Investigating the structural biofunctionality of antibodies conjugated to magnetic nanoparticles†
Emanuela
Occhipinti‡
a,
Paolo
Verderio‡
b,
Antonino
Natalello
a,
Elisabetta
Galbiati
a,
Miriam
Colombo
ab,
Serena
Mazzucchelli
b,
Agnese
Salvadè
b,
Paolo
Tortora
a,
Silvia Maria
Doglia
a and
Davide
Prosperi
*ab
aDipartimento di Biotecnologie e Bioscienze, Università di Milano-Bicocca, P.za della Scienza 2, 20126, Milano, Italy. E-mail: davide.prosperi@unimib.it; Fax: +39 026448 3565; Tel: +39 026448 3302
bCentro di Microscopia Elettronica per le Nanotecnologie applicate alla medicina, Università di Milano, Via G.B. Grassi 74, 20157, Milano, Italy
First published on 29th September 2010
Abstract
We present the synthesis of trastuzumab-functionalized pegylated iron oxide nanoparticles and provide an FTIR-based approach to gain a direct evidence of the actual conservation of the native structure of conjugated antibody. Their target-selectivity to specific cancer cell receptors has been also assessed.
The successful development of targeted nanoprobes for noninvasive medical imaging represents a new frontier in the prevention and treatment of malignant diseases.1 Among the available choices to obtain an effective targeting action, the conjugation of nanoparticles with monoclonal antibodies (mAbs) is a well-established strategy to deliver the nanoprobe to specific cell types.2 This approach combines the unique physical properties of nanoparticles, with the specific and selective recognition capability of mAbs to cells and tissues. Moreover, the potential improvement in the cellular uptake and the enhanced intracellular stability could be two of the major advantages of using mAb-conjugated nanoparticles.
Trastuzumab (TZ) is a recombinant, humanized IgG mAb that selectively binds with high affinity to the extracellular domain of the human epidermal growth factor receptor 2 (HER2), which is expressed in several primary tumors, including breast, ovarian, gastric and salivary cancers, and in metastatic sites.3 For this reason, TZ has been developed for clinical immunotherapy and the conjugation of TZ with chemotherapeutics may offer an excellent strategy for targeted delivery of drugs to malignant cells.4 The therapeutic action of TZ mainly consists in blocking the dimerization of the two components of HER2 complex resulting in an interruption of the signal transduction. Colloidal systems, such as those based on inorganic nanomaterials, provide higher drug carrier capacities than individual mAbs, concomitantly acting as signal emitters for medical diagnosis. Therefore, in view of the outstanding potential of mAb-based hybrid nanovehicles, the conjugation of mAbs to nanoparticles is now a widespread practice to gain selective delivery and localization of nanoparticle probes to the desired target site.
However, despite the huge interest for mAb nanoconjugates, only poor evidence is presently available on the actual conservation of the protein biofunctionality at the molecular structural level, once mAb has been covalently conjugated to the nanoparticle. Such difficulty mainly resides in the lack of reliable methods capable of providing exhaustive information on structural/conformational features sustaining the protein functionalities of bioconjugate systems. This is indeed a crucial point, which urgently needs to be addressed. Weiss et al. exploited circular dichroism to investigate proteins immobilized on gold nanoparticles.5 Unfortunately, this technique is expected to fail with magnetic nanoparticles due to the strong absorption of iron oxide in the UV range. The purpose of the present study is to provide a direct evidence on the extent of preservation of the structural bioactivity of IgGs immobilized onto the surface of iron oxide nanoparticles deduced by accurate analysis of the essential folding features obtained by Fourier-transform infrared (FTIR) spectroscopy. As a model for this study, PEG-stabilized, TZ-modified magnetite nanoparticles (TMNP) were developed (Scheme 1). Thanks to the unique magnetic properties of nanoscale iron oxides, this class of nanoparticles could be useful as targeted contrast agents for magnetic resonance imaging of cancer cells.6–8
