Exposure to multiple metals from groundwater—a global crisis: Geology, climate change, health effects, testing, and mitigation
Erika
Mitchell
a,
Seth
Frisbie
ab and
Bibudhendra
Sarkar
*cd
aBetter Life Laboratories, E. Calais, VT, USA. E-mail: em63@cornell.edu
bDepartment of Chemistry and Biochemistry, Norwich University, Northfield VT, USA. E-mail: sfrisbie@norwich.edu; Fax: 802-485-2333; Tel: 802-485-2614
cMolecular Structure and Function Program, The Hospital for Sick Children, Toronto, ON, Canada. E-mail: bsarkar@sickkids.ca; Fax: 416-813-5022; Tel: 416-813-5921
dDepartment of Biochemistry, University of Toronto, Toronto, ON, Canada
First published on 18th July 2011
Abstract
This paper presents an overview of the global extent of naturally occurring toxic metals in groundwater. Adverse health effects attributed to the toxic metals most commonly found in groundwater are reviewed, as well as chemical, biochemical, and physiological interactions between these metals. Synergistic and antagonistic effects that have been reported between the toxic metals found in groundwater and the dietary trace elements are highlighted, and common behavioural, cultural, and dietary practices that are likely to significantly modify health risks due to use of metal-contaminated groundwater are reviewed. Methods for analytical testing of samples containing multiple metals are discussed, with special attention to analytical interferences between metals and reagents. An overview is presented of approaches to providing safe water when groundwater contains multiple metallic toxins.
 Erika Mitchell | Erika Mitchell has worked as a researcher with Better Life Laboratories, a non-profit research and training institute in Vermont, since getting her PhD at Cornell University in 1993. She has been a part of an international team of volunteer scientists in Bangladesh since 1998, mapping the extent of arsenic and other toxic metals in the groundwater and developing methods for groundwater testing. She has strong interests in societal factors involving diet and nutrition, and societal responses to proposed technological solutions for groundwater remediation. |
 Seth Frisbie | Dr Seth H. Frisbie is an environmental and analytical chemist at Norwich University, and the president of a nonprofit corporation, Better Life Laboratories, Inc. He received his doctoral and master's degrees from Cornell University. He has studied drinking water for over 30 years. He has worked on drinking water and public health in Bangladesh and other developing countries since 1997. |
 Bibudhendra Sarkar | Bibudhendra Sarkar received his PhD in biochemistry from the University of Southern California and did his further studies in protein chemistry in Cambridge University and quantum biochemistry in the Université de Paris-Sorbonne. At the University of Toronto and the Hospital for Sick Children, he established his research career in metal-caused diseases. He became full professor in 1978, department head of Structural Biology and Biochemistry 1990–2002 and director of the Advanced Protein Technology Center 1998–2002. Since 1997 he has been leading a team of international volunteer scientists researching the health crisis caused by multiple metals contamination of drinking water in South Asia. Erika Mitchell and Seth Frisbie are members of this team. |
Introduction
Over the past 25 years, a health crisis of epic proportions has emerged in the Bengal Delta due to populations switching to groundwater for drinking, cooking, and irrigation rather than surface water. The switch away from using surface water for drinking and cooking was initially hailed as a tremendous boon for public health because of dramatic declines in mortality due to water-borne diseases. However, unbeknownst to the installers of the wells which made this switch possible, groundwater in the region is commonly contaminated with arsenic, manganese, uranium and other toxic metals of hydro-geological origin. In this paper, we present an overview of the global extent of naturally occurring toxic metals in groundwater, noting that multiple metal contamination of groundwater is an issue of global concern, and the risks may be further magnified by climate change.Several metals that occur as natural contaminants of groundwater are associated with severe and potentially lethal adverse health effects, including arsenic, manganese, and uranium. We review the adverse health effects attributed to the toxic metals most commonly found in groundwater, both individually and in combination with each other. We emphasize that exposure to these toxic metals most often involves exposure to a combination of toxic metals rather than to just a single metal; thus, accurate assessment of health risks due to drinking metal-contaminated groundwater must take into account potential interactions between the metals on the chemical, biochemical, and physiological levels.
Health effects due to co-exposures to toxic metals are further affected by behavioural and cultural practices (e.g. smoking, sun avoidance) and dietary habits. In this paper, we highlight synergistic and antagonistic effects that have been reported between the toxic metals found in groundwater and the dietary trace elements and we also discuss behavioural, cultural, and dietary practices that are likely to significantly modify health risks due to use of metal-contaminated groundwater. We provide an overview of analytical testing for multiple metals, noting the special considerations that must be taken to avoid analytical interferences when samples contain mixtures of metals. We conclude with a brief summary of current approaches and technologies for providing safe water when groundwater contains multiple metallic toxins.
Toxic metals in groundwater
The discovery of toxic metals in groundwater
Finding sources of pure water is an age-old problem for humans. In desert regions, people have long resorted to wells in order to access water. Folk wisdom of the desert, however, advises users to observe the behaviour of animals, and to avoid wells that animals would not drink from.1 In 1918, this folk wisdom received its first scientific confirmation that water from certain wells is contaminated and causes disease.2 Dr Abel Ayerza, a professor at the Buenos Aires Medical School, reported that a number of villagers from Cordoba had developed palmar keratosis and melanoma, and he argued that this was due to arsenic in the region's groundwater. He described a patient with weeping sores on his lower legs and discoloured toes, whose feet eventually had to be amputated, and patients with liver and heart problems or general weakness. He suggested that problems with the arterial system and the heart were due to the effects of arsenic and vanadium in combination. He noted that villagers were very much aware that some waters in the region were unhealthy despite having no bad taste or colour, and they would go to great lengths to access safe water, including harvesting rainwater from their “zinc” (galvanized) roofs or carrying water from afar. Ayerza observed that the spatial distribution of the contaminants seemed to be quite unpredictable, with some wells heavily contaminated while neighbouring wells had safe water, and he cautioned that foods in the area were also likely to contain high levels of arsenic. Subsequent research in many other parts of the globe has confirmed and replicated Ayerza's observations made almost a century ago on the serious health risks of drinking groundwater contaminated with toxic metals and the difficulty of predicting which wells may be affected.Because groundwater is generally safe, and is almost universally preferable to surface water in terms of biological contaminants, the possibility that it may contain toxic metals has not been considered until widespread health problems have arisen. Throughout the twentieth century, increasing dependence on groundwater due to migration, population pressure, or the introduction of new water technologies, has brought about epidemics of keratosis, blackfoot disease, skeletal fluorosis and other results of metal contamination due to natural sources (e.g. arsenic: Taiwan,3 Chile,4 China,5 Vietnam,6 Burkina Faso.7 In terms of numbers of people affected, arsenic contamination of groundwater in the Bengal Basin has presented the most alarming case, where 60–65 million people8 are estimated to be drinking water with excess levels of arsenic.
Due to the extent of the contamination and the numbers of people affected, groundwater contamination has been examined more methodically and documented to a greater extent in Bangladesh than in any other country, with national surveys for arsenic in 19979 and multiple metals in 1998–1999.10 These national surveys detected high concentrations of manganese and other toxic metals besides arsenic before any resulting health effects were observed. In other regions, groundwater has typically been tested only after health has been compromised, and sampling has been regional rather than national. However, more researchers are now considering the possibility of multiple-metal contamination, and routinely screening their samples for the presence of such multiple toxins such as manganese, lead, and fluoride, rather than testing for only a single element such as arsenic. As a result, multiple metal contamination of groundwater is now being reported in many regions; see Table 1, Fig. 1.
| Cancers | Skin cancer60 |
| Lung cancer61 | |
| Bladder and urinary tract cancers60a,62 | |
| Digestive tract cancers60a | |
| Liver cancer63 | |
| Prostate cancer64 | |
| Breast cancer65 | |
| Skin conditions | Keratosis and pigment change66 |
| Liver disease | Enlargement of the liver, cirrhosis and fatty degeneration11,66a,67 |
| Lung disease | Pulmonary obstructive and restrictive effects, chronic cough and bronchitis67,68 |
| Changes in mouse lung cells69 and cultured human lung cells70 | |
| Heart disease | Arteriosclerosis, myocardial infarction, ischemic heart disease, vascular constriction, and blackfoot disease64a,71 |
| Hypertension68d,72 | |
| Diabetes | Increased risk of diabetes72b,73 |
| Blood disorders | Anaemia or leucopoenia68c |
| Kidney disease | Renal damage74 |
| Eye disease | Pterygium75 |
| Cataracts76 | |
| Conjunctivitis77 | |
| Immune effects | Reduced immune function78 |
| Reproductive effects | Endocrine disruption79 |
| Decreased testosterone levels and increased rates of erectile dysfunction80 | |
| Aberrant morphology and decreased motility of sperm in rabbits81 | |
| Neurological effects | Peripheral neuropathy of both sensory and motor neurons, causing numbness, loss of reflexes, and muscle weakness2,66a,68d,82 |
| Learning disabilities in children and reduced intellectual function83 | |
| Developmental effects | Adverse pregnancy outcomes: spontaneous abortion, stillbirth, and preterm delivery,84 congenital malformations, and low birth weight85 |
| Increased infant mortality86 | |
| Decreased weight, height, and lung capacities of children87 |
| Neurological effects | Decreased brain function in domestic animals102 |
| Neurological deficits in nonhuman primates103 | |
| Disruptions in dopamine, norepinephrin, and acetylcholinesterase levels103 | |
| Manganism, a condition characterized by a cock-like walk,104 hyperirritability, violent acts, hallucinations, disturbances of libido, incoordination,102 and ultimately, Parkinson-like symptoms including tremors and difficulties with balance105 | |
| Compulsive behaviours, emotional lability and hallucinations, and somatisation disorder106 | |
| Violent or criminal behaviour107 | |
| Hyperactivity in children, learning disabilities, and deficits in children’s manual dexterity and rapidity, short-term memory, and visual identification56,108 | |
| Amyotrophic lateral sclerosis102 | |
| Possible increased susceptibility to prion diseases109 | |
| Liver disease | Liver damage and cirrhosis in rats104 |
| Kidney disease | Nephritis in rats104 |
| Reproductive effects | Degenerative changes in rat testes104,110 |
| Cardiovascular effects | Decreased heart muscle respiration in rats111 |
| Ear disorders | Hearing loss in mice112 |
| Developmental effects | Increased infant mortality, especially with concurrent pregnancy-induced iron deficiency anaemia113 |
| Bone effects | Decreased bone formation133 |
| Increased bone resorption134 | |
| Increased osteocalcin levels in men, particularly with current smokers134 | |
| Kidney disease | Decreased kidney function135 |
| Cardiovascular effects | Increased blood pressure136 |
| Neurological effects | Changes in the brain cholinergic system in rats137 |
| Hormonal effects | Endocrine-disruptor138 |
| Reproductive effects | Disturbed follicogenesis, oocyte meiosis and oocyte morphology in mice139 |
| Possible fertility problems and reproductive cancers138 |
| Neurological effects | Reduced children’s capacity to learn, even at very low levels of exposure141 |
| Reduced cognitive function in older adults142 | |
| Degenerative dementia143 | |
| Bone effects | Toxic systemic effects as lead is released from bones during pregnancy or menopause144 |
| Dental caries and tooth loss in adults145 | |
| Cardiovascular effects | Hypertension146 |
| Ischemic heart disease143 | |
| Kidney disease | Kidney damage in rats and humans143,147 |
| Liver disease | Liver damage in rats147 |
| Diabetes | Increased incidence of diabetes146b |
| Reproductive disorders | Reduced fertility with paternal exposure148 |
| Eye disorders | Cataracts149 |
| Cancer | Bladder cancer150 |
| Glucose balance and weight effects | Decreased fluid intake161 |
| Weight loss in rats162 | |
| Lowered BMI in women163 | |
| Insulin mimicker, improved glucose tolerance164 | |
| Bone effects | Increased bone growth165 |
| Inhibited cancer metastasis in bone cells166 | |
| Cardiovascular effects | Decreased heart rate in animals167 |
| Lowered or increased blood pressure in animals and humans167,168 | |
| Increased total cholesterol levels in rats169 | |
| Increased triglyceride levels in impaired glucose tolerance patients170 | |
| Reproductive effects | Impaired sperm morphology and motility in rabbits81 |
| Reduced sperm count and fertility in mice171 | |
| Neurological effects | Neurotoxic effects in dopaminergic neuronal cells172 |
| Kidney effects | Kidney damage in rats162,168b |
| Immune effects | Reduced immune response in rats162 |
| Developmental effects | Adverse development in rats162 |
| Necrotic death in neonatal cardiomyocytes in rats173 | |
| Cancer | Increased chromosome mutations and DNA strand breaks in mammalian cells174 |
| Reduced chromosome mutations in rats175 |
 | ||
| Fig. 1 Regions with naturally occurring multiple toxic metals in groundwater. Note: Many regions have not been systematically tested for multiple toxic metals in groundwater. | ||
The distribution and geology of toxic metals in groundwater
