Kinetic investigation of bioresponsive nanoparticle assembly as a function of ligand design†
Heiko
Andresen‡
,
Shalini
Gupta‡
and
Molly M.
Stevens
*
Imperial College London, Department of Materials and Institute for Biomedical Engineering, Prince Consort Road, London, SW7 2AZ, UK. E-mail: m.stevens@imperial.ac.uk; Fax: +44 (0)207 594 6757; Tel: +44 (0)207 594 6804
First published on 23rd August 2010
Abstract
Homogeneous and heterogeneous nanoparticle (NP) assembly induced by ligand-specific immunorecognition is commonly used for biosensing applications. We investigated how the structural design of the peptide ligands used to functionalise gold NPs affected the kinetics of NP assembly and hence biodetection. We observed that aggregation rates varied up to 20-fold for the surface binding and 120-fold for the solution-phase assembly of NPs as a function of peptide design. Our results show how the fundamental difference in NP assembly on surfaces and in solution requires different optimised ligand designs. This increased understanding of the specifics of ligand-triggered NP aggregation should help in the design of faster and more efficient bioassays in the future.
Engineered bioresponsive NP systems commonly use particles functionalised with specific molecular receptors and/or ligands to systematically induce their aggregation in a variety of formats.1 For instance, metallic and semiconducting NPs can be specifically assembled to report analyte binding events by fluorescence elimination.2 In the case of homogeneous gold NP assays, one can exploit the colorimetric shift of analyte-triggered NP aggregation to detect biomolecular activity as gold NP suspensions exhibit dispersion-dependent absorbance due to interparticle plasmon coupling.3 Similarly, heterogeneous surface-based biodetection formats take advantage of other intrinsic properties of NPs relating to their high surface-to-volume ratio such as high specific mass, redox potential or light scattering to generate or amplify binding signals.1,4 An important parameter known to affect the efficacy of the NP assembly process is the design of the ligand used to biofunctionalise the NPs, particularly in terms of length and structure of spacer molecules, as it dictates the accessibility of the biorecognition sites,5 can influence the ligand's functional integrity6 as well as improve colloidal stability and resistance to unspecific binding.7 An as important yet so far less investigated consideration is the differing impact of the ligand design on the bioresponsive surface and solution-phase assembly of NPs due to key differences that might be inherent between both aggregation mechanisms.
Considering the importance of the ligand presentation and the ubiquity of immunological-based detection methods in biological and medical applications, we sought to systematically investigate the influence of ligand design for NP functionalisation on antibody-triggered NP association, and how the ligand design may be differently optimised in homogeneous and heterogeneous immunocomplex formation. We used a synthetic phosphorylatable peptide antigen and a cognate monoclonal anti-phosphopeptide antibody in order to address this fundamental question with a biologically and medically relevant system. The enzymatic phosphorylation of synthetic peptides is frequently utilised in kinase detection assays as well as in kinase inhibitor screening.8 We used this immunorecognition system in two different assay formats (Fig. 1). In the first approach, an antibody-functionalised sensor chip was used to capture peptide-coated NPs from solution. The binding progress was monitored in real-time using the surface plasmon resonance (SPR) technique (Fig. 1A). In the second approach, a population of gold NPs functionalised with antibodies was added to the dispersion of gold NPs coated with the peptide antigen to induce interparticle crosslinking, which was then semi-continuously detected by measuring the changes in absorbance intensity over the 400–750 nm wavelength range (Fig. 1B). Both systems (Fig. 1A and B) were used to characterise the aggregation behaviour as a function of the peptide design, in which the peptide ligands were equipped with spacers of different structural properties and lengths, and immobilised either N- or C-terminally on the NPs (Fig. 1C). The peptidic spacers used in pY-1 through pY-3 were considered rigid, whereas the alkyl chain spacer in pY-4 added flexibility and rotational freedom. The polyethylene glycol (PEG) spacers in pY-5 and pY-6 were the longest and most flexible spacers used in this study.