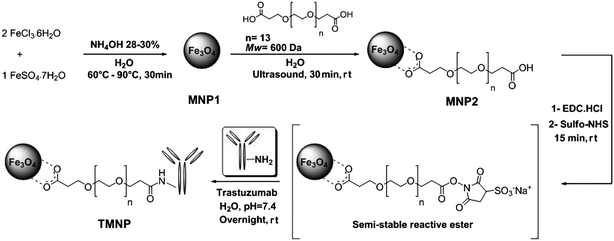 | ||
| Scheme 1 Schematic representation of the synthesis of pegylated trastuzumab-modified iron oxide nanoparticles (TMNP). | ||
Bare monodisperse magnetite nanoparticles (MNP1) were obtained by aqueous alkaline coprecipitation of Fe2+ and Fe3+ ions in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 molar ratio, as described in a previous work.9 A bifunctional linker α-ω-dicarboxyl-terminated polyethylene glycol (PEG, Mw 600 Da) was grafted on the Fe3O4 surface by ultrasound-assisted reaction and the unreacted excess of PEG was removed by dialysis. The synthesized carboxyPEG-functionalized nanoparticles (MNP2) were highly soluble in water, thus the MNP2 suspension was diluted to a final concentration of 1 mg mL−1, which was stable for several months at room temperature. According to previous reports, PEG-coated nanoparticles are more biodegradable, non-antigenic, non-irritative for tissues and less toxic than unmodified nanoparticles.10–12 At the same time, the PEG chains are responsible for the so-called “stealth effect”, preventing nonspecific adsorption of opsonin proteins.13
2 molar ratio, as described in a previous work.9 A bifunctional linker α-ω-dicarboxyl-terminated polyethylene glycol (PEG, Mw 600 Da) was grafted on the Fe3O4 surface by ultrasound-assisted reaction and the unreacted excess of PEG was removed by dialysis. The synthesized carboxyPEG-functionalized nanoparticles (MNP2) were highly soluble in water, thus the MNP2 suspension was diluted to a final concentration of 1 mg mL−1, which was stable for several months at room temperature. According to previous reports, PEG-coated nanoparticles are more biodegradable, non-antigenic, non-irritative for tissues and less toxic than unmodified nanoparticles.10–12 At the same time, the PEG chains are responsible for the so-called “stealth effect”, preventing nonspecific adsorption of opsonin proteins.13
TEM image showed that the core size of MNP2 was unchanged after PEG grafting (10 ± 3 nm, Fig. S2, ESI†). The hydrodynamic diameter of MNP2 was determined to be 82.4 ± 1.0 nm by dynamic light scattering (DLS, Fig. S3a, ESI†). A good contrast power for MNP2 in PBS was deduced by the relaxivity value r2, which was calculated to be 131.9 mM−1 s−1 (Fig. S4, ESI†). The pH-dependent stability of MNP2 in water was also investigated by UV-vis absorption spectra (Fig. S5 and Table S1, ESI†). MNP2 were dispersed in deionized water and evaluated in the pH range from 1 to 11, in which they did not display any aggregation up to pH 7–8. A critical formation of large clusters of nanoparticles was instead observed above pH 9 right after 90 min incubation, which resulted in collapsing of the colloidal suspension (Fig. S6, ESI†). Zeta potential (ζ) of MNP2 in water in a range of pH between 3 and 9 was also investigated (Fig. S3b, ESI†). At pH 3, MNP2 were strongly positively charged with ζ = +42.0 ± 3.2 mV, probably due to a tendency of PEG to capture acidic protons. In contrast, at pH 9, MNP2 exhibited a strongly negative surface charge of ζ = −43.3 ± 1.3 mV, confirming the presence of completely deprotonated carboxylate groups on the external surface of nanoparticles. Similar results were obtained in PBS: as expected, in this case, MNP2 were less negatively charged, with ζ = −19.5 ± 0.5 mV.