In a worldwide review of regions with arsenic in groundwater, Smedley and Kinnibergh observed that arsenic-containing reservoirs tend to be either strongly reducing aquifers derived from alluvium or loess, or inland aquifers in arid or semi-arid environments.47 Both of these types of environments tend to be in geologically young sediments, in flat low-lying areas where groundwater movement is slow, thus allowing for the accumulation rather than flushing of toxic metals released from sediments. In high pH aquifers typical of arid regions, there is desorption of arsenic and other anion-forming elements, including vanadium, fluoride, selenium and uranium. In the strongly reducing aquifers found in some deltaic regions, there is desorption of arsenic and reductive dissolution of iron and manganese oxides.47 Limited flushing is also a factor that may promote elevated concentrations of manganese, particularly where there is manganese enrichment of bedrock.48 The presence of fresh organic matter is likely to be the cause of the reducing conditions which promote the release of iron and manganese as well as arsenic.12 Iron and manganese levels may also be elevated in high pH coastal plains.14,49 Heavy use of phosphate fertilizers can increase uranium content in groundwater,50 and extensive extraction of groundwater can result in increased concentration of uranium in semi-arid areas.51Research on groundwater has found correlations and co-occurrences between metal contaminants that generally accord with the redox condition predictions. Combinations including arsenic, fluoride and/or vanadium are frequent in arid, high pH environments (e.g., Argentina,45 Mongolia12), and combinations of arsenic, manganese, lead and/or cadmium are frequent in deltaic, low pH environments (e.g. Bangladesh,10b Cambodia52). However, some common contaminants such as manganese and uranium are found in both arid and deltaic environments, often in combination with arsenic (e.g. manganese + arsenic in Argentina,45 uranium + arsenic in Bangladesh21). One strategy that has been suggested for accessing groundwater with safe levels of arsenic in deltaic regions such as Bangladesh is to drill deeper wells. However, it must be kept in mind that arsenic is not the only potential toxic metal contaminant, and that water from deeper wells may contain other toxic metals. In one study in the Kushtia region of Bangladesh, while deeper wells tended to have less arsenic, they were found to have increased levels of uranium.21b
Smedley and Kinnibergh emphasized that while arsenic regions tend to share certain geological characteristics, the specific spatial distribution of arsenic contamination is highly variable,47 necessitating the testing of individual wells in high-arsenic regions. The geology and spatial distribution of other toxic contaminants in groundwater reservoirs and wells has received much less attention, but may follow the same pattern of extreme local variability. In a case of neighbourhood uranium groundwater contamination in the United States, concentrations differed widely from one well to the next.53 Unambiguous geological indicators have not been identified for predicting the presence or concentration of one element based on the presence or concentration of another element. It is not clear whether such potential indicators even exist, although Winkel et al. argue that Holocene deltaic and organic-rich surface sediments are key indicators that arsenic contamination of the aquifer is likely.54 Slow moving groundwater with limited flushing is a major risk factor for metal contamination of groundwater, especially when the aquifer occurs in newly deposited sediments. Given the unpredictability of groundwater contamination, the prudent practice would be to screen for multiple toxic elements, including especially arsenic, manganese, lead, fluoride, and uranium, whenever new groundwaters are accessed, whether for public water supply or individual private wells.53,55
Smedley and Kinnibergh noted that arsenic and fluoride are recognized as the most dangerous natural contaminants of groundwater,47 but subsequent research has shown that manganese and uranium should also be of concern. It should be noted that even in developed countries such as the United States and Canada, which have laws governing the testing of public water supplies for toxic elements, private wells are generally unregulated and are not routinely tested for toxic metals. Furthermore, established permissible limits for manganese contamination do not take into consideration recent research demonstrating that manganese is much more toxic than previously believed, especially for children.56 In developed countries, increasing migration away from urban centres is resulting in the drilling of more private wells, and in developing countries, economic gains are permitting more people to drill private wells, increasing the risk that more people will be exposed to toxic elements in groundwater in the future.
Climate change and multi-metal exposure through groundwater
Global climate change may also increase the numbers of people exposed to combinations of toxic elements through groundwater. With climate change, many arid regions are expected to become more arid,57 increasing dependence on groundwater while simultaneously decreasing aquifer flushing and possibly increasing uranium concentration in groundwater.51 Rising sea levels may promote migration from coastal to arid regions, increasing populations exposed to contaminated groundwater in those regions. In coastal areas, as sea levels rise, there will likely be less flushing of aquifers, resulting in greater accumulation of arsenic, manganese and other contaminants in aquifers where conditions make these elements soluble. Seawater intrusion into aquifers may also promote the release of arsenic into groundwater.58For example, Bangladesh is located at one of the largest river deltas in the world. The Ganges, Brahmaputra, and Meghna rivers flow south through Bangladesh to the Bay of Bengal and the Indian Ocean. Most of this country is less than 12 meters above sea level. In a normal monsoon season one-third of the cultivated land is flooded with a mixture of fresh and saltwater.59 This flooding is likely to promote the spread of arsenic and other metals in groundwater through enhancing reducing conditions in the aquifers, promoting anion exchange, and causing mixing of waters from deep and shallow aquifers. A graph of arsenic concentration versus oxidation-reduction potential is shown in Fig. 2. This graph suggests that arsenic is released from solids to Bangladesh's groundwater by reduction9 (see Fig. 3). If so, by enhancing reducing conditions, flooding also promotes the release of a wide variety of other ions, such as Fe+2 and Mn+2, into groundwater. Fig. 3 and 4 show contour maps of arsenic and chloride concentration in groundwater from shallow wells (less than 30.5 meters below ground surface). The correlation of arsenic and chloride concentrations shown in these maps suggest that arsenic may be released in the presence of chloride through anion exchange.9 Thus, saltwater intrusion from seawater flooding likely releases a wide variety of other ions, such as U+4, U+6, Mn+2, Ni+2, Cr+2, or Pb+2, into groundwater by both anion and cation exchange. Currently, Bangladesh's deep aquifer is largely free of arsenic,16a enabling the population to access safe water in many parts of the country by drilling deep wells. However, as sea levels rise, the deep aquifer may become contaminated, and deep wells may no longer provide safe water, making water treatment a necessity in large portions of the country. Thus, as global climate change takes effect, there will be an increased need for understanding metal contaminants of groundwater and their potential health effects.
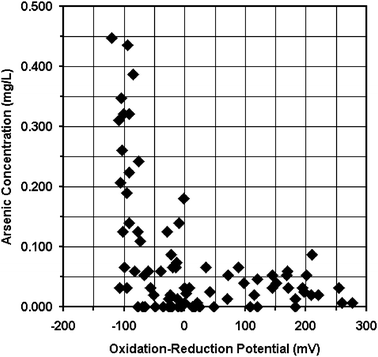 | ||
| Fig. 2 Graph of arsenic concentration (mg L−1) versus oxidation-reduction potential in water from sampled Bangladeshi wells.9a | ||
 | ||
| Fig. 3 Map of Bangladesh showing the average arsenic concentration (mg L−1) in water from tubewells less than 30.5 meters below ground surface (● = village location; Intensity of colour indicates increasing concentration).9a | ||
 | ||
| Fig. 4 Map of Bangladesh showing the average chloride concentration (mg L−1) in water from tubewells less than 30.5 meters below ground surface (● = village location; Intensity of colour indicates increasing concentration).9a | ||
Health effects of toxic metals from groundwater
Health effects of individual toxic metals from groundwater
Arsenic is known to cross the placenta of laboratory animals and similar arsenic concentrations have been found in cord blood and maternal blood of maternal-infant pairs exposed to arsenic in drinking water.11 Prenatal arsenic exposure has been associated with increased incidence of arteriosclerosis, carcinogenesis and lung cancer in mature mice88 and young adults.89 Prenatal arsenic exposure is also associated with increased frequency of acute respiratory infections in infants.78c
Gender differences with arsenic toxicity are commonly reported. Male subjects tend to show arsenic lesions before women, men have a higher risk of skin cancer associated with arsenic exposure, despite similar exposure levels90 and pulmonary effects associated with arsenic exposure were found to be higher in men than women.68a In contrast, bladder cancer mortality in arsenic-exposed areas in Chile was found to be higher among women than men.91 Women of child-bearing age have been found to methylate arsenic more efficiently than men,90f,92 particularly during pregnancy.93 Arsenic methylation efficiency in pregnant women was found to be resistant to effects of malnutrition.93 Overweight women had highly efficient arsenic methylation compared to males of normal weight;94 high body mass index (BMI) is associated with increased oestrogen levels,95 thus, it is likely that oestrogen plays a role in the gender differences with arsenic methylation and toxicity. Despite any potential protective benefit offered by increased oestrogen levels during pregnancy, epidemiological studies still show adverse effects of arsenic on pregnancy outcomes and infant health. In addition, gestational diabetes rates were found to increase with exposure to arsenic.73f Thus, although oestrogen may provide some protection against the toxic effects of arsenic, it does not eliminate health risks due to arsenic exposure. It is also unclear whether the mother's increased methylation of arsenic extends beneficial effects to the foetus' health. More direct research on the possible role of oestrogen providing some protection against arsenic toxicity is needed, especially research that directly compares human oestrogen levels to arsenic methylation efficiency. Susceptibility to toxic effects from arsenic has also been shown to be affected by genetic polymorphisms.92a,96
No effective treatments for chronic arsenic poisoning have been found,68a,c,97 although early health effect of arsenic (melanosis and keratosis) can be reversed by switching to water with safe levels of arsenic.98 In Taiwan, some improvement in vascular dysfunction was found after patients switched to arsenic-free water,99 but in other cases, the adverse health effects due to exposure to arsenic persist long after exposure ceases.83c Infant mortality continued to be elevated in an arsenic-affected city in Chile for 5–8 years after exposure to arsenic ceased.86 In Argentina,100 Chile,91 China,101 and Taiwan,90a excess mortality continued to be observed in arsenic-affected cities 5–25 years after arsenic treatment systems were installed; in Taiwan increased heart disease risk in arsenic affected areas began to fall appreciably only 17–25 years after exposure ceased.71d Although some participants in a study in Bangladesh of arsenic exposure and intellectual deficits in children switched to safer wells following initial testing and their urinary arsenic levels decreased, the well-switching did not eliminate the intellectual deficits associated with the arsenic exposure from the original wells.83b
Despite the fact that foetuses, infants, and children might be presumed to be highly susceptible to neurological toxins, until very recently, little research has specifically examined the possible health effects of manganese exposure for children. In rats and humans manganese absorption is higher in neonates than with adults due to the immaturity of the biliary manganese excretion pathways.102,114 Manganese has been found to accumulate in the brain of infants and young animals.109 Manganese effects in the brain may be particularly of concern for foetuses and infants, due to the high number of transferrin receptors elaborated by neuronal cells during development, coupled with the neural cells' putative need for transferrin for their differentiation and proliferation.102 Neonatal rats have been shown to be more likely to show symptoms of manganese neurotoxicity at the same dosing level than adults.115 Formula-fed infants had higher levels of learning disabilities than breast-fed infants; one possible explanation could be the higher manganese content in infant formulas, particularly those that are soy-based.116 Hair manganese was elevated for formula-fed but not breast-fed infants.117 In two case studies of the exposure of single families exposed to manganese through drinking water, in each case, the youngest family member was the first to develop clinical symptoms of manganese poisoning.108b,118
In a population-based study, 10-year-old Bangladeshi children whose drinking water contained high levels of manganese were found to have decreased intellectual function; this effect persisted even when co-exposure to arsenic was controlled for.56a It should be noted that this study only examined children attending school, and may have inadvertently excluded those children with intellectual or behavioural impairments severe enough to prevent them from attending school. Thus, the actual deleterious effects on behaviour and learning for the total population could potentially have been stronger than those reported in the study, which only considered the subset of the population enrolled in school. In a study involving schoolchildren in Quebec, children whose water contained high levels of manganese also had elevated levels of manganese in their hair, and a greater likelihood of having learning disabilities, problematical classroom behaviours, and hyperactivity.56b One possible mechanism for manganese increasing risk of hyperactivity may be its effects on the dopaminergic and GABAergic systems.56b,119 It should be noted that the WHO drinking water guidelines and US EPA RfDs for manganese are based solely on studies of adult exposures109,120—according to the WHO (World Health Organization) manganese taken by the oral route is “one of the least toxic elements”,109 but this document does take into account recent findings of neurological impairment or elevated infant mortality due to manganese exposure in children.56,113 Significantly, the cognitive deficits associated with manganese exposure in Bangladeshi children, were found with total daily intake of manganese less than 4.5 mg g−1, less than the Institute of Medicine Upper Limit (IOM UL) of 6 mg g−1 for children, which was interpolated from adult risk studies.56a
Blood121 and hair56b,120b,122 manganese levels have been reported higher in girls and women, and more manganese is absorbed by women than by men given similar levels of exposures.123 One possible mechanism for manganese concentrations being higher in girls and women is that low iron stores increase manganese absorption,124 and iron deficiency is common amongst menstruating women and girls. Paradoxically, saliva manganese levels have been reported higher in boys than girls125 and symptoms of manganese toxicity were observed at a greater frequency with men than with women given similar levels of exposure.120b,126 In general, hyperactivity is found to be more common amongst boys than girls;127 however, epidemiological data have not been studied to see whether this pattern might correlate with manganese exposure and body burden.
