mater. Res., Part A, 2007, 80a, 602 CrossRef CAS.
- A. G. Secchi, et al. , J. Biomed. Mater. Res., Part A, 2007, 83a, 577 CrossRef CAS.
- J. Auernheimer, et al. , BioChem., 2005, 6, 2034 CAS.
- T. Douglas, S. Heinemann, U. Hempel, C. Mietrach, C. Knieb, S. Bierbaum, D. Scharnweber and H. Worch, J. Mater. Sci.: Mater. Med., 2008, 19, 1653 CrossRef CAS.
- S. A. Hacking, M. Zuraw, E. J. Harvey, M. Tanzer, J. J. Krygier and J. D. Bobyn, J. Biomed. Mater. Res. A, 2007, 82, 179 CrossRef CAS.
- J. Takagi, B. M. Petre, T. Walz and T. A. Springer, Cell, 2002, 110, 599 CrossRef CAS.
- B. Alberts, Molecular biology of the cell, 5th edn, 2008 Search PubMed.
- Y.-L. Wang and D. E. Discher, Methods in Cell Biology - Cell Mechanics, Academic Press - Elsevier, Oxford, UK, 2007 Search PubMed.
- M. J. P. Biggs, R. G. Richards and M. J. Dalby, Nanomedicine: Nanotechnology, Biology, and Medicine, 2010, 6, 619 CrossRef.
- P. P. Girard, E. A. Cavalcanti-Adam, R. Kemkemer and J. P. Spatz, Soft Matter, 2007, 3, 307 RSC.
- X. R. Bustelo, V. Sauzeau and I. M. Berenjeno, BioEssays, 2007, 29, 356 CrossRef CAS.
- F. M. Watt, P. W. Jordan and C. H. O'Neill, Proc. Natl. Acad. Sci. U. S. A., 1988, 85, 5576 CrossRef CAS.
- J. C. Adams and F. M. Watt, Development, 1993, 117, 1183 CAS.
- C. D. Roskelley, P. Y. Desprez and M. J. Bissell, Proc. Natl. Acad. Sci. U. S. A., 1994, 91, 12378 CrossRef CAS.
- C. S. Chen, M. Mrksich, S. Huang, G. M. Whitesides and D. E. Ingber, Science, 1997, 276, 1425 CrossRef CAS.
- C. S. Chen, J. L. Alonso, E. Ostuni, G. M. Whitesides and D. E. Ingber, Biochem. Biophys. Res. Commun., 2003, 307, 355 CrossRef CAS.
- R. McBeath, D. M. Pirone, C. M. Nelson, K. Bhadriraju and C. S. Chen, Dev. Cell, 2004, 6, 483 CrossRef CAS.
- T. Albrektsson and A. Wennerberg, Int J Prosthodont., 2004, 17, 544 Search PubMed.
- M. M. Shalabi, A. Gortemaker, M. A. V. t. Hof, J. A. Jansen and N. H. Creugers, J. Dent. Res., 2006, 85, 496 CrossRef CAS.
- Y. T. Sul, C. Johansson, A. Wennerberg, L. R. Cho, B. S. Chang and T. Albrektsson, Int. J. Oral Maxillofac. Implants, 2005, 20, 349 Search PubMed.
- J. E. Davies, J Dent. Educ., 2003, 67, 932 Search PubMed.
- J. Y. Park and J. E. Davies, Clin. Oral Implants Res., 2000, 11, 530 CrossRef CAS.
- B. D. Boyan, S. Lossdorfer, L. Wang, G. Zhao, C. H. Lohmann, D. L. Cochran and Z. Schwartz, Eur. Cell Mater., 2003, 6, 22 CAS.
- K. Kieswetter, Z. Schwartz, T. W. Hummert, D. L. Cochran, J. Simpson, D. D. Dean and B. D. Boyan, J. Biomed. Mater. Res., 1996, 32, 55 CrossRef CAS.
- C. v. Wilmowsky, S. Bauer, R. Lutz, M. Meisel, F. W. Neukam and T. Toyoshima, et al. , J. Biomed. Mater. Res. B Appl. Biomater., 2009, 89, 165 CrossRef.
- M. Jäger, C. Zilkens, K. Zanger and R. Krauspe, J. Biomed. Biotechnol., 2007, 2007, 69036 Search PubMed.
- L. L. Guehennec, A. Soueidan, P. Layrolle and Y. Amouriq, Dent. Mater., 2007, 23, 844 CrossRef CAS.