MNP2 were ready for conjugation with amine-containing biomolecules by amide coupling owing to the high surface density of carboxyl functionalities. To examine their potential in mAb conjugation, purified TZ was covalently bound to MNP2 at pH 7.4 in the presence of coupling agents, such as sulfo-NHS and EDC, exploiting the lysine amine groups present on the peptide sequence of the antibody. The resulting TMNP were washed with PBS to remove unbound antibodies, which would lead to false-positives in biological experiments, resuspended in PBS at a final concentration of 0.8 mg mL−1 and stored at 4 °C. The average number of TZ loaded on TMNP was estimated to be about 2 mAb per nanoparticle by a Bradford protein assay. The hydrodynamic size of these mAb-conjugates was 224.9 ± 2.7 nm (Fig. S8, ESI†), which was remarkably higher than that of MNP2, while only a slight decrease in relaxivity was observed (Table 1). In addition, ζ values showed a marked decrease in the negative charge to ζ = −7.8 ± 1.1 mV (Table S2, ESI†), which demonstrated that several carboxylate groups from the surface PEG chains were involved in the linkage with TZ, and the residual negative charges were partially compensated by the positive charges on the IgG molecule, consistent with the basic isoelectric point of TZ (IPTZ = 8.45). The physical/chemical characteristics of MNP2 and TMNP are summarized in Table 1.
| Parameter | Unit | MNP2 | TMNP |
|---|---|---|---|
| Particle size (from DLS) | nm | 82.2 ± 1.0 | 224.9 ± 2.7 |
| Polydispersity | 0.239 ± 0.01 | 0.194 ± 0.02 | |
| ζ-Potential | mV | −19.5 ± 0.5 | −7.8 ± 1.1 |
| T 2 relaxivity (r2) | mM−1 s−1 | 131.9 | 103.0 |
| Ab binding efficiency (Ab/NP) | µg mg−1 | — | 217 |
| — | 2 |
An important prerequisite to keep in mind when designing an appropriate conjugation strategy is maintaining intact, as much as possible, the biological activity and full receptor binding capability of immobilized mAbs. We had a first immediate evidence of the immunoreactivity of mAbs in TMNP performing a dot blot analysis (Fig. S9, ESI†). Our results confirmed that TMNP were able to cross-react with anti-human antibody with the same sensitivity of free TZ.
In order to explore the structural properties of the conjugated antibody, we applied FTIR spectroscopy to obtain information on protein secondary structure.14,15 This method has been seldom exploited to investigate protein molecules physically adsorbed onto nanoscale gold surfaces. Shang et al. observed remarkable conformational changes in the protein secondary structure occurring upon adsorption.16 However, to the best of our knowledge, the present study represents the first example aimed at assessing the conformational effects caused by covalent conjugation of antibodies to nanoparticles. Herein, the secondary structure and stability of the conjugated mAb were investigated by FTIR spectroscopy, which has previously been used to determine the folding features of antibodies.17 In Fig. 1a, the absorption spectra of free TZ (A), of a mixture of MNP2 and free TZ (B), of TMNP (C), and of MNP2 (D) are reported in the 1500–1700 cm−1 spectral region, where the amide I and amide II bands occur. These bands are due, respectively, to the absorption of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching and N–H bending vibrations of the protein backbone.14,15 The presence of these two bands in the TMNP spectrum confirmed that the conjugation reaction occurred successfully. Indeed, unconjugated MNP2 displayed a completely different profile in this spectral region, with only a broad absorption centered at about 1606 cm−1.
O stretching and N–H bending vibrations of the protein backbone.14,15 The presence of these two bands in the TMNP spectrum confirmed that the conjugation reaction occurred successfully. Indeed, unconjugated MNP2 displayed a completely different profile in this spectral region, with only a broad absorption centered at about 1606 cm−1.