Genetic factors seem to at least partially control susceptibility to manganese toxicity. Studies of occupational and environmental exposures to manganese have suggested a genetic involvement128 and there is a great degree of inter-individual variation in manganese retention.129 Mice with Huntington's genes were found have reduced susceptibility to manganese exposure, but enhanced susceptibility to cadmium toxicity.130
It should be noted that as with arsenic exposure, many of the neurological symptoms associated with manganese exposure persist after manganese levels return to normal,102,131 although some recovery has also been observed.132 In a case study involving learning disabilities possibly associated with manganese exposure, the learning problems persisted at least 18 months after the manganese exposure ceased.108b Chelation has not been shown to reduce symptoms of neurological toxicity.105b
Children may be particularly sensitive to this toxic metal both because they consume more water per body weight than adults and because the kidneys mature postnatally.53 In a case study in which a family was exposed to high levels of uranium in their drinking water, it was the youngest child in the family (age 3) who first manifested symptoms of kidney damage.53 Incidence of reproductive or gonadal cancer is significantly higher for New Mexico Native American children than age-matched non-Native American children.140 It has been suggested that this may be at least partially due to elevated uranium concentrations in drinking water.138
Early in the twentieth century, the adverse effects of lead exposure on reproductive health were obvious enough for authorities to restrict women of reproductive age from working in the lead industry.151 Adverse effects of lead are often worse prenatally than postnatally.152 In utero exposure to lead is associated with increased rates of schizophrenia.153 Lead is particularly toxic for neonates because of the immaturity of the blood-brain barrier.104 Cord blood levels were found to be associated with greater neurological problems with boys than with girls.154 Boys may be more susceptible to neurotoxic effects of early lead exposure, while girls may be more susceptible to immuntoxic effects.144b Genetic polymorphisms have been reported to affect lead metabolism;155 in particular, variants in genes associated with iron metabolism modify lead metabolism.156 The neurotoxicological effects of lead exposure persist after the exposure ceases.157 Evidence for renal damage can persist years after exposure to lead ceases.158
Vanadium has been shown to promote oxidative stress in rats176 and in cell culture.172,177 Hair vanadium levels were reported higher for women than men in one study.163
Combinations of toxic metals
Environmental exposure to metals almost always involves simultaneous exposure to a combination of metals as well as other environmental factors.206 When water from high arsenic areas is screened for other metals, it is quite often found to have multiple elements exceeding drinking water guidelines (e.g. arsenic + manganese, uranium, lead, nickel, chromium, barium, and/or cadmium in South and Southeast Asia,10b,16,21,56a,207 arsenic + uranium, and/or vanadium in Argentina,2,45,100 arsenic + manganese in China:68c Studies of metal exposure frequently note evidence of co-exposure by other metals.53,56a,83a,83c,113,120a,208In addition to exposure through drinking water, populations are also commonly exposed to various toxic metal through diet, smoking, drug-therapies, and alcohol intake. Smoking and use of tobacco products, including exposure to second-hand smoke, implies significant exposure to cadmium,209 and may also involve lead,145,209e vanadium,171 arsenic,209e uranium,210 antimony,209g or barium.209g For these reasons, heavy metal interactions with drinking water contaminants including cadmium and other metals must be considered when populations exposed to metals through drinking water include smokers. Environmental lead exposure is also common in countries with metal-contaminated drinking water, such as Bangladesh, where many children have blood lead levels above 10 μg/dl WHO guideline.211
When subjects are exposed to metals in combination, the metals may each have their own typical health effects; they may also have synergistic or antagonistic effects on each other. In calculating cancer risks due to drinking water contaminants, it is vital to consider not only the health risks due to individual contaminants, but also those risks due to combinations of contaminants.
Effects on absorption and excretion
Ingestion of metals in combination may increase or decrease the absorption of the individual metals in the digestive tract. Some metals promote the excretion of other metals. The absorption and excretion effects of metals are summarized in Table 7.| Element | Interaction effects on absorption and excretion of metals (arrow shows likely direction of net interaction effect on body burden) |
|---|---|
| Zinc | ↓ Arsenic212 |
| ↓ Lead157,213 | |
| ↓ Manganese214 | |
| ↓ chromium215 | |
| ↓ Iron216 and haemoglobin217 | |
| Selenium | ↓ Arsenic218 |
| ↓ Lead157 | |
| Calcium | ↓ Manganese219 |
| ↓ Lead220 | |
| ↓ Chromium221 | |
| ↓ Iron222 | |
| ↓ Zinc223 | |
| Iron | ↓ Arsenic69,195,224 |
| ↓ Manganese214 | |
| ↓ Lead225 | |
| ↓ Cadmium226 | |
| ↓ Chromium215 | |
| ↓ Calcium222 | |
| Chromium | ↓ Lead157 |
| Vanadium | ↓ Chromium227 |
| Barium | ↑ Calcium204b |
| Aluminium | ↓ Fluoride228 |
| Arsenic | ↓ Selenium218a,c |
| Uranium | ↓ Calcium134 |
| Nickel | ↓ Manganese, zinc and magnesium229 |
| ↑ Iron230 | |
| ↓ Calcium231 | |
| ↓ Ascorbic acid232 | |
| Manganese | ↑ Cadmium157 |
| ↓ Iron104,233 | |
| ↓ Zinc214 | |
| ↓ Calcium231 | |
| Lead | ↓ Arsenic from bones234 |
| ↓ Zinc, iron and manganese from the brain235 | |
| ↓ Zinc213a | |
| ↓ Selenium157 | |
| Cadmium | ↑ Nickel236 |
| ↓ Selenium237 | |
| ↓ Iron237,238 | |
| ↓ Zinc214,239 | |
| ↓ Calcium231,238,240 |
Zinc absorption is also increased by concurrent protein consumption,223 but reduced by concurrent casein consumption,239 phytates, or dietary fibre.238,239 Zinc promotes absorption of vitamin A.241 During pregnancy, zinc and cadmium absorption from oral routes are increased in rats and humans.242 Iron absorption is increased by concurrent consumption of ascorbic acid243 or vitamin A,244 but decreased by concurrent consumption of chilli245 or tea.246 Chromium absorption is reduced by concurrent phytate consumption but increased by concurrent oxalate consumption.247 Manganese absorption is reduced by concurrent consumption of dietary fibre, phytates, phosphorus, oxalic acid or tannic acid.248
Since lead is associated with reduced levels of brain manganese, co-exposure to lead might reduce the neurotoxic effects of manganese. However, it is not clearly whether any potential benefits due to such exposure would outweigh the neurotoxic effects of the lead exposure itself. If nickel also reduces tissue concentrations of manganese in humans as with animals, concurrent exposure to nickel may provide some protection against exposure to manganese; however, it is also possible that ingestion of nickel could increase manganese absorption by reducing calcium stores, since concurrent calcium consumption reduces manganese absorption. It is also possible that ingestion of uranium or cadmium may increase manganese absorption by reducing calcium stores.
Deficiencies of essential trace metal elements may also affect absorption of toxic metals, as summarized in Table 8.