- K. H. Park, S. J. Heo, J. Y. Koak, S. K. Kim, J. B. Lee, S. H. Kim and Y. J. Lim, J. Oral Rehabil., 2007, 34, 517 CrossRef CAS.
- G. L. Yang, F.M.H, J. A. Hu, X. X. Wang and S. F. Zhao, Oral Surg., Oral Med., Oral Pathol., Oral Radiol. Endodontol., 2009, 107, 782 Search PubMed.
- L. Meirelles, A. Arvidsson, M. Andersson, P. Kjellin, T. Albrektsson and A. Wennerberg, J. Biomed. Mater. Res. A, 2008, 87, 299 CrossRef.
- L. Meirelles, L. Melin, T. Peltola, P. Kjellin, I. Kangasniemi, F. Currie, M. Andersson, T. Albrektsson and A. Wennerberg, Clin. Implant Dent. Relat. Res., 2008, 10, 254 Search PubMed.
- Y. T. Sul, J. Jönsson, G. S. Yoon and C. Johansson, Clin. Oral Implants Res., 2009, 20, 1146 CrossRef.
- Y. T. Sul, B. S. Kang, B. Johansson, H. S. Um, C. J. Park and T. Albrektsson, J. Biomed. Mater. Res. A, 2009, 89, 942 CrossRef.
- Y. T. Sul, C. B. Johansson, E. Byon and T. Albrektsson, Biomaterials, 2005, 26, 6720 CrossRef CAS.
- Y. T. Sul, C. B. Johansson and T. Albrektsson, Int. J. Prosthodont., 2006, 19, 319 Search PubMed.
- M. G. Tavares, P. T. Oliveira, A. Nanci, A. C. Hawthorne, A. L. Rosa and S. P. Xavier, Clin. Oral Implants Res., 2007, 18, 452 CrossRef.
- J. Guo, R. J. Padilla, W. Ambrose, I. J. D. Kok and L. F. Cooper, Biomaterials, 2007, 28, 5418 CrossRef CAS.
- J. E. Ellingsen, C. B. Johansson, A. Wennerberg and A. Holmen, Int. J. Oral. Maxillofac. Implants, 2004, 19, 656 Search PubMed.
- T. Berglundh, I. Abrahamsson, J. P. Albouy and J. Lindhe, Clin. Oral Implants Res., 2007, 18, 147 CrossRef CAS.
- I. Abrahamsson, J. P. Albouy and T. Berglundh, Clin. Oral Implants Res., 2008, 19, 153 CrossRef.
- C. M. Stanford, G. K. Johnson, A. Fakhry, D. Gratton, J. T. Mellonig and W. Wanger, Appl. Osseointegration Res., 2006, 5, 50 Search PubMed.
- G. Oxby, J. Lindqvist and P. Nilsson, Appl. Osseointegration Res., 2006, 5, 68 Search PubMed.
- V. C. Mendes, R. Moineddin and J. E. Davies, Biomaterials, 2007, 28, 4748 CrossRef CAS.
- G. Orsini, M. Piattelli, A. Scarano, G. Petrone, J. Kenealy and A. Piattelli, et al. , J. Periodontol., 2007, 78, 209 CrossRef CAS.
- R. J. Goené, T. Testori and P. Trisi, Int. J. Periodontics Restorative Dent., 2007, 27, 211 Search PubMed.
- F. Vignoletti, M. d. Sanctis, T. Berglundh, I. Abrahamsson and M. Sanz, J. Clin. Periodontol., 2009, 36, 688 CrossRef.
- P. G. Coelho, G. Cardaropoli, M. Suzuki and J. E. Lemons, J. Biomed. Mater. Res. B Appl. Biomater., 2009, 88, 387 CrossRef.
- R. Granato, C. Marin, J. N. Gil, S. K. Chuang, T. B. Dodson, M. Suzuki and P. G. Coelho, Clin. Implant Dent. Relat. Res., 2009 DOI:10.1111/j.1708-8208.2009.00186.x.
- J. W. Park, H. K. Kim, Y. J. Kim, C. H. An and T. Hanawa, Clin. Oral Implants Res., 2009, 20, 684 CrossRef.
- J.-W. Park, J.-Y. Suh and H.-J. Chung, J. Biomed. Mater. Res. A, 2008, 86, 117 CrossRef CAS.