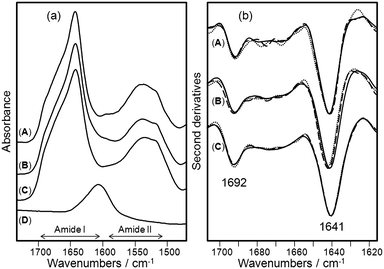 | ||
| Fig. 1 Structural characterization of TZ, TMNP, and MNP2 by FTIR spectroscopy. (a) FTIR absorption spectra in the amide I and amide II regions of (A) free TZ at 1.0 mg mL−1, (B) a mixture of free TZ at 1.0 mg mL−1 and free MNP2 at 4.7 mg mL−1, (C) TMNP at 4.7 mg mL−1 (1.0 mg mL−1TZ), and (D) MNP2 at 7.0 mg mL−1. In this spectral region, MNP2 (D) displayed only a broad absorption around 1606 cm−1, while TMNP (C) showed the protein amide I and amide II bands confirming the success of the bioconjugation. The spectra were collected immediately after sample preparation. (b) Second derivatives of the spectra reported in (a). Spectra were collected at time 0 (continuous lines), after 24 h (dashed lines), and after 96 h (dotted lines) of incubation at 37 °C. The spectra are dominated by two components at 1692 cm−1 and 1641 cm−1 assigned to the native β-sheet structure of TZ that were found to be stable upon incubation at 37 °C both in the free and in the bioconjugated protein. | ||
Information on the secondary structure of the protein can be obtained through the analysis of the amide I band, as it is the spectral region most sensitive to the structural conformation changes of the protein.15,18 In order to resolve the amide I band components, we performed the second derivatives of the measured spectra (Fig. 1b).14 The spectrum of freshly purified TZ is dominated by two main components, which appear as negative peaks in the second derivative, at 1692 cm−1 and 1641 cm−1 that can be both assigned to the native β-sheet structure of the protein.15,17 The spectrum of TMNP was almost identical to that of free TZ under native condition indicating that the amidic conjugation used in this work did not affect significantly the protein secondary structure. To monitor the stability of the conjugated mAb, we collected the FTIR spectra of the samples at different times of incubation up to 96 h at 37 °C. The comparison of the FTIR second derivative spectra of native TZ at the beginning of incubation with that after 96 h indicates that the protein secondary structures were substantially unchanged. The same result was obtained for conjugated TZ after incubation at 37 °C. We tested the storage stability of TMNP, and found that conjugated TZ was stable even after two months incubation at 4 °C without any additional stabilizing agents.
Next, we examined the FTIR absorption of all the above samples after being lyophilized and resuspended in buffered heavy water (PBS/D2O), pH 7.4, to simulate the physiological environment. In this way, it was possible to monitor in real time the stability of the conjugated antibodies by transmission FTIR at 37 °C, exploiting the low absorbance of D2O in the amide I region.15,17 These results confirmed once again that conjugation did not affect the native protein secondary structure, which was found to be stable over 24 h incubation at 37 °C (data not shown). Altogether, these data provided a compelling evidence on the preserved stability of mAb in TMNP system and demonstrated that under these synthetic conditions the conjugated protein did not change its physiological biofunctionality.
To quantitatively assess the secondary structure components of the conjugated antibodies, we performed a Gaussian curve fitting of the absorption spectra of free TZ and of TMNP, whose results are reported in the ESI† (Fig. S11). The relative weights of the β-sheet components (1641 and 1692 cm−1) and of the random coil component (R.c. 1663 cm−1) were very similar, with only minor differences. In particular, TMNP seems to display a slightly larger R.c. that could account for a partially reduced bioactivity. Next, we examined the infrared absorption spectra of TZ physically adsorbed on bare MNP1 (Fig. S10 and S11 in ESI†). As expected, the bioactivity of the antibody was strongly reduced. The spectra were very different in the amide I band, whose maximum shifted from 1641 cm−1 for free TZ to 1650 cm−1 for adsorbed TZ. Indeed, the Gaussian curve fitting of these spectra indicates a strong change in the antibody secondary structures. The β-sheet components were reduced from ∼50% in free TZ to ∼34% in the adsorbed TZ immediately after preparation, and to ∼22% after 96 h incubation at 37 °C. R.c. increased from ∼ 44% in free TZ to ∼55% and ∼66% in the adsorbed TZ at 0 and 96 h incubation, respectively. In this case, the loss of native secondary structure predicted by the above FTIR analysis is in agreement with the decreased bioactivity observed for the adsorbed TZ.
To check whether TMNP were able to maintain effectively the specific targeting capability of TZ for HER2-positive breast cancer cells, we investigated the TMNP binding to HER2 in HER2-overexpressed MCF7 cells.19 Two MCF7 whole cell extracts were incubated in parallel at 4 °C with TMNP and with unconjugated MNP2 (control), respectively. Immunoprecipitants (bound) and the supernatants (unbound) were first analyzed by western blotting with anti-HER2 antibody in order to reveal TMNP binding to HER2 receptor (Fig. 2, upper). The comparison between TMNP and MNP2 confirmed that the conjugation with TZ was necessary to immunoprecipitate HER2 membrane receptor in MCF7 whole cell extract. Next, calnexin (Clnx), an integral protein of the endoplasmic reticulum,20 was tested as negative control to assess the possible nonspecific interaction of TMNP with undesired biomolecules. To this aim, a labeled anti-Clnx mAb was used, which displayed a detectable signal only in unbound sample (Fig. 2, lower). Such immunoprecipitation assay revealed that Clnx was completely absent in the TMNP sample whereas it was detected in the unbound supernatant, suggesting that the interaction between TMNP and HER2 is specific and correlated to antibody conjugation.