| Deficiency | Interaction effects on absorption and excretion of metals (arrow shows likely direction of net interaction effect on body burden) |
|---|---|
| Iron | ↑ Manganese absorption214,249 |
| ↑ Arsenic in liver250 | |
| ↑ Lead absorption104,157,214,237,251 | |
| ↑ Cadmium104,214,237,252 | |
| ↑ Zinc214 | |
| Zinc | ↑ Lead213a |
| ↑ Cadmium237 | |
| Calcium | ↑ Lead157,253 |
Women with similar exposure histories tend to have higher levels of blood cadmium levels than men, possibly because of lower iron stores.254
Chemical interactions
Elements with similar physical and chemical properties may act antagonistically by competing for transport and storage sites and displacing each other from enzymatic and receptor proteins.157,255 Conversely, they may act synergistically by promoting oxidative and other cellular damage. While additivity is generally assumed for low level co-exposures,256 synergistic cytotoxicity effects have been reported with human keratinocyte cells with chemical mixtures including arsenic, cadmium, chromium, and lead at low doses.257 Cadmium, chromium, nickel and lead may interfere with DNA repair, leading to damage not directly attributable to the heavy metals.258Selenium protects against oxidative stress through glutathione peroxidase and other antioxidant activity.218a Selenium and arsenic are chemically similar; thus, they act antagonistically in certain reactions.268 Arsenic inhibits the action of selenium, causing an apparent deficiency in the glutathione peroxidase system.11 Selenium has been found to reduce the toxicity of arsenic,259a,269 selenium deficiencies have been linked to arsenic-induced skin cancers,270 and dietary supplements with selenium may counter some degree of arsenic toxicity.271 Selenium has also been shown to have antagonistic effects on cadmium, lead and nickel,157,263,272 although in order for selenium to have significant protective effects against lead, selenium must be given at levels high enough to have its own toxic effects235 and high doses of selenium appear to exaggerate lead toxicity in rats.273 Smokers are reported to have lower serum selenium levels, and heavy smokers also have depressed serum zinc levels.274
Arsenic is methylated in the liver with an enzymatic reaction that requires glutathione and S-adenosylmethionine.275 Thus, metals that interfere with the required methylation reactions reduce the elimination of arsenic and prolong exposure, potentially increasing the toxic effects. In particular, selenium and zinc may be required for methylation reactions,276 and selenium may increase arsenic methylation capacity,94 so exposure to arsenic may increase requirements for selenium and zinc; selenium and zinc deficiencies may increase arsenic toxicity. Oestrogen increases choline synthesis,277 which enables the formation of methionine, a necessary amino acid for arsenic methylation. Smokers and chewers of betel quid were found to have reduced arsenic methylation efficiency;92b smokers of bidis, hand-rolled cigarettes popular in South Asia, were found to methylate arsenic less efficiently than smokers of mass produced cigarettes.90f
Chisholm suggested that heavy metals are likely to interact with immune proteins, particularly those containing sulphur.262 The toxic effects of concurrent exposure to arsenic and antimony may be sub-additive because of competition for sulfhydryl groups.278 Arsenic inhibits the increase in serum creatinine associated with cadmium,279 and lead tends to antagonize the cadmium-induced rise in N-acetyl-β-D-glucosaminidase,279 suggesting that co-exposure to arsenic and/or lead might provide some degree of protection against the renal damage due to cadmium exposure. However, synergistic effects between arsenic and cadmium on nephrotoxicity in a human population co-exposed to both metals have been reported.280 The trivalent arsenical phenylarsine oxide forms thioarsenite complexes with vicinal or paired thio groups of cellular proteins, and this has been shown to inhibit glucose transport in adipocytes.281 Zinc and selenium have been found to protect against cadmium-induced alterations in glucose tolerance282 in rats, but it is not known whether zinc and selenium can protect against arsenic-induced diabetes. Nickel antagonizes manganese-induced neurotoxicity in rats283 and depletes intracellular ascorbate.232
Mimicry is one type of competitive mechanism through which toxic metals may gain access to cells.255 Selenium may be a functional mimicker of oestrogen.255 Oestrogen has been found to provide some protection against oxidative stress mechanisms in mice.284 Other metallomimics of oestrogen include cadmium,285 antimony, arsenic, barium, chromium, lead, nickel, uranium, and vanadium.286
Arsenic may also act as a co-genotoxin for methyl methanesulfonate and UV-induced pyrimidine dimers.293 Mice exposed to arsenic in addition to UV-light showed greater incidence of skin cancers than mice exposed to UV-light alone287f,h,294 and human keratinocytes showed synergistic effects when exposed to UV-light in the presence of arsenic.266,295 A dose-response effect with UV and arsenic exposure was noted in humans,90e while selenium was found to block the cancer enhancement effect of arsenic but not of UVR in mice.296 Exposure to sunlight was also found to predict arsenic lesions in a Bangladeshi population.90d,297 Chen et al. also reported a possible relationship between arsenic toxicity and exposure to fertilizers, but it was not noted in that study whether socio-economic status (SES) had been taken into consideration as a potential confounding factor.90d Ingestion of arsenic has been found to increase the incidence of spontaneous viral cancers.218a Pretreatment with arsenic at low levels has been found to dramatically increase liver damage in mice exposed to lipopolysaccharide.298
Nickel may also require exposure to another toxin to potentiate its mutagenic effects;178 arsenic and nickel may act synergistically in the lungs to produce lung cancer. Nickel has been found to increase skin cancer risk with mice exposed to UVR.299 On its own, chromium III is not known to have carcinogenic effects;300 however, it could potentiate mutagenic effects of other metals by causing DNA damage.188a,301 A synergistic effect on the teratogenecity of lead and cadmium has been reported;302 lead and cadmium are also reported to interact synergistically with testicular effects,303 but co-exposure to zinc may decrease the testicular effects.304 An interaction effect on children's IQ has been reported for lead and manganese.305 A risk assessment study of metal mixtures predicted that lead may have a greater than additive effect when combined with arsenic or cadmium for neurological problems.303 It has been suggested that there could be possible synergistic effects between arsenic and antimony, due to similarities between these metals.306
Exacerbation of health effects
The effective toxicity of metals may be increased when multiple metals have adverse effects on the same organs or physiological systems. Effects that might ordinarily be minor may result in critical damage when multi-metal exposure occurs. Conversely, in some instances, multi-metal exposure may provide some degree of protection against adverse effects of some metals.Iron deficiency anaemia increases manganese concentrations in the brain,308 and manganese exposure with concurrent iron deficiency magnified deleterious manganese effects in the rat brain,309 making manganese exposure particularly problematic for those with iron deficiency anaemia or low iron intake, especially since iron deficiency anaemia is also linked to increased absorption of lead and cadmium, which each have their own toxic neurological effects. This is of special concern in South Asia where iron deficiency anaemia is endemic310 and manganese concentrations in the drinking water are frequently high, and concurrent exposures to lead or cadmium are common. In addition, iron deficiency anaemia is independently associated with problems of neurological development and lower mental and motor test scores311 and increased levels of neuropsychiatric symptoms106 and iron stores, like manganese, affect the dopaminergic system.106
Zinc is essential for the development and function of the nervous system.261 Zinc deficiency impairs brain function216,261,312 and is associated with increased levels of depression;313 since nickel, lead, and cadmium exposure reduce zinc absorption and increase zinc excretion, they may indirectly affect brain function by reducing essential zinc stores.
Neurobehavioral effects and learning disabilities associated with childhood exposure to one toxic metal may be increased synergistically when exposure is to multiple metals. The blood-brain barrier develops only during the first year of life, increasing risks associated with toxic metal exposures to foetus and neonates. Visual-motor performance deficits were reported in children exposed to arsenic and lead in combination, as well as possible problems with attention deficit and distractibility.83a,208a,314 Mentally retarded and learning disabled children show higher than average levels of toxic metals, particularly cadmium and lead.314a,315 Arsenic, and manganese have been independently linked to intelligence deficits and learning disabilities in children; in the absence of evidence for antagonistic effects between these two metals, it is likely that adverse effects due to co-exposure to these metals is at least additive, and further exacerbated by co-exposure to lead.
A risk assessment study of mixtures of metals predicted that cadmium, arsenic, lead and chromium(VI) will have a less than additive risk for renal effects;303 concurrent exposure to lead and cadmium resulted in lower than expected renal effects in rats.288 In contrast, mice who consumed both arsenic and cadmium showed greater renal effects than when fed either arsenic or cadmium alone.317
Persons with renal insufficiency show increased levels of blood vanadate with vanadium exposure,318 thus co-exposure to metals associated with kidney damage is of special concern when there is exposure to vanadium. Exposure to uranium through drinking water has been associated with disturbances of iron homeostasis in the kidney in rats319 and causes kidney damage.135a,320 Nickel, lead, chromium and vanadium exposure are also associated with kidney damage.162,178,321 Patients with kidney disease are advised to restrict intake of chromium because of increased susceptibility to its toxic effects,322 thus exposure to chromium at levels that might ordinarily be considered safe may have increased adverse effects for persons with concurrent exposure to arsenic, uranium, nickel, or lead exposure. Renal disease is also associated with zinc deficiency,323 which is promoted by ingestion of lead, nickel, or cadmium.
Chronic renal insufficiency is associated with changes in bone metabolism.324 Kidney damage due to cadmium exposure increases levels of bone resorption markers and may induce osteoporosis through increasing excretion of calcium.240a,325 Cadmium exposure concurrent with low calcium intake may result in Itai-Itai disease.326 Exposure to uranium likewise increases bone resorption markers;134 this is of particular concern for smokers drinking uranium-enriched water due to their concurrent exposure to cadmium through smoking. Iron deficiency has been shown to decrease bone density in rats.327 Through causing renal insufficiency, metals associated with kidney damage, including uranium, nickel, lead, and chromium may increase risk of osteoporosis, particularly with smokers, who have additional exposure to cadmium, and exposure to uranium, nickel, lead, or chromium may be of added concern for populations with high rates of iron deficiency.
Arsenic,72a,328 uranium136 and lead146b exposure have been independently shown to be associated with hypertension. Hypertension is known to be a risk factor for kidney disease and kidney disease itself is also associated with increased levels of hypertension.329 Thus, concurrent exposure to a combination of metals including arsenic, uranium, manganese, nickel, lead, or chromium may synergistically bring about both hypertension and kidney disease; these effects may be further exacerbated by smoking, which is also independently associated with increased hypertension and additional exposure to cadmium, which is itself associated with renal damage.330
Population fertility rates are likely to be affected through exposures to combinations of metals. Population fertility is likely to be lowered because of reduced testosterone levels and erectile dysfunction due to arsenic exposure,80 testes damage due to manganese exposure,110 reduced male fertility due to lead,148 chromium,322 vanadium171 or molybdenum335 exposure, reduced sperm motility due to arsenic, chromium, or vanadium exposure,81 oocyte or follicular changes due to uranium exposure,138,139 reduced implantation due to chromium exposure,193 delayed sexual maturity due to chromium exposure,193 and increased chance of spontaneous abortion and infant mortality due to arsenic, manganese or nickel exposure, acting alone or in combination.84a,b,d,86,180,336 However, in one study in Bangladesh, excess infant mortality due to manganese exposure may have been reduced in areas with co-exposure to arsenic,113 although SES may have been a potential confounding factor in that study.
Dietary interactions with groundwater toxic metals
While total dietary exposure to manganese is generally highest from food sources,349 manganese has greatest bioavailability in water.350 In foods, manganese levels tend to be relatively low in meats and poultry and higher in nuts, cereals, legumes, and leafy greens;248 blood manganese concentration has been found to be related to frequency of consumption of grains and leafy greens.121 Vegetarian diets may be higher in manganese than non-vegetarian diets.351 Tea is particularly high in manganese, but tannins greatly reduce its bioavailability from this source.248,352 Cadmium levels in food, while generally low, are highest in grains, leafy vegetables, root vegetables, organ meats, and shellfish,353 and rice grown in cadmium-contaminated soils.354 Uranium levels tend to be higher in root vegetables than other foods.50 Certain meat products in Pakistan and India have been found to contain unacceptably high levels of arsenic, cadmium, and lead; the Pakistani products also contain relatively low levels of zinc.355
Drinking water provides only a minor component of overall intake of zinc.216 Zinc is most commonly obtained from and most absorbable from animal products261,356 and a high-protein diet is probably necessary in order to obtain recommended daily allowances of zinc from food.357 Vegetarian diets tend to result in less zinc absorbance than diets that include meat.358 The only significant dietary source of selenium is animal protein, and the levels of selenium in animal protein are dependent on the selenium levels of the plants consumed by the animals, which are in turn, dependent on the selenium content of the soils and irrigation water.218a
Elevated levels of arsenic, iron, and manganese were found in soils that received irrigation from groundwater with high concentrations of these contaminants, which could lead to elevated levels of these contaminants in crops.359 Vegetables irrigated with arsenic-rich water have elevated levels of arsenic,346d,k,360 as does tobacco.361 Rice straw is used as animal feed in Bangladesh, and may contribute to arsenic levels in beef.360a Foods grown in Bangladesh and imported to the UK contained 2 to 100 times as much arsenic as similar foods grown in the UK.346c Arsenic content of food was also found to be high in a region of Thailand.362 It has also been suggested that fertilizer and pesticide use may contribute to heavy metal contamination of soils.363 Both soy and rice are known to accumulate manganese.114 Grains and rice may accumulate cadmium if grown in polluted soil or irrigated with contaminated water157,364 and tobacco naturally accumulates cadmium.365 Cadmium levels in Bangladeshi vegetables were lower than those reported in the developed world, but lead levels were higher; zinc levels were also lower.360b In a market basket survey in Bangladesh, dietary intake of manganese and nickel was high, while zinc intake was low;346a however, zinc content of vegetables grown in one region of Bangladesh was high.363 Lead content in chicken eggs has been found to correlate with lead concentrations in soil.366
Preparation and cooking methods can alter the arsenic content and arsenic species found in food.367 Rice cooked in high-arsenic water contained twice as much arsenic as raw rice;347a,368 milling rice or parboiling in arsenic-free water can reduce the arsenic content of cooked rice.360c Even when safe drinking water is provided, arsenic levels in hair may still be elevated due to continuing to cook food in arsenic-contaminated water.369 Arsenic levels in cooked food in Bangladesh correlated with arsenic levels in household drinking water.346h Beans and legumes absorb arsenic when cooked in water containing arsenic.370 This is of particular concern for vegetarians and people of lower SES in South Asia who often eat legumes in place of meat. Some studies have found reduced arsenic symptoms amongst people who eat animal protein, but one explanation for this finding could be that people who consume little animal protein also consume relatively large amounts of beans and legumes, which when cooked in arsenic-contaminated water, may increase their arsenic exposure. The concentrations of other metals in cooked foods and possible effects of cooking methods have not been studied; for instance, it is not known how total manganese content and bioavailability in tea may be affected by the manganese concentration of the water with which the tea is made.