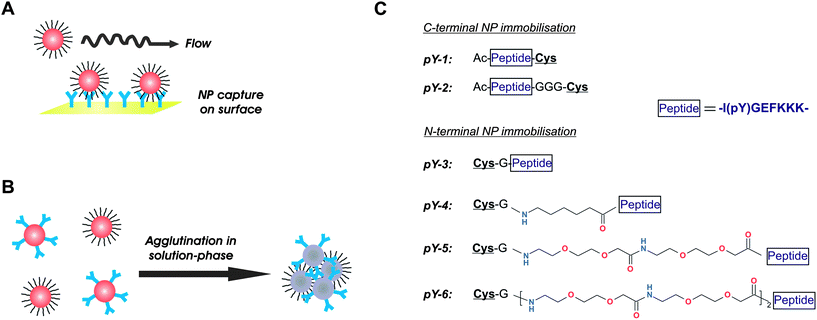 | ||
| Fig. 1 Schematic overview of the immunoassay formats and peptide structures used in this study to investigate the influence of ligand design on NP assembly. (A) Heterogeneous assay format with NP capture by surface-immobilised antibodies and real-time monitoring of the binding progress. (B) Homogeneous dual-NP format with antibody-mediated interparticle crosslinking and semi-continuous detection by spectrophotometric measurement. (C) Structure of peptide ligands used in this study. A core peptide with the phosphotyrosine residue is equipped with different spacers and immobilised to gold NPs either C- or N-terminally via the cysteine residue. Note how the immobilisation direction itself has an impact on the distance of the phosphotyrosine epitope from the NP surface. | ||
The binding of peptide-functionalised NPs to antibodies was interrogated using SPR as a generic surrogate for heterogeneous, solid-phase formats, and to obtain real-time kinetic data. The use of metallic NPs in SPR has become increasingly popular as they provide a unique means to induce large resonance angle shifts that can be used to substantially amplify or fine-tune the resonance signal response.9 We immobilised the antibodies on the sensor surface at high surface packing, so as to provide a non-limiting number of binding sites for the subsequent kinetic titration of functionalised NPs. It was predicted that within the first 60 seconds of association no mass transport limitation would occur if a sufficiently high flow rate was used at the same time. Following the injections of the NP dilutions, we observed a linear increase in the resonance signals for the association phase, which is consistent with a non-limiting availability of surface binding sites, and a concomitant irreversibility of the formed immunocomplexes, as evidenced by the steady-state signal level during the dissociation phase (Fig. 2A). The collective data revealed an increase in the apparent association rate constants as a function of the increase in the spacer lengths of the peptide ligands, or, more precisely, with the distance of the phosphotyrosine epitope from the NP surface (Fig. 3—top). The observed rate constants ranged from 3.0 × 105 M−1s−1 for the N-terminally immobilised peptide without spacer (pY-3) to 5.8 × 106 M−1s−1 for the peptide with the longest PEG spacer (pY-6). The peptide design could thus accelerate the complex formation by 20-fold, which could substantially decrease the analysis time of surface-based NP assays. We performed additional control experiments with SPR to evaluate the packing density of the peptides on gold surfaces and found a similar packing regardless of the peptide sequence (see ESI†, Fig. S1). The distance-related differences in the association rates observed can therefore most likely be attributed to an enhanced accessibility of the epitope accompanied by a higher structural flexibility of the ligand, which compensates for the diminished degree of freedom of the surface-immobilised antibody.
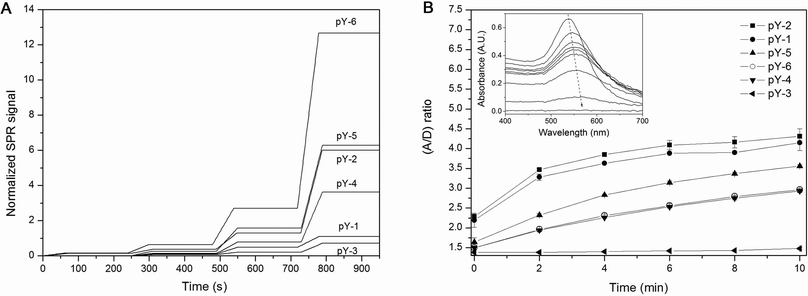 | ||
| Fig. 2 Determination of the kinetic rate constants of surface and solution-phase NP assembly as a function of peptide ligand design. (A) Qualitative reproduction of SPR traces obtained by kinetic titration of increasing concentrations of the peptide-functionalised NPs sequentially injected over surface-immobilised antibodies at 25 °C. For easier comparison the data are presented after subtracting the unspecific binding signal from the reference channel, and normalisation of the resonance signal responses to their respective Bmax values. (B) Immunoaggregation process of gold NPs in solution plotted as a change in the A/D ratio over time as determined by UV-Vis spectroscopy at room temperature. 7 nM peptide-functionalised NPs were mixed with an equimolar amount of antibody-functionalised NPs to induce interparticle crosslinking. The inset illustrates the change of UV-Visible spectra over time as agglutination progresses (here: pY-1). | ||
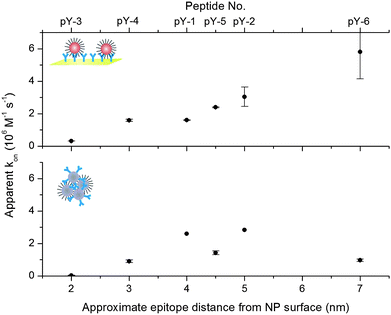 | ||