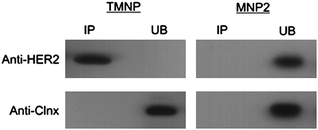 | ||
| Fig. 2 Immunoprecipitation of HER2 in MCF7 cells. Immuno-precipitation was performed using anti-HER2 or anti-calnexin antibodies cross-linked to TMNP and MNP2 (as negative control). Nanoparticles were incubated overnight at 4 °C in a whole extract of MCF7 cells. Bound (IP) and unbound (UB) proteins were eluted in SDS-PAGE application buffer, electrophoresed and immunoblotted. | ||
The immunoprecipitation data were confirmed by relaxivity measurements, which provided evidence on iron oxide capture by MCF7 (Table S3, ESI†), and by immunofluorescence after TMNP incubation with living cells. Since HER2 is a trans-membrane receptor,3TMNP were expected to localize on the membrane of HER2 positive MCF7 cells. In order to assess HER2 surface distribution, MCF7 cells were first treated with TZ (Fig. 3d–f). TMNP were then incubated with MCF7. Next, TZ and TMNP were labeled with anti-human FITC-labeled secondary antibodies (green), whereas cell membranes were stained with DiD oil (mb, blue). After 20 min incubation, TMNP were observed in MCF7 cell surfaces (Fig. 3a–c), demonstrating that they localized selectively in correspondence of trans-membrane receptors. The combined results of these experiments clearly evidenced the target-selectivity of TMNP towards HER2 breast cancer marker.
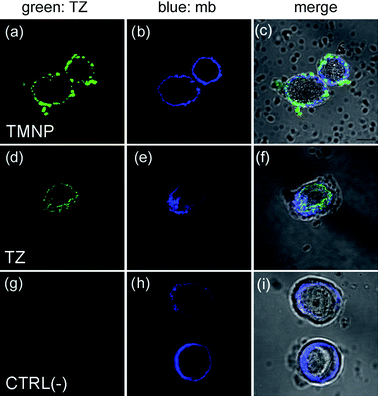 | ||
| Fig. 3 Confocal laser images of MCF7 cells cultured with TMNP or free TZ. MCF7 were incubated for 20 min with TMNP (100 µg mL−1; a–c) or TZ (11 µg mL−1; d–f). As negative control, MCF7 were stained with secondary antibodies anti-human FITC (g–i). Cell membranes (mb) were stained with DiD oil (blue). TMNP and TZ were labeled with anti-human FITC secondary antibodies. Scale bar = 10 µm. | ||
In summary, we have synthesized a trastuzumab-modified, fully pegylated iron oxide nanoparticle (TMNP) as a model for the study of the interaction between a mAb nanoconjugate and the relevant natural receptor. In particular, we have demonstrated for the first time that accurate analysis of the FTIR signals in the 1500–1700 cm−1 absorption range may be a valuable method enabling the determination of protein conformational changes in mAb conjugate. As a proof of concept, testing our TMNP by FTIR revealed that, different from physically adsorbed protein, mAb conjugated to the nanoparticle surface via a dicarboxylic PEG linker did not change its structural properties maintaining the folding characteristics basically intact. This simple and fast structural characterization tool is versatile and of wide utility, as it might be easily applied to other typologies of proteins and nanoparticles different from the ones used in this study. As the functionality of a protein is essentially related to its native folding, the approach presented here is able to provide a preliminary evidence on the actual bioactivity expected for a protein in a biohybrid nanoconjugate system. Such rapid evidence could be of large utility in designing and testing novel hybrid targeted nanovectors, preventively providing a chance to reduce those side effects, which usually lead to nonspecific uptake by normal tissues.