The metallic composition of cooking and serving dishes and potential for leaching metals also needs to be considered when calculating total metal exposure. Leaching from kitchen utensils and water pipes can add significantly to daily intake of nickel, chromium, and lead.227,371 Chromium exposure may be increased when acidic food is prepared or served in stainless steel utensils;186 such utensils are commonly used as serving dishes throughout South Asia. Glass dinnerware, glazed ceramic tea mugs, and pressure cookers produced in India were found to leach lead, cadmium, manganese, nickel, chromium, iron, and/or zinc.372
Protein. S-adenosylmethionine levels in rats are dependent on dietary consumption of methionine and choline;373 adequate protein intake has been identified as the most important dietary factor affecting arsenic toxicity.263 Low protein diets have also been found to be associated with reduced arsenic methylation efficiency and greater arsenic toxicity71a,289b,374 and subjects who consumed higher levels of animal protein seemed to have decreased adverse health effects due to arsenic exposure.346h Protein intake also affects lead toxicity375 and low protein diets increase absorption of cadmium in animals.263 It should be noted that studies comparing arsenic toxicity to animal protein intake may have zinc and/or selenium intake as a confounding factor, since intake of zinc and selenium are strongly correlated with animal protein intake, and zinc and selenium likely reduce arsenic toxicity. Thus, further study is needed to differentiate the effects that zinc, selenium, protein, and animal protein have on arsenic and other metal toxicity.
Vitamins. B vitamins have been identified as essential for arsenic methylation reactions.297,346h,376 Increased intake of B vitamins reduces arsenic toxicity297,346h and people who consume diets with low amounts of niacin or folate were found to have reduced arsenic methylation efficiency.374d Folate supplementation has been found to increase arsenic methylation efficiency377 and lower blood arsenic levels. People with higher plasma folate levels were found to have decreased risk of urothelial carcinoma378 and skin lesions.379 Diets low in folate were associated with increased chance of arsenic-induced skin lesions.374c Thus a deficiency in B vitamins may increase the toxic effects of arsenic, and exposure to arsenic may increase dietary requirements for B vitamins.374c Smoking is associated with decreased levels of vitamin B12.380
Increased consumption of antioxidants (in particular, vitamins A, C, and E and beta-carotene) reduces skin cancer incidence and arsenic toxicity,90a,272b,297,346h and vitamin E reduces skin cancer risk for mice exposed to nickel and UVR299 and lead toxicity for rabbits exposed to lead.375 Increased consumption of fruits and yellow and light green vegetables was associated with reduced odds of lung cancer in tin miners exposed to arsenic.263 Smoking, both active and passive, has been associated with decreased blood levels of vitamins A, C, iron, zinc, and selenium.381 Vitamin D increases absorption of iron, zinc, and lead.263
Dietary fibre and other dietary constituents. Consumption of large quantities of whole grains, with their high phytate and lignin content, can result in zinc deficiency through interfering with zinc absorption, especially if there is low consumption of animal products.261 Thus, diets high in whole grains, legumes, and other sources of dietary fibre and low in animal products may exacerbate adverse health effects from toxic metals by promoting zinc deficiency.
Arsenic has been found to decrease absorption of nutrients and has been associated with weight loss;11 thus, consumption of arsenic may promote nutritional deficiencies that increase absorption and adverse effects of other toxic metals. Higher total food and fat intake are associated with decreased lead uptake.263
Dietary deficiencies, malnutrition, and metal toxicity. Dietary deficiencies can increase absorption of toxic metals and present shortfalls of antioxidants important for counteracting toxic effects of metals.157 An animal suffering from multiple nutritional deficiencies may be “highly vulnerable” to the toxic effects of heavy metals and nutritional status plays a significant role in determining risk response measures for a given metal.268 Adverse effects of trace elements usually begin to appear at 20 or more times their required intake levels, but with nutrient deficiencies, this may be reduced to only 2 to 3 times the required intake levels.238
Deficiency in methionine, folate, vitamin B12, or selenium decrease arsenic methylation ability and increase arsenic toxicity,289b,61b while zinc deficiency is associated with decreased ability for DNA repair.261 Iron deficiency increases lead deposition in the bones, and calcium deficiency increases lead and cadmium deposition in kidneys, bones and other tissues.268 Vitamin E deficiency has been shown to increase the toxic effects of lead in rats; concurrent calcium deficiency further amplifies this effect.268 Zinc, iron, folate, vitamin B12, vitamin A, and selenium deficiencies are common throughout the developing world;382 in particular, they are common in regions affected by multiple metal contamination of groundwater (Bangladesh,310,383 India,384 Nepal,385 Cambodia,386 Vietnam;387 Thailand;388 Mongolia389), factors which may be exacerbating the toxic effects of metal exposures. Smokers have been found to have decreased levels of beta-carotene, vitamin C and selenium compared to non-smokers,390 and female smokers and chewers of betel quid in Pakistan were found to have elevated levels of hair and blood cadmium and depressed levels of zinc,391 further increasing risks of metal toxicity for tobacco users in regions with metal-contaminated drinking water.
Low-level exposures to metals that would otherwise produce at most subtle effects in healthy subjects may produce full-blown toxicity effects in malnourished subjects.208a Malnutrition is associated with increased sensitivity to arsenic toxicity in animals and humans71a,263,374c,d,392 and good nutrition347a,393 and higher BMI90c are associated with reduced incidence of arsenical skin lesions. Children with poor nutritional status had more arsenic lesions and at lower rates of exposure than well-nourished children.394 Increased learning problems are associated with arsenic-exposure in children with chronic malnutrition.395 However, even being well-nourished is not sufficient to prevent the effects of arsenic altogether.83c,206b,263
South Asian diets, environment, cultural factors and metal toxicity. Diets in South Asia are heavily based on rice. Regular access to animal protein is limited to people of middle and upper socioeconomic SES,383e and many individuals follow vegetarian diets. Thus, there is relatively high consumption of legumes for protein. Access to fresh fruit is also somewhat limited to people of middle and upper SES.383e Consumption of dairy products is low396 and tea and chilli peppers high. Consumption of fruits and vegetables is lower among South Asian smokers than non-smokers.397 A market basket survey of foods in West Bengal found high levels of arsenic, manganese and nickel, and low levels of zinc.346a Parasitic infestations398 and H. pylori infection399 are not uncommon in South Asia. In much of South Asia, women cover head, arms and legs in public, which reduces sunlight exposure. Periodic fasting is a common and required practice for many communities.
These factors are likely to exacerbate the adverse effects of multi-metal exposure. The high consumption of rice adds to the arsenic load, especially if the rice is irrigated with, and then cooked in, arsenic-contaminated water. The high consumption of legumes also adds to the arsenic load, especially when cooked in arsenic-contaminated water. The primarily vegetarian diet is likely to include relatively high amounts of manganese. While the lignins and hemicelluloses in the legumes may decrease the absorption of manganese, they also decrease absorption of zinc. The low consumption of animal products and high consumption of tea and chilli peppers245 increase likelihood of iron deficiency, which further increases absorption of manganese and lead and also increases absorption of cadmium. The low consumption of animal products also increases the likelihood of zinc and selenium deficiencies, a condition made even more serious by the increased need for zinc and selenium under conditions of toxic metal exposure. Parasitic infestations and H. pylori infections further increase the likelihood of zinc and iron deficiencies.216,383d,399 In turn, iron deficiency may increase absorption of lead, chromium and manganese; manganese and lead absorption may also be increased by the low calcium intake of the diets. The general low overall consumption of food may promote higher absorption of lead. While restricting exposure to sunlight may reduce the incidence of arsenic-caused skin lesions, it may also promote vitamin D deficiency, further exacerbating iron and zinc deficiencies. Fasting alters arsenic methylation processes400 and increases uranium absorption.401
Intake of riboflavin, pyridoxine, vitamin A, vitamin C, calcium and zinc in Bangladesh fall below RDAs for India.297,374c,383e,402 It has been demonstrated that iron deficiency anaemia could be addressed through ferrous sulphate supplementation of drinking water,403 but the extent of iron deficiency anaemia in South Asia suggests that although the groundwater in the region often contains high levels of iron, the iron may not be in a bio-available form. Vitamin D levels have been found to be low in Bangladeshi women,404 which may exacerbate arsenic and manganese toxicity by decreasing absorption of iron and zinc.
In South Asia, poverty is sometimes measured in terms of calories available for consumption on a daily basis. By this measure, approximately 33–50% of Bangladeshis are “poor”, with fewer than 2122 kilocalories per family member available on a daily basis.405 In Bangladesh, low BMI is associated with poverty and lack of schooling;406 34% of Bangladeshi women have a BMI of less than 18.5.406b In South Asian populations exposed to high levels of arsenic, BMI has been reported to be inversely correlated with incidence of skin lesions.407 BMI has been reported to be lower among tobacco users in India.408 Incidence of underweight is decreasing throughout South Asia, but is still high by world standards;406b,408c,409 one recent study in rural Bangladesh found 56% of children ages 2–6 underweight.383g Effects of multi-metal exposures seem to be more pronounced in undernourished children in Bangladesh.410 It has also been argued that exposure to arsenic may reduce BMI in children.411 If oestrogen does indeed provide some protection from arsenic, then people with decreased oestrogen levels due to low BMI may show increased risk of adverse health effects due to arsenic exposure; this may provide an additional reason for why the poor of South Asia seem particularly susceptible to the effects of arsenic exposure.
Research on diet and metal toxicity. The dietary factors surrounding individual dietary components and their effects on arsenic toxicity are extremely complex and require further study to disentangle the conflating factors. For instance, an inverse correlation was found between consumption of fruit and canned foods in Bangladesh and incidence of arsenic lesions amongst those exposed to arsenic through drinking water.289b However, consumption of fruit and canned foods are more likely with higher SES, and those with higher SES have been shown to be less susceptible to arsenic toxicity,297,412 to have better overall nutritional levels, and higher BMI. Thus, before it can be concluded that consumption of fruit and canned foods can protect against arsenic lesions, the effects of SES and overall good nutrition must be differentiated. Similarly, increased consumption of animal protein was correlated with decreased arsenic toxicity in a Bangladeshi population,346h but consumption of animal protein is also associated with higher SES, possibly reduced consumption of legumes, higher general protein intake, higher general nutrition levels, and higher BMI. Before concluding that animal protein provides a benefit against arsenic toxicity, animal protein consumption must be separated statistically from the other factors, especially legume consumption, since consumption of animal protein in place of legumes implies reduced dietary arsenic exposure, and increased zinc consumption, since zinc is often contained in animal products and may reduce arsenic toxicity.
Human epidemiological studies may be the only way to determine the health effects of metal exposures on humans, however, these studies cannot uncover the complete picture if they do not screen for co-exposure to other metals and account for such co-exposures statistically. Even studies that have found a clear link between exposure to a single metal and a direct health effect would benefit from being re-examined in the light of possible multiple metal effects. For example, some authors have reported that the expected correlation between lead exposure in children and neurological symptoms disappeared when co-exposure to other metals was partialed out.83a,208a A variety of toxic metals can cause similar symptoms making it impossible to differentiate metal exposure by the toxic effects that are observed. Where evidence for a direct effect is clear and the biological pathway or mechanism underlying the effect is identified, screening for multiple metal effects would provide additional confirmation that the single metal is indeed the one responsible for the effect. At the same time, multiple metal screening may provide explanation for any observed variation in predicted health effects152 and ultimately lead to a better understanding of the other metals to which subjects may have been exposed. Studies of effects of toxic metals must assume that the possibility of multiple metal exposure is possible, test for multiple metal exposures, and ensure that there is enough variation in population exposure of the studied metals to provide statistical power for factoring the contributions of the various metals alone and in combination.