| Fig. 3 Plot of the rate constants determined for the immunoaggregation of functionalised NPs on the surface (top) and in solution (bottom) in relation to the epitope distance from the NP surface (x-axis, the corresponding peptide design is denoted on the secondary x-axis). The surface aggregation rates increase monotonically with increasing spacer lengths, whereas the solution-phase aggregation shows a non-linear relationship between the spacer lengths and the aggregation rate. | ||
The peptide-coated NPs were also used in a homogeneous assay format, in which equimolar amounts of antibody-functionalised NPs were added to induce aggregation and eventually precipitation due to interparticle crosslinking. Such examples in which the NPs serve both as a substrate and a reporter at the same time are particularly attractive, since they allow the development of a variety of almost unprecedentedly simple, single-step bioassays with high sensitivity. Upon addition of the antibody-NPs, the aggregation/precipitation progress was semi-continuously monitored by recording the samples' concomitant change in absorbance. Plotting the ratio of aggregated vs. dispersed particles over time (Fig. 2B) and applying our previously published kinetic model to the data10 revealed differences in the aggregation behaviour of the NPs and provided the apparent rate constants of the NP complex formation. Whilst there was a discernable increase in the association rate with increasing spacer length for the N-terminally immobilised peptides pY-3, pY-4 and pY-5 that mirrored the trend observed in the heterogeneous surface-based NP capture assay, the further elongation of the spacer length (pY-6) in contrast resulted in a significant slowing of the NP aggregation (Fig. 3—bottom). Interestingly and again in contrast to the surface-based NP assay, the C-terminally immobilised peptides pY-1 and pY-2 were associated with the relatively highest association rates in this format with 2.60 × 106 M−1s−1 and 2.83 × 106 M−1s−1, respectively. Notably, pY-5 which includes a short PEG spacer results in a significantly lower association rate than pY-1 and pY-2, although the distance of the epitope from the NP surface is almost identical for these three designs. We presume that the observed kinetic effects are characteristic of the homogeneous NP aggregation mechanism; in contrast to the heterogeneous format where the ligand binds unidirectionally from the solution-phase to the surface and isolated binding events are detected, the homogeneous NP assay is dependent on a multi-directional binding mechanism and the formation of multivalent particle aggregates. It appears that a rather rigid design of the peptide ligands, represented by pY-1 and pY-2, benefits the multi-directional binding better in this system than an elongated and flexible structure, as long as the general accessibility of the epitope is not compromised. This is in good agreement with previous investigations of multivalent binding of biomolecules, which have shown that not only is there a system-specific optimum linker length, but also that long and flexible ligand tethers can impair the efficient formation of higher ordered complexes due to a loss in conformational entropy, whereas rigid or moderately flexible structures may be thermodynamically more conducive to multivalent binding.11
Three peptide designs, pY-2, pY-3 and pY-6, were further used to investigate the influence of temperature on the aggregation rate in the homogeneous assay format (Table 1). An elevated temperature was predicted to increase the efficiency of multimeric NP aggregate formation and thus accelerate the assay response. The rise in temperature from 20 °C to 37 °C resulted in an increased agglutination rate of approximately 25% and 45% for peptide designs pY-2 and pY-6, respectively, whereas for the peptide pY-3 a considerably stronger increase of 400% was registered. This result suggests particularly good accessibility and spacing of the peptide ligand pY-2, and similarly of pY-6, for unhindered immunocomplex formation. The altogether different aggregation of the pY-3 ligand at the higher temperature likely reflects a spatially obstructed surface presentation of the peptide epitope, which can be significantly compensated for by the temperature associated mobility gain of the binding partners.