Acknowledgements
We thank R. Allevi for TEM images. This work was supported by “Fondazione Romeo ed Enrica Invernizzi”, CMENA (University of Milano) and Regione Lombardia (NanoMeDia Project). S.M.D., P.T., and D.P. acknowledge the financial support of F.A.R. and A.N. the post-doctoral fellowship of the University of Milano-Bicocca.Notes and references
- J. V. Frangioni, J. Clin. Oncol., 2008, 26, 4012–4021 CrossRef.
- D. Peer, J. M. Karp, S. Hong, O. C. Farokhzad, R. Margalit and R. Langer, Nat. Nanotechnol., 2007, 2, 751–760 CrossRef CAS.
- M. Colombo, F. Corsi, D. Foschi, E. Mazzantini, S. Mazzuchelli, C. Morasso, E. Occhipinti, L. Polito, D. Prosperi, S. Ronchi and P. Verderio, Pharmacol. Res., 2010, 62, 150–165 CrossRef CAS.
- G. L. Plosker and S. J. Keam, Drugs, 2006, 66, 449–475 CrossRef CAS.
- A. Weiss, T. C. Preston, J. Popov, Q. Li, S. Wu, K. C. Chou, H. M. Burt, M. B. Bally and R. Signorell, J. Phys. Chem. C, 2009, 113, 20252–20258 CrossRef CAS.
- M. V. Yezhelyev, X. Gao, Y. Xing, A. Al-Hajj, S. Nie and R. M. O'Regan, Lancet Oncol., 2006, 7, 657–667 CrossRef CAS.
- M. G. Harisinghani, J. Barentsz, P. F. Hahn, W. M. Deserno, S. Tabatabaei, C. H. van de Kaa, J. de la Rosette and R. Weissleder, N. Engl. J. Med., 2003, 348, 2491–2499 CrossRef.
- M. Zhao, D. A. Beauregard, L. Loizou, B. Davletov and K. M. Brindle, Nat. Med. (N. Y.), 2001, 11, 1241–1244 CrossRef.
- L. Polito, M. Colombo, D. Monti, S. Melato, E. Caneva and D. Prosperi, J. Am. Chem. Soc., 2008, 130, 12712–12724 CrossRef CAS.
- F. Corsi, C. De Palma, M. Colombo, R. Allevi, M. Nebuloni, S. Ronchi, G. Rizzi, A. Tosoni, E. Trabucchi, E. Clementi and D. Prosperi, Small, 2009, 5, 2555–2564 CrossRef.
- C. Sun, K. Du, C. Fang, N. Bhattarai, O. Veiseh, F. Kievit, Z. Stephen, D. Lee, R. G. Ellenbogen, B. Ratner and M. Zhang, ACS Nano, 2010, 4, 2402–2410 CrossRef CAS.
- F. Hu, L. Wei, Z. Zhou, Y. Ran, Z. Li and M. Gao, Adv. Mater., 2006, 18, 2553–2556 CrossRef CAS.
- W. Lin, M. C. Garnett, E. Schacht, S. S. Davis and L. Illum, Int. J. Pharm., 1999, 189, 161–170 CrossRef CAS.
- H. Susi and D. M. Byler, Methods Enzymol., 1986, 130, 290–311 Search PubMed.
- J. L. R. Arrondo and F. M. Goni, Prog. Biophys. Mol. Biol., 1999, 72, 367–405 CrossRef CAS.
- L. Shang, Y. Wang, J. Jiang and S. Dong, Langmuir, 2007, 23, 2714–2721 CrossRef CAS.
- A. Dong, P. Huang and W. S. Caughey, Biochemistry, 1990, 29, 3303–3308 CrossRef CAS.
- A. Natalello, D. Ami, S. Brocca, M. Lotti and S. M. Doglia, Biochem. J., 2005, 385, 511–517 CrossRef CAS.
- L. Yuste, J. C. Montero, A. Esparís-Ogando and A. Pandiella, Cancer Res., 2005, 65, 6801–6810 CrossRef CAS.
- J. J. Carmelo and A. J. Parodi, J. Biol. Chem., 2008, 283, 10221–10225 CrossRef.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental details, TEM images, DLS and relaxivity plots, additional FT-IR spectra and analyses. See DOI: 10.1039/c0nr00436g |
| ‡ These authors contributed equally to the research. |
| This journal is © The Royal Society of Chemistry 2011 |