At present, because of lack of research data, US recommended daily allowances (RDAs) and United States Environmental Protection Agency (U.S. E.P.A) reference doses (RfDs for metals do not take into account possible interactions between one trace element and another, even though the normal context of exposure is with multiple elements.423 A common assumption for risk assessment of mixtures is that the effects will be additive, not synergistic. In order for a model to properly predict risk, published research must be available detailing the nature of effects for chemicals in combination (additive, subtractive, or synergistic), but such data are rarely available.424 Some risk assessment models address mixtures of metals, but are limited by the paucity of research that has explored the toxicity of metals in combination.303 A number of alternative approaches to risk assessment with combinations have been proposed.425 Monitoring for biomarkers of oxidative stress may be vital for understanding the effects of chemical mixtures, particularly at low doses.141i
Many researchers have observed that lead, cadmium, and manganese are more toxic to neonates than adults.104,147,308,431 The bioavailability of manganese is greatly increased for infants than adults102,116,128,350 and manganese exposure causes more damage to mice exposed as juveniles than as adults;432 uranium is also absorbed more readily by infant animals50 and uranium is more likely to be deposited in bone with children than with adults.50 In general, prenatal exposure to heavy metals results in adverse health and neurological effects152 and it has been suggested that early exposure to neurotoxic metals may be resulting in a “global pandemic” of neuro-developmental disorders.206b
Nevertheless, studies on the health effects of toxic metals have, by design, almost exclusively included only adults, and sometimes only older adults. Epidemiological studies involving children and metals to date have mostly focused solely on learning and intellectual deficits rather than on more general health effects. While the results of the adult health studies are vital for understanding the effects of metal exposure on humans, they do not shed light on any special health risks that may arise when children are exposed to metals. Since no research has been done on the health effects of toxic metals on children, drinking water guidelines and RfDs have been calculated solely on the basis of the adult health risk data. While estimates have been made to extend these data to cover children's risks, there has been no research available to confirm whether such extensions provide adequate protection for children;351 it should be noted that adverse effects of manganese exposure in children were found at manganese levels below the Institute of Medicine Upper Level (IOM UL) interpolated from adult risk studies.56a
Clinical observations have noted that children seem to develop adverse health effects due to toxic metals before adults given the same level of exposure.2,4,25a,433 In case studies involving single North American families with multiple family members exposed to high levels of metals in private wells, it was the youngest member of each family to show health symptoms, and who became the sickest from the exposure.53,108b,118 Clinical manifestations of arsenic poisoning may not be as obvious with young patients and hence may be overlooked.426b Children may be more sensitive than adults to arsenic-induced toxicity,434 and instances of myocardial infarction, myocardial ischemia, arterial thickening, and other cardiac problems as well as pulmonary effects have been noted in children exposed to arsenic through drinking water.11,435 Thus, it is urgent that epidemiological studies be undertaken to develop understanding of the health risks of metal exposure with children. It is also imperative that current drinking water guidelines be re-examined in the light of recent research involving learning deficits caused by children's exposure to metals.56,83b
Testing for multiple metals in water
The storage, preservation, and analysis of drinking water samples is complex. This is especially true when these samples are analyzed for more than one analyte. For example, samples collected for boron analyses must be stored in polyethylene containers and cannot be stored in glass containers because glass is both a potential boron source and sink. In contrast, samples collected for total organic carbon analyses must be stored in glass containers and cannot be stored in polyethylene containers because polyethylene is both a potential carbon source and sink. Therefore, the same sample may need to be divided into several different containers at the time of collection.436The preservation method chosen for a sample is determined by the analytical method and equipment available for analysis. For example, samples collected for arsenic analyses by atomic absorption (AA) spectrometry or inductively coupled plasma mass spectrometry (ICPMS) must be preserved with nitric acid. However, samples collected for arsenic analyses by colorimetric methods such as silver diethyldithiocarbamate cannot be preserved with nitric acid, since oxidizing agents such as nitrate interfere with this method,436 so they must be preserved with hydrochloric acid. In contrast, if a sample is to be analyzed for arsenic by ICPMS, hydrochloric acid must not be used for preservation since the chloride may combine with calcium from the sample matrix to form the polyatomic interference 40Ca35Cl+ at 75 atomic mass units, which is the same mass as the only natural isotope for arsenic, resulting in inflated measured values for arsenic concentration.437 Thus, a sampling plan for drinking water that may contain multiple trace and/or toxic elements must be carefully designed, taking into account the special storage and preservation requirements of the different elements as well as potential analytical interferences.
Mitigation of contaminated groundwater
Alternative sources of drinking water
In general, it is usually easier and less expensive to find alternative sources of water than treat contaminated water. Possible alternative sources include deeper wells,9a,21a,438 water sharing,438a,439 rain-water harvesting,2,55 or relying on surface water.55,440 Each of these methods may have utility in a given context; however, none is universally available or effective at providing safe water, and risks inherent with the alternative methods must be compared against potential benefits.441 Even where applicable, water from alternative sources must still be tested to ensure that levels of biological pathogens,440 multiple metal contaminants,442 and carcinogenic by-products from treatment443 meet established drinking water guidelines. It should be noted that surface water may be additionally contaminated by arsenic-containing irrigation run-off,21a,442b,444 and while deeper wells in Bangladesh and Vietnam may have less arsenic,21a,444,445 deeper wells in China may have more arsenic.446Drinking water treatment
Treatment technologies include both home scale and community systems. A number of low-cost home treatment technologies for arsenic have recently been developed for Bangladesh.447 Arsenic can be removed from drinking water using adsorbents.448 In the developed world, home-scale arsenic treatment systems are also available. These vary in cost and effectiveness, but are not regulated by governments.449 Some home-scale treatment technologies are reported to be effective at removing manganese as well as arsenic (e.g. Bangladesh SONO filter447a), however, no independent peer-reviewed data are available concerning the effectiveness of home scale technologies for removing uranium, nickel, or lead. Home treatment systems must also be regularly monitored for bacteriological contamination, particularly in the developing world where chances of such contamination are high.441 An additional concern with home-scale systems is the problem of toxic sludge disposal once the adsorbents have exceeded their utility; it is essential that proper disposal solutions be made, developed and put in place before home-scale treatment systems are adopted for widespread use.Community treatment systems have often been designed to remove a combination of metals, since community water supplies must typically meet multiple drinking water guidelines. The most successful community treatment systems are not mass-produced, but rather designed specifically to meet the unique treatment needs of the community. For example, a treatment system in a Greek community was designed to remove arsenic, manganese, and iron, using a combination of biological and chemical treatments.36 One challenge for community-scale water treatment facilities is that maintenance and water quality standards must be rigorously adhered to. Community-scale arsenic treatment facilities in the West Bengal basin do not have a good track record for supplying safe water.450
Recommendations and conclusions
Given the severity of adverse health effects caused by potential exposure to metals in groundwater it is vital for governments to increase their understanding of the specific risks faced by their populations and how these risks can be avoided. Systematic national water testing surveys based on stratified random sampling should be undertaken to test groundwater for multiple elements, including especially those known to commonly appear in groundwater that have recognized toxic effects: arsenic, manganese, fluoride, uranium, lead, and nickel. Laws must also be promulgated requiring testing of public water supplies and governments must ensure that the laws are followed. These laws need to be re-examined regularly and updated to take into consideration continuing research on the toxicity of single and multiple metals. Stakeholders must be educated about the need for testing of private wells, putting special emphasis on the need for testing in areas identified as possible “hot spots” of contamination by national surveys. The reliability of drinking water test results must be ensured by monitoring and regulating private water testing laboratories. Governments may find it cost-effective to subsidize water testing for private wells. In terms of public health, it is less expensive to help populations avoid exposure to toxic metals than to treat any resulting diseases or provide support for families if disability or death occurs as a result of exposure.For too many people worldwide, drinking water contains biological pathogens, carcinogenic treatment residues, or toxic metals. A variety of factors are pushing populations to rely on groundwater, including increased access to well technology, improved understanding of biological pathogens in surface water, increasing population pressures, migration to decentralized areas, political upheavals, and global climate change. Whenever a new source of water is accessed, particularly groundwater, it is crucial for public health that the water be tested for the presence of multiple metals as well as biological pathogens. It must be kept in mind that multiple metal exposure is the natural form of environmental metal exposure. In human epidemiological research, it must be assumed that multiple metal exposure has occurred, and thus research on single metal effects must test for multiple metals and then factor out other metals and combinations of metals before concluding that a given symptom, disease, or condition is due to exposure to a single specific metal. Mitigation efforts to reduce metal exposure through drinking water must consider all possible toxic metals known to occur in the region's water. Before a particular method is declared effective at providing safe water, it must pass tests for all toxins known to occur in the region as well as tests for biological pathogens.
Notes and references
- S. Sambu and R. Wilson, Arsenic in food and water—a brief history, Toxicol. Ind. Health, 2008, 24, 217–226 CrossRef CAS.
- A. Ayerza, Arsenicismo regional endémico, Bol. Acad. Nac. Medicina., 1918, 1, 11–24 Search PubMed.
- T. L. Kuo, Arsenic content of artesian well water in endemic area of chronic arsenic poisoning, Rep. Inst. Pathol. Natl. Taiwan Univ., 1968, 20, 7–13 Search PubMed.
- J. M. a. G. Borgoño and Rosa, in University of Missouri 5th Annual Conference on Trace Substances in Environmental Health, ed. D. D. Hemphill. University of Missouri, Columbia, MO, 1971, pp. 13–24 Search PubMed.
- Q. X. Huang Y and G. Wang, et al., A survey of endemic chronic arsenism, Acta Academiac Medicinae Xinjiang, 1983, 6, 91–96 Search PubMed.
- M. Berg, H. C. Tran, T. C. Nguyen, H. V. Pham, R. Schertenleib and W. Giger, Arsenic contamination of groundwater and drinking water in Vietnam: a human health threat, Environ. Sci. Technol., 2001, 35, 2621–6 CrossRef CAS.
- P. L. Smedley, J. Knudson and D. Maiga, Arsenic in groundwater from mineralised Proterozoic basement rocks of Burkino Faso, Appl. Geochem., 2007, 22, 1074–1092 CrossRef CAS.
- A. Mukherjee, Fryar, E. Alan, O'Shea and M. Bethany, in Arsenic: Environmental Chemistry, Health Threats and Waste Treatment, ed. K. R. Henke, John Wiley, West Sussex, 2009, pp. 303–350 Search PubMed.
- (a) D. M. Maynard, Frisbie, H. Seth, Hoque and A. Bilqis, Report of the Impact of the Bangladesh Rural Electrification Program on Groundwater Quality, Bangladesh Rural Electrification Board, Dhaka, Bangladesh, 1997 Search PubMed; (b) S. H. Frisbie, Maynard, M. Donald, Hoque and A. Bilqis, in Metals and Genetics, ed. B. Sarkar, Plenum Publishing Company, New York, 1999, pp. 67–85 Search PubMed.
- (a) British Geological Survey Phase 1 groundwater studies of arsenic contamination in Bangladesh; British Geological Survey: Nottingham, 1999; (b) S. H. Frisbie, R. Ortega, D. M. Maynard and B. Sarkar, The concentrations of arsenic and other toxic elements in Bangladesh's drinking water, Environ. Health Perspect., 2002, 110, 1147–1153 CrossRef CAS.
- B. K. Mandal and K. T. Suzuki, Arsenic round the world: a review, Talanta, 2002, 58, 201–235 CrossRef CAS.
- P. L. Smedley, M. Zhang, G. Zhang and Z. Luo, Mobilisation of arsenic and other trace elements in fluviolacustrine aquifers of the Huhhot Basin, Inner Mongolia, Applied Geochemistry, 2003, 18, 1453–1477 Search PubMed.
- J. Virkutyte and M. Sillanpää, Chemical evaluation of potable water in Eastern Qinghai Province, China: Human health aspects, Environment International, 2006, 32, 80–86 Search PubMed.
- B. Nath, J.-S. Jean, M.-K. Lee, H.-J. Yang and C.-C. Liu, Geochemistry of high arsenic groundwater in Chia-Nan plain, Southwestern Taiwan: possible sources and reactive transport of arsenic, J. Contam. Hydrol., 2008, 99, 85–96 CrossRef CAS.
- M. Kato, S. Onuma, Y. Kato, N. D. Thang, I. Yajima, M. Z. Hoque and H. U. Shekhar, Toxic elements in well water from Malaysia, Toxicological & Environmental Chemistry, 2010, 92, 1609–1612 Search PubMed.