| Peptide | k on,app in solution (RT) /M−1s−1 | Time to completiona | |
|---|---|---|---|
| RT | 37 °C | ||
| a Complete precipitation of NPs. | |||
| pY-2 | 2.83 × 106 | 45 min | 30 min |
| pY-3 | 2.38 × 104 | ≫24 h | 24 h |
| pY-6 | 9.68 × 105 | 6 h | 4 h |
Conclusions
This communication reports the kinetic differences of bioresponsive NP assembly as a function of the ligand design in homogeneous and heterogeneous immunoassay formats. We found that the rate of the unidirectional binding of functionalised NPs to a sensor surface increases monotonically with increasing linker length and gain in structural flexibility. This is explained by the fact that long and flexible linkers can adopt multiple conformations which allow an optimised binding to a surface-immobilised and thus sterically obstructed receptor antibody. In contrast, the rate of homogeneous NP aggregation reveals a maximum for a rather short, more rigid ligand design and decreases significantly with further increase of the spacer length and/or added spacer flexibility. This notable difference in aggregation behaviour reflects the specific characteristic of the homogeneous NP assay, which depends on a multi-directional binding mechanism to form larger aggregates. This mechanism proceeds fastest for an optimal spacer length which is concomitant with an optimal distance between ligands, because these conditions create a high effective molarity of ligands on the NP surface and minimise the conformational entropy loss in multivalent binding. Although this optimal design is system-dependent, our results can be readily extended to similar NP systems and be used as an instructive guideline for other biological and medical NP applications.Acknowledgements
HA gratefully acknowledges the support of the European Community under a Marie Curie Intra-European Fellowship. MMS thanks the EPSRC and ERC grant “Naturale” for funding.References
- N. L. Rosi and C. A. Mirkin, Chem. Rev., 2005, 105, 1547 CrossRef CAS.
- T. Pons, I. L. Medintz, K. E. Sapsford, S. Higashiya, A. F. Grimes, D. S. English and H. Mattoussi, Nano Lett., 2007, 7, 3157 CrossRef CAS.
- C. A. Mirkin, R. L. Letsinger, R. C. Mucic and J. J. Storhoff, Nature, 1996, 382, 607 CrossRef CAS.
- M. E. Stewart, C. R. Anderton, L. B. Thompson, J. Maria, S. K. Gray, J. A. Rogers and R. G. Nuzzo, Chem. Rev., 2008, 108, 494 CrossRef CAS.
- K. Inamori, M. Kyo, K. Matsukawa, Y. Inoue, T. Sonoda, K. Tatematsu, K. Tanizawa, T. Mori and Y. Katayama, Anal. Chem., 2008, 80, 643 CrossRef CAS.
- R. Hong, N. O. Fischer, A. Verma, C. M. Goodman, T. Emrick and V. M. Rotello, J. Am. Chem. Soc., 2004, 126, 739 CrossRef CAS; C. F. W. Becker, Y. Marsac, P. Hazarika, J. Moser, R. S. Goody and C. M. Niemeyer, ChemBioChem, 2007, 8, 32 CrossRef CAS.
- B. C. Mei, K. Susumu, I. L. Medintz and H. Mattoussi, Nat. Protoc., 2009, 4, 412 Search PubMed.
- D. M. Olive, Expert Rev. Proteomics, 2004, 1, 89 Search PubMed; J. E. Ghadiali and M. M. Stevens, Adv. Mater., 2008, 20, 4359 CrossRef CAS; J. E. Ghadiali, B. E. Cohen and M. M. Stevens, ACS Nano, 2010 DOI:10.1021/nn101293s.
- L. A. Lyon, M. D. Musick and M. J. Natan, Anal. Chem., 1998, 70, 5177 CrossRef CAS; Y. Li, A. W. Wark, H. J. Lee and R. M. Corn, Anal. Chem., 2006, 78, 3158 CrossRef CAS; L. He, M. D. Musick, S. R. Nicewarner, F. G. Salinas, S. J. Benkovic, M. J. Natan and C. D. Keating, J. Am. Chem. Soc., 2000, 122, 9071 CrossRef CAS.
- S. Gupta, H. Andresen, J. E. Ghadiali and M. M. Stevens, Small, 2010, 6, 1509 CrossRef CAS.
- V. M. Krishnamurthy, V. Semetey, P. J. Bracher, N. Shen and G. M. Whitesides, J. Am. Chem. Soc., 2007, 129, 1312 CrossRef CAS; R. S. Kane, Langmuir, 2010, 26, 8636 CrossRef CAS; T. A. Shewmake, F. J. Solis, R. J. Gillies and M. R. Caplan, Biomacromolecules, 2008, 9, 3057 CrossRef CAS; P. I. Kitov and D. R. Bundle, J. Am. Chem. Soc., 2003, 125, 16271 CrossRef CAS; G. D. Glick and J. R. Knowles, J. Am. Chem. Soc., 1991, 113, 4701 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental section and complementary results. See DOI: 10.1039/c0nr00469c |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2011 |