- J.-Y. Suh, O.-C. Jeung, B.-J. Choi and J.-W. Park, Clin. Oral Implants Res., 2007, 18, 362 CrossRef.
- C. Marin, R. Granato, M. Suzuki, J. N. Gil, A. Piattelli and P. G. Coelho, J. Periodontol., 2008, 79, 1942 CrossRef.
- J. A. Shibli, S. Grassi, A. Piattelli, G. E. Pecora, D. S. Ferrari, T. Onuma, S. d'Avila, P. G. Coelho, R. Barros and G. Iezzi, Clin. Implant Dent. Relat. Res., 2009 Search PubMed.
- E. M. Hetrick and M. H. Schoenfisch, Chem. Soc. Rev., 2006, 35, 780 RSC.
- L. Zhao, P. K. Chu, Y. Zhang and Z. Wu, J. Biomed. Mater. Res., Part B, 2009, 91b, 470 CrossRef CAS.
- M. Lucke, G. Schmidmaier, S. Sadoni, B. Wildemann, R. Schiller, N. P. Haas and M. Raschke, Bone, 2003, 32, 521 CrossRef CAS.
- T. Hanawa, J. Artif. Organs, 2009, 12, 73 Search PubMed.
- J. E. Puskas, L. G. Muñoz-Robledo, R. A. Hoerr, J. Foley, S. P. Schmidt, M. Evancho-Chapman, J. Dong, C. Frethem and G. Haugstad, Nanomedicine and Nanobiotechnology, 2009, 1, 451 Search PubMed.
- I. Tsujino, J. Ako, Y. Honda and P. J. Fitzgerald, Expert Opin. Drug Delivery, 2007, 4, 287 Search PubMed.
- H. Wieneke, O. Dirsch and T. Sawitowski, et al. , Catheter. Cardiovasc. Interventions, 2003, 60, 399 Search PubMed.
- E. Grube, American College of Cardiology meeting. Chicago, USA, 2003 Search PubMed.
- J. Gottlow, The implant design and biological response, Wiley-Blackwell, Iowa, 2009 Search PubMed.
- C. A. Simmons, S. A. Meguid and R. M. Pilliar, J. Biomed. Mater. Res., 2001, 55, 63 CrossRef CAS.
- O. Mouzin, K. Soballe and J. E. Bechtold, J. Biomed. Mater. Res., 2001, 58, 61 CrossRef CAS.
- C. A. Simmons, S. A. Meguid and R. M. Pilliar, J. Orthop. Res., 2001, 19, 187 CrossRef CAS.
- O. H. Van, J. Duyck, S. J. Vander, G. V. D. Perre, S. L. C. M. De, R. Puers and I. Naert, Clin. Oral Implants. Res., 1998, 9, 407.
- S. E. De, S. Jaecques, K. Vandamme, S. J. Vander and I. Naert, Clin. Oral Implants Res., 2005, 16, 402 CrossRef.
- J. Duyck, E. Slaets, K. Sasaguri, K. Vandamme and I. Naert, J. Clin. Periodontol., 2007, 34, 998 CrossRef.
- J. Duyck, K. Vandamme, L. Geris, O. H. Van, C. M. De, J. Vandersloten, R. Puers and I. Naert, Arch. Oral Biol., 2006, 51, 1 CrossRef CAS.
- C. T. Rubin and K. J. McLeod, Clin. Orthop. Relat Res., 1994, 298, 165.
- S. Szmukler-Moncler, H. Salama, Y. Reingewirtz and J. H. Dubruille, J. Biomed. Mater. Res., 1998, 43, 192 CrossRef CAS.
- K. Vandamme, I. Naert, L. Geris, S. J. Vander, R. Puers and J. Duyck, J. Clin. Periodontol., 2007, 34, 172.
- J. B. Brunski, Adv. Dent. Res., 1999, 13, 99 Search PubMed.
- P. Leucht, J. B. Kim, R. Wazen, J. A. Currey, A. Nanci, J. B. Brunski and J. A. Helms, Bone, 2006, 40, 919.
- A. Jokstad, U. Braegger, J. B. Brunski, A. B. Carr, I. Naert and A. Wennerberg, Int. Dent. J., 2003, 53, 409 Search PubMed.
- E. G. Loboa, T. D. Fang, S. M. Warren, D. P. Lindsey, K. D. Fong, M. T. Longaker and D. R. Carter, Bone, 2004, 34, 336 CrossRef.
- J. D. Humphrey, J. Biomech. Eng., 2001, 123, 638 CrossRef CAS.