- J. Buschmann, M. Berg, C. Stengel, L. Winkel, M. L. Sampson, P. T. K. Trang and P. H. Viet, Contamination of drinking water resources in the Mekong delta floodplains: arsenic and other trace metals pose serious health risks to population, Environ. Int., 2008, 34, 756–764 CrossRef CAS.
- T. T. G. Luu, S. Sthiannopkao and K.-W. Kim, Arsenic and other trace elements contamination in groundwater and a risk assessment study for the residents in the Kandal Province of Cambodia, Environ. Int., 2009, 35, 455–460 CrossRef CAS.
- T. Agusa, T. Kunito, J. Fujihara, R. Kubota, T. B. Minh, P. T. K. Trang, H. Iwata, A. Subramanian, P. H. Viet and S. Tanabe, Contamination by arsenic and other trace elements in tube-well water and its risk assessment to humans in Hanoi, Vietnam, Environ. Pollut., 2006, 139, 95–106 CrossRef CAS.
- V. A. Nguyen, S. Bang, P. H. Viet and K.-W. Kim, Contamination of groundwater and risk assessment for arsenic exposure in Ha Nam province, Vietnam, Environ. Int., 2009, 35, 466–472 CrossRef CAS.
- P. R. Feldman, J.-W. Rosenboom, M. Saray, P. Navuth, C. Samnang and S. Iddings, Assessment of the chemical quality of drinking water in Cambodia, J. Water Health, 2007, 5, 101–116 CrossRef CAS.
- (a) British Geological Survey Phase 2 groundwater studies of arsenic contamination in Bangladesh; British Geological Survey: Nottingham, 2001; (b) S. H. Frisbie, E. J. Mitchell, L. J. Mastera, D. M. Maynard, A. Z. Yusuf, M. Y. Siddiq, R. Ortega, R. K. Dunn, D. S. Westerman, T. Bacquart and B. Sarkar, Public health strategies for western Bangladesh that address arsenic, manganese, uranium, and other toxic elements in drinking water, Environ. Health Perspect., 2009, 117, 410–416 CAS.
- A. van Geen, Z. Cheng, Q. Jia, Y. Zheng, A. A. Seddique, M. W. Rahman, M. M. Rahman and K. M. Ahmed, Monitoring 51 community wells in Araihazar, Bangladesh, for up to 5 years: Implications for arsenic mitigation, J. Environ. Sci. Health, Part A: Toxic/Hazard. Subst. Environ. Eng., 2007, 42, 1729–1740 Search PubMed.
- M. Buragohain, B. Bhuyan and H. P. Sarma, Seasonal variations of lead, arsenic, cadmium and aluminium contamination of groundwater in Dhemaji district, Assam, India, Environ. Monit. Assess., 2009 Search PubMed.
- S. Bhattacharjee, S. Chakravarty, S. Maity, V. Dureja and K. K. Gupta, Metal contents in the groundwater of Sahebgunj district, Jharkhand, India, with special reference to arsenic, Chemosphere, 2005, 58, 1203–1217 CrossRef CAS.
- (a) D. Chakraborti, S. C. Mukherjee, S. Pati, M. K. Sengupta, M. M. Rahman, U. K. Chowdhury, D. Lodh, C. R. Chanda, A. K. Chakraborti and G. K. Basu, Arsenic groundwater contamination in Middle Ganga Plain, Bihar, India: a future danger?, Environ. Health Perspect., 2003, 111, 1194–1201 CAS; (b) A. K. Singh, S. Bhagowati, T. K. Das, D. Yubbe, B. Rahman, M. Nath, P. Obing, W. S. K. singh, C. Z. Renthlei, L. Pachuau and R. Thakur, Assessment of arsenic, fluoride, iron, nitrate and heavy metals in drinking water of northeastern India, ENVIS Bulletin, 2008, 16, 2008 Search PubMed.
- (a) C. K. Jain, A. Bandyopadhyay and A. Bhadra, Assessment of ground water quality for drinking purpose, District Nainital, Uttarakhand, India, Environ. Monit. Assess., 2010, 166, 663–676 CrossRef CAS; (b) K. K. Borah, B. Bhuyan and H. P. Sarma, Lead, arsenic, fluoride, and iron contamination of drinking water in the tea garden belt of Darrang district, Assam, India, Environ. Monit. Assess., 2009, 169, 347–352.
- N. Rajmohan and L. Elango, Distribution of iron, manganese, zinc and atrazine in groundwater in parts of Palar and Cheyyar river basins, South India, Environ. Monit. Assess., 2005, 107, 115–131 CrossRef CAS.
- A. H. Barati, A. Maleki and M. Alasvand, Multi-trace elements level in drinking water and the prevalence of multi-chronic arsenical poisoning in residents in the west area of Iran, Sci. Total Environ., 2010, 408, 1523–1529 CrossRef CAS.
- R. G. Taylor and K. W. F. Howard, in Goundwater Quality, ed. H. Nash and G. J. H. McCall, Chapman & Hall, London, UK, 1994, pp. 31–44 Search PubMed.
- British Geological Survey Groundwater Quality: Mali; British Geological Survey: London, UK, 2002.
- R. Buamah, Adsorptive Removal of Manganese, Arsenic and Iron From Groundwater, Wageningen University, Delft, The Netherlands, 2009 Search PubMed.
- P. W. Lahermo, G. Alfthan and D. Wang, Selenium and arsenic in the environment in Finland, J. Environ. Pathol. Toxicol. Oncol., 1998, 17, 205–216 Search PubMed.
- (a) M. Muikku, M. Puhakainen, T. Heikkinen and T. Ilus, The mean concentration of uranium in drinking water, urine, and hair of the occupationally unexposed Finnish working population, Health Phys., 2009, 96, 646–54 CrossRef CAS; (b) O. Prat, T. Vercouter, E. Ansoborlo, P. Fichet, P. Perret, P. Kurttio and L. Salonen, Uranium speciation in drinking water from drilled wells in southern Finland and its potential links to health effects, Environ. Sci. Technol., 2009, 43, 3941–3946 CrossRef CAS.
- T. Hatva, Iron and Manganese in Groundwater in Finland: Occurence in Glacifluvial Aquifers and Removal by Biofiltration, National Board of Waters and the Environment, Helsinki, Finland, 1989 Search PubMed.
- R. Vivona, E. Presiosi, B. Madé and G. Giuliano, Occurrence of minor toxic elements in volcanic-sedimentary aquifers: a case study in central Italy, Hydrogeol. J., 2007, 15, 1183–1196 CrossRef CAS.
- I. A. Katsoyiannis, S. J. Hug, A. Ammann, A. Zikoudi and C. Hatziliontos, Arsenic speciation and uranium concentrations in drinking water supply wells in Northern Greece: correlations with redox indicative parameters and implications for groundwater treatment, Sci. Total Environ., 2007, 383, 128–140 CrossRef CAS.
- A. Kelepertsis, D. Alexakis and K. Skordas, Arsenic, antimony and other toxic elements in the drinking water of Eastern Thessaly in Greece and its possible effects on human health, Environ. Geol., 2006, 50, 76–84 CrossRef CAS.
- British Columbia Ministry of the Environment. British Columbia Ministry of the Environment, Surrey, B.C., 2010, vol. 2011.
- (a) CBC News, in CBC News, Canada. CBC Radio-Canada: Toronto, ON, 2008; (b) Government of Nova Scotia. Government of Nova Scotia: Halifax, NS, 2010, vol. 2011.
- F. N. Robertson, Arsenic in ground-water under oxidizing conditions, southwest United States, Environ. Geochem. Health, 1989, 11, 171–185 CrossRef CAS.
- (a) J. S. Stanton and S. L. Qi, Ground-Water Quality of the Northern High Plains Aquifer, 1997, 2002–04, Publications of the US Geological Survey US Geological Survey, Lincoln, NE, 2006 Search PubMed; (b) J. D. Ayotte, M. G. Nielsen, G. Robinson, R. and R. B. Moore, Relation of Arsenic, Iron, and Manganese in Ground Water to Aquifer Type, Bedrock Lithogeochemistry, and Land Use in the New England Coastal Basins, Water-Resources Investigations Report U.S. Geological Survey, National Water-Quality Assessment Program, Pembroke, NH, 1999 Search PubMed; (c) J. A. Colman, Arsenic and Uranium in Water from Private Wells Completed in Bedrock of East-Central Massachusetts—Concentrations, Correlations with Bedrock Units, and Estimated Probability Maps, U.S. Geological Survey Scientific Investigations Report U.S. Geological Survey, Northborough, MA, 2011 Search PubMed.
- T. Kresse and J. Fazio, Occurence of Arsenic in Ground Waters of Arkansas and Implications for Source and Release Mechanisms, Arkansas Department of Environmental Quality, Arkansas Ambient Ground-Water Monitoring Program, Little Rock, AR, 2003 Search PubMed.
- P. F. Hudak, Boron and selenium contamination in south Texas groundwater, J. Environ. Sci. Health, Part A: Toxic/Hazard. Subst. Environ. Eng., 2004, 39, 2827–2834 Search PubMed.
- S. S. Farías, V. A. Casa, C. Vázquez, L. Ferpozzi, G. N. Pucci and I. M. Cohen, Natural contamination with arsenic and other trace elements in ground waters of Argentine Pampean Plain, Sci. Total Environ., 2003, 309, 187–199 CrossRef CAS.
- H. B. Nicolli, J. Suriano, M. Gomez Peral, L. H. Ferpozzi and O. A. Balean, Groundwater contamination with arsenic and other trace elements in an area of the Pampa, Province of Córdoba, Environmental Geology and Water Sciences, 1989, 14, 3–16 Search PubMed.
- G. Concha, K. Broberg, M. Grandér, A. Cardozo, B. Palm and M. Vahter, High-level exposure to lithium, boron, cesium, and arsenic via drinking water in the Andes of northern Argentina, Environ. Sci. Technol., 2010, 44, 6875–80 CrossRef CAS.
- P. L. Smedley and D. G. Kinniburgh, A review of the source, behaviour and distribution of arsenic in natural waters, Appl. Geochem., 2002, 17, 517–568 CrossRef CAS.
- J. Hamilton-Taylor and N. B. Price, The geochemistry of iron and manganese in the waters and sediments of Bolstadfjord, S.W. Norway, Estuarine, Coastal Shelf Sci., 1983, 17, 1–19 CrossRef CAS.
- M.-K. Lee, J. Griffin, J. Saunders, Y. Wang and J.-S. Jean, Reactive transport of trace elements and isotopes in the Eutaw coastal plain aquifer, Alabama, J. Geophys. Res., 2007, 112 Search PubMed.
- ATSDR, Draft Toxicological Profile for Uranium; US Department of Health and Human Services. Public Health Service. Agency for Toxic Substances and Disease Registry, Atlanta, GA, 1999.
- B. C. Jurgens, M. S. Fram, K. Belitz, K. R. Burow and M. K. Landon, Effects of Groundwater Development on Uranium, Central Valley, California, USA, Groundwater, 2009 Search PubMed.
- J. Buschmann, M. Berg, C. Stengel and M. L. Sampson, Arsenic and manganese contamination of drinking water resources in Cambodia: coincidence of risk areas with low relief topography, Environ. Sci. Technol., 2007, 41, 2146–2152 CrossRef CAS.
- H. S. Magdo, J. Forman, N. Graber, B. Newman, K. Klein, L. Satlin, R. W. Amler, J. A. Winston and P. J. Landrigan, Grand rounds: nephrotoxicity in a young child exposed to uranium from contaminated well water, Environ. Health Perspect., 2007, 115, 1237–1241 CrossRef CAS.
- L. Winkel, M. Berg, M. Amini, S. J. Hug and C. A. Johnson, Predicting groundwater arsenic contamination in Southeast Asia from surface parameters, Nat. Geosci., 2008, 1, 536–542 Search PubMed.
- M. Rahman, International research on arsenic contamination and health, J. Health Popul. Nutr., 2006, 24, 123–128 Search PubMed.
