Modeling the cellular impact of nanoshell-based biosensors using mouse alveolar macrophage cultures†‡
Abstract
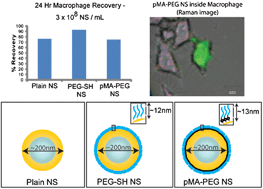
In this study, the relative toxicity of native gold-
- This article is part of the themed collection: Metal Toxicity
* Corresponding authors
a
Biological, Chemical, and Physical Sciences Department, Illinois Institute of Technology, 3101 S. Dearborn St., Chicago, Illinois 60616
E-mail:
bishnoi@iit.edu
b Cancer Biology Division, IIT Research Institute, Chicago, IL
c Graduate Program in Areas of Basic and Applied Biology, University of Porto, Porto, Portugal
In this study, the relative toxicity of native gold-

 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
 Fetching data from CrossRef.
Fetching data from CrossRef.
This may take some time to load.
Loading related content