- J. B. Brunski, J. A. Currey, J. A. Helms, P. Leucht, A. Nanci and R. M. Wazen, The healing bone-implant interface: role of micromotion and related strain levels in tissue, A. Jokstad, Iowa, 2008 Search PubMed.
- L. Claes, R. Blakytny, M. Göckelmann, M. Schoen, A. Ignatius and B. Willie, J. Orthop. Res., 2009, 27, 22 CrossRef.
- L. E. Claes, H. J. Wilke, P. Augat, S. Rübenacker and K. J. Margevicius, Clin. Biomech., 1995, 10, 227 CrossRef.
- L. Claes, P.A.P, S. Schorlemmer, C. Konrads, A. Ignatius and C. Ehrnthaller, J. Orthop. Res., 2008, 26, 772 CrossRef.
- T. Albrektsson and A. Wennerberg, Int. J. Prosthodont., 2004, 17, 536 Search PubMed.
- R. M. Leven, A. S. Virdi and D. R. Sumner, J. Biomed. Mater. Res., 2004, 70a, 391 Search PubMed.
- C. Masaki, G. B. Schneider, R. Zaharias, D. Seabold and C. Stanford, Clin. Oral Implants Res., 2005, 16, 650 CrossRef.
- T. Ogawa and I. Nishimura, J. Dent. Res., 2006, 85, 566 CrossRef CAS.
- Z. M. Isa, G. B. Schneider, R. Zaharias, D. Seabold and C. M. Stanford, Int. J. Maxillofac. Implants, 2006, 21, 203 Search PubMed.
- D. E. Discher, D. J. Mooney and P. W. Zandstra, Science, 2009, 324, 1673 CrossRef CAS.
- P. Leucht, J. B. Kim, J. A. Currey, J. B. Brunski and J. A. Helms, PLoS One, 2007, 2 Search PubMed e390-391-e390.
- E. K. Yim and K. W. Leong, Nanomed.: Nanotechnol., Biol. Med., 2005, 1, 10 CrossRef CAS.
- M. J. Dalby, D. McCloy, M. Robertson, H. Agheli, D. Sutherland, S. Affrossman and R. O. Oreffo, Biomaterials, 2006, 27, 2980 CrossRef CAS.
- J. C. Hansen, J. Y. Lim, L. C. Xu, C. A. Siedlecki, D. T. Mauger and H. J. Donahue, J. Biomech., 2007, 40, 2865 CrossRef.
- A. Sargeant and T. Goswami, Mater. Des., 2007, 28, 155 CrossRef CAS.
- Y. Li, C. Wong, J. Xiong, P. Hodgson and C. Wen, J. Dent. Res., 2010, 89, 493 CrossRef CAS.
- T. A. G. Donato, L. H. d. Almeida, R. A. Nogueira, T. C. Niemeyer, C. R. Grandini, R. Caram, S. G. Schneider and A. R. Santos, Mater. Sci. Eng., C, 2009, 29, 1365 CrossRef CAS.
- J. Y. Lim, A. D. Dreiss, Z. Zhou, J. C. Hansen, C. A. Siedlecki, R. W. Hengstebeck, J. Cheng, N. Winograd and H. J. Donahue, Biomaterials, 2007, 28, 1787 CrossRef CAS.
- K. Das, S. Bose and A. Bandyopadhyay, J. Biomed. Mater. Res., Part A, 2009, 90a, 225 CrossRef CAS.
- H. Noh and E. A. Vogler, Biomaterials, 2007, 28, 405 CrossRef CAS.
- C. M. Alves, R. L. Reis and J. A. Hunt, J. R. Soc. Interface, 2010, 7, 1367 CrossRef CAS.
- Anderson, et al. , Semin. Immunol., 2008, 20, 86 CrossRef CAS.
- R. M. Wazen, C. E. Tye, H. A. Goldberg, G. K. Hunter, C. E. Smith and A. Nanci, J. Histochem. Cytochem., 2007, 55, 35 CAS.
- D. L. Cochran, J. Periodontol., 1999, 70, 1523 CrossRef CAS.
- D. Morton, R. Jaffin and H. P. Weber, Int. J. Oral. Maxillofac. Implants, 2004, 19(Suppl.), 103 Search PubMed.
- L. Tolstunov, Implant Dent., 2006, 15, 341 CrossRef.
- R. A. Jaffin and C. L. Berman, J. Periodontol., 1991, 62, 2 CAS.
- C. A. Bain, Int. J. Oral Maxillofac. Implants, 1996, 11, 756 Search PubMed.
- J. P. Fiorellini, P. K. Chen, M. Nevins and M. L. Nevins, Int. J. Periodontics Restorative Dent., 2000, 20, 366 Search PubMed.
- P.-O. Ostman, M. Hellman, T. Albrektsson and L. Sennerby, Clin. Oral Implants Res., 2007, 18, 409 CrossRef.
- T. Albrektsson, in Annual Meeting of the Academy of Dental Materials, Trieste (Italy), Editon edn, 2010 Search PubMed.
- L. M. Svanborg, A.W and M. Andersson, J. Biomed. Mater. Res. B Appl. Biomater., 2010, 92, 462.
- A. Wennerberg and T. Albrektsson, Clin. Oral Implants Res., 2009, 20, 172 CrossRef.
- S. J. Marshall, S. C. Bayne, R. Baier, A. P. Tomsi and G. W. Marshall, Dent. Mater., 2010, 26, e11 CrossRef.
- K. C. Popat, M. Eltgroth and T. A. Desai, Small, 2007, 3, 1878 CrossRef CAS.
- S. A. Redey, S. Razzouk, C. Rey, D. Bernache-Assollant, G. Leroy, M. Nardin and G. Cournot, J. Biomed. Mater. Res., 1999, 45, 140 CrossRef CAS.
- J. Wei, M. Yoshinari, S. Takemoto, M. Hattori, E. Kawada, B. Liu and Y. Oda, J. Biomed. Mater. Res., Part B, 2007, 81b, 66 CrossRef CAS.
- S. Brandon, N. Haimovich, E. Yeger and A. Marmur, J. Colloid Interface Sci., 2003, 263, 237 CrossRef CAS.
- A. Marmur, Soft Matter, 2006, 2, 12 RSC.
- T. S. Meiron, A. Marmur and I. S. Saguy, J. Colloid Interface Sci., 2004, 274, 637 CrossRef CAS.
- S. Choudhary, K. M. Haberstroh and T. J. Webster, Tissue Eng., 2007, 13, 1421 CrossRef CAS.
- E. Fine, L. Zhang, H. Fenniri and T. J. Webster, Int. J. Nanomedicine, 2009, 4, 91 Search PubMed.
- G. Lewis, J. Biomed. Mater. Res., Part B, 2008, 86b, 569 CrossRef CAS.
- N. Kukreja, Y. Onuma, J. Daemen and P. W. Serruys, Pharmacol. Res., 2008, 57, 171 CrossRef CAS.
- L. Yang and T. J. Webster, Expert Opin. Drug Delivery, 2009, 6, 851 Search PubMed.
- N. Naguib, H. Ye, Y. Gogotsi, A. G. Yazicioglu, C. M. Megaridis and M. Yoshimura, Nano Lett., 2004, 4, 2237 CrossRef CAS.
- S. Clair, F. Variola, M. Kondratenko, P. Jedrzejowski, A. Nanci, F. Rosei and D. F. Perepichka, J. Chem. Phys., 2008, 128, 144705 CrossRef.
- G. Avila, K. Misch, P. Galindo-Moreno and H. L. Wang, Implant Dent., 2009, 18, 17 Search PubMed.
- A. Palmieri, F. Pezzetti, G. Brunelli, M. Martinelli, L. L. Muzio, A. Scarano, M. Degidi, A. Piattelli and F. Carinci, Implant Dent., 2008, 17, 100 Search PubMed.
- M. Mizuno, R. Fujisawa and Y. Kuboki, J. Cell. Physiol., 2000, 184, 207 CrossRef CAS.
- C. D. Reyes, T. A. Petrie, K. L. Burns, Z. Schwartz and A. J. García, Biomaterials, 2007, 28, 3228 CrossRef CAS.
- M. J. Dalby, N. Gadegaard, R. Tare, A. Andar, M. O. Riehle, P. Herzyk, C. D. W. Wilkison and R. O. C. Oreffo, Nat. Mater., 2008, 6, 997.
- T. Hodgkinson, X. F. Yuan and A. Bayat, Expert Rev. Med. Devices, 2009, 6, 621 Search PubMed.
- T. J. Webster, C. Ergun, R. H. Doremus, R. W. Siegel and R. Bizios, J. Biomed. Mater. Res., 2000, 51, 475 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2011 |