- (a) G. A. Wasserman, X. Liu, F. Parvez, H. Ahsan, D. Levy, P. Factor-Litvak, J. Kline, A. van Geen, V. Slavkovich, N. J. LoIacono, Z. Cheng, Y. Zheng and J. H. Graziano, Water manganese exposure and children's intellectual function in Araihazar, Bangladesh, Environ. Health Perspect., 2006, 114, 124–129 CAS; (b) M. Bouchard, F. Laforest, L. Vandelac, D. Bellinger and D. Mergler, Hair manganese and hyperactive behaviors: pilot study of school-age children exposed through tap water, Environ. Health Perspect., 2007, 115, 122–127 CrossRef CAS.
- B. C. Bates, Z. W. Kundzewicz, S. Wu, J. P. Palutik, of Climate change and water, Technical paper of the Intergovernmental Panel on Climate Change, IPCC, Geneva, 2008.
- (a) M. M. Sherif and V. P. singh, Effect of climate change on sea water intrusion in coastal aquifers, Hydrol. Processes, 1999, 13, 1277–1287 CrossRef; (b) Y.-B. Lin, Y.-P. Lin, C.-W. Liu and Y.-C. Tan, Mapping of spatial multi-scale sources of arsenic variation in groundwater on ChiaNan floodplain of Taiwan, Sci. Total Environ., 2006, 370, 168–181 CrossRef CAS.
- J. Monan, Bangladesh: The strength to succeed, Oxfam, Oxford, 1995 Search PubMed.
- (a) S. C. Besuschio, A. C. Perez Desanzo and M. Croci, Epidemiological associations between arsenic and cancer in Argentina, Biol. Trace Elem. Res., 1980, 2, 41–55 CrossRef; (b) A. H. Smith and C. M. Steinmaus, Health effects of arsenic and chromium in drinking water: recent human findings, Annu. Rev. Public Health, 2009, 30, 107–22, DOI:10.1146/annurev.publhealth.031308.100143.
- (a) H. Y. Chiou, Y. M. Hsueh, K. F. Liaw, S. F. Horng, M. H. Chiang, Y. S. Pu, J. S. Lin, C. H. Huang and C. J. Chen, Incidence of internal cancers and ingested inorganic arsenic: a seven-year follow-up study in Taiwan, Cancer Res., 1995, 55, 1296–1300 CAS; (b) Y. Chen, F. Parvez, M. Gamble, T. Islam, A. Ahmed, M. Argos, J. H. Graziano and H. Ahsan, Arsenic exposure at low-to-moderate levels and skin lesions, arsenic metabolism, neurological functions, and biomarkers for respiratory and cardiovascular diseases: review of recent findings from the Health Effects of Arsenic Longitudinal Study (HEALS) in Bangladesh, Toxicol. Appl. Pharmacol., 2009, 239, 184–192 CrossRef CAS; (c) J. E. Heck, A. S. Andrew, T. Onega, J. R. Rigas, B. P. Jackson, M. R. Karagas and E. J. Duell, Lung cancer in a U.S. population with low to moderate arsenic exposure, Environ. Health Perspect., 2009, 117, 1718–1723 CAS.
- C.-Y. Yang, H.-F. Chiu, C.-C. Chang, S.-C. Ho and T.-N. Wu, Bladder cancer mortality reduction after installation of a tap-water supply system in an arsenious-endemic area in southwestern Taiwan, Environ. Res., 2005, 98, 127–132 CrossRef CAS.
- (a) H.-F. Chiu, S.-C. Ho, L. Y. Wang, T. N. Wu and C.-Y. Yang, Does arsenic exposure increase the risk for liver cancer?, J. Toxicol. Environ. Health, Part A, 2004, 8, 1491–1500 Search PubMed; (b) J. Liaw, G. Marshall, Y. Yuan, C. Ferreccio, C. Steinmaus and A. H. Smith, Increased childhood liver cancer mortality and arsenic in drinking water in northern Chile, Cancer Epidemiol., Biomarkers Prev., 2008, 17, 1982–1987 Search PubMed; (c) J. Liu and M. P. Waalkes, Liver is a target of arsenic carcinogenesis, Toxicol. Sci., 2008, 105, 24–32 CrossRef CAS.
- (a) D. R. Lewis, J. W. Southwick, R. Ouellet-Hellstrom, J. Rench and R. L. Calderon, Drinking water arsenic in Utah: A cohort mortality study, Environ. Health Perspect., 1999, 107, 359–365 CrossRef CAS; (b) L. Benbrahim-Tallaa and M. P. Waalkes, Inorganic arsenic and human prostate cancer, Environ. Health Perspect., 2008, 116, 158–164 CAS.
- N.-S. Joo, S.-M. Kim, Y.-S. Jung and K.-M. Kim, Hair iron and other minerals' level in breast cancer patients, Biol. Trace Elem. Res., 2009, 129, 28–35 CrossRef CAS.
- (a) D. N. Guha Mazumder, Chronic arsenic toxicity: Clinical features, epidemiology, and treatment: Experience in West Bengal, J. Environ. Sci. Health, Part A: Toxic/Hazard. Subst. Environ. Eng., 2003, 38, 141–163 Search PubMed; (b) M. M. Rahman, J. C. Ng and R. Naidu, Chronic exposure of arsenic via drinking water and its adverse health impacts on humans, Environ. Geochem. Health, 2009, 31, 189–200 Search PubMed.
- K. K. Majumdar, D. N. G. Mazumder, N. Ghose, A. Ghose and S. Lahiri, Systemic manifestations in chronic arsenic toxicity in absence of skin lesions in West Bengal, Indian J. Med. Res., 2009, 129, 75–82 Search PubMed.
- (a) D. N. Guha Mazumder, R. Haque, N. Ghosh, B. K. De, A. Santra, D. Chakraborti and A. H. Smith, Arsenic in drinking water and the prevalence of respiratory effects in West Bengal, India, Int. J. Epidemiol., 2000, 29, 1047–52 Search PubMed; (b) D. N. G. Mazumder, C. Steinmaus, P. Bhattacharya, O. S. von Ehrenstein, N. Ghosh, M. Gotway, A. Sil, J. R. Balmes, R. Haque, M. M. Hira-Smith and A. H. Smith, Bronchiectasis in persons with skin lesions resulting from arsenic in drinking water, Epidemiology, 2005, 16, 760–765 CrossRef; (c) S. Kapaj, H. Peterson, K. Liber and P. Bhattacharya, Human health effects from chronic arsenic poisoning—a review, J. Environ. Sci. Health A Tox. Hazard Subst. Environ. Eng., 2006, 41, 2399–2428 Search PubMed; (d) J. X. Guo, L. Hu, P. Z. Yand, K. Tanabe, M. Miyatalre and Y. Chen, Chronic arsenic poisoning in drinking water in Inner Mongolia and its associated health effects, J. Environ. Sci. Health A Tox Hazard Subst. Environ. Eng., 2007, 42, 1853–1858 Search PubMed; (e) L. N. Islam, A. H. M. N. Nabi, M. M. Rahman and M. S. H. Zahid, Association of respiratory complications and elevated serum immunoglobulins with drinking water arsenic toxicity in human, J. Environ. Sci. Health A Tox. Hazard Subst Environ. Eng., 2007, 42, 1807–1814 Search PubMed; (f) F. Parvez, Y. Chen, P. W. Brandt-Rauf, A. Bernard, X. Dumont, V. Slavkovich, M. Argos, J. D'Armiento, R. Foronjy, M. R. Hasan, H. E. M. M. Eunus, J. H. Graziano and H. Ahsan, Nonmalignant respiratory effects of chronic arsenic exposure from drinking water among never-smokers in Bangladesh, Environ. Health Perspect., 2008, 116, 190–195 CAS.
- A. S. Andrew, V. Bernardo, L. A. Warnke, J. C. Davey, T. Hampton, R. A. Mason, J. E. Thorpe, M. A. Ihnat and J. W. Hamilton, Exposure to arsenic at levels found in U.S. drinking water modifies expression in the mouse lung, Toxicol. Sci., 2007, 100, 75–87 CrossRef CAS.
- A. S. Andrew, R. A. Mason, V. Memoli and E. J. Duell, Arsenic activates EGFR pathway signaling in the lung, Toxicol. Sci., 2009, 109, 350–357 CrossRef CAS.
- (a) C. J. Chen, M. M. Wu, S. S. Lee, J. D. Wang, S. H. Cheng and H. Y. Wu, Atherogenicity and carcinogenicity of high-arsenic artesian well water. Multiple risk factors and related malignant neoplasms of blackfoot disease, Arteriosclerosis, 1988, 8, 452–460 CAS; (b) C.-H. Tseng, Blackfoot disease and arsenic: a never-ending story, J. Environ. Sci. Health C Environ. Carcinog Ecotoxicol. Rev., 2005, 23, 55–74 Search PubMed; (c) H.-S. Yu, C.-H. Lee and G.-S. Chen, Peripheral vascular diseases resulting from chronic arsenical poisoning, J. Dermatol., 2002, 29, 123–130 Search PubMed; (d) C.-C. Chang, S.-C. Ho, S.-S. Tsai and C.-Y. Yang, Ischemic heart disease mortality reduction in an arseniasis-endemic area in southwestern Taiwan after a switch in the tap-water supply system, J. Toxicol. Environ. Health, Part A, 2004, 67, 1353–1361 Search PubMed; (e) P. P. Simeonova and M. I. Luster, Arsenic and atherosclerosis, Toxicol. Appl. Pharmacol., 2004, 198, 444–449 CrossRef CAS; (f) A. Navas-Acien, A. R. Sharrett, E. K. Silbergeld, B. S. Schwartz, K. E. Nachman, T. A. Burke and E. Guallar, Arsenic exposure and cardiovascular disease: a systematic review of the epidemiologic evidence, Am. J. Epidemiol., 2005, 162, 1037–1049 CrossRef; (g) C.-Y. Yang, Does arsenic exposure increase the risk of development of peripheral vascular diseases in humans?, J. Toxicol. Environ. Health, Part A, 2006, 69, 1797–1804 Search PubMed; (h) C.-H. Wang, C.-L. Chen, C. K. Hsiao, F.-T. Chiang, L.-I. Hsu, H.-Y. Chiou, Y.-M. Hsueh, M.-M. Wu and C.-J. Chen, Increased risk of QT prolongation associated with atherosclerotic diseases in arseniasis-endemic area in southwestern coast of Taiwan, Toxicol. Appl. Pharmacol., 2009, 239, 320–324 CrossRef CAS; (i) J. C. States, S. Srivastava, Y. Chen and A. Barchowsky, Arsenic and cardiovascular disease, Toxicol. Sci., 2009, 107, 312–323 CAS.
- (a) C. J. Chen, Y. M. Hsueh, M. S. Lai, M. P. Shyu, S. Y. Chen, M. M. Wu, T. L. Kuo and T. Y. Tai, Increased prevalence of hypertension and long-term arsenic exposure, Hypertension, 1995, 25, 53–60 CAS; (b) C.-J. Chen, S.-L. Wang, J.-M. Chiou, C.-H. Tseng, H.-Y. Chiou, Y.-M. Hsueh, S.-Y. Chen, M.-M. Wu and M.-S. Lai, Arsenic and diabetes and hypertension in human populations: a review, Toxicol. Appl. Pharmacol., 2007, 222, 298–304 CrossRef CAS.
- (a) M. S. Lai, Y. M. Hsueh, C. J. Chen, M. P. Shyu, S. Y. Chen, T. L. Kuo, M. M. Wu and T. Y. Tai, Ingested inorganic arsenic and prevalence of diabetes mellitus, Am J Epidemiol, 1994, 139, 484–492 CAS; (b) C. H. Tseng, T. Y. Tai, C. K. Chong, C. P. Tseng, M. S. Lai, B. J. Lin, H. Y. Chiou, Y. M. Hsueh, K. H. Hsu and C. J. Chen, Long-term arsenic exposure and incidence of non-insulin-dependent diabetes mellitus: a cohort study in arseniasis-hyperendemic villages in Taiwan, Environ. Health Perspect., 2000, 108, 847–851 CAS; (c) C.-H. Tseng, The potential biological mechanisms of arsenic-induced diabetes mellitus, Toxicol. Appl. Pharmacol., 2004, 197, 67–83 CrossRef CAS; (d) A. Díaz-Villaseñor, A. L. Burns, M. Hiriart, M. E. Cebrián and P. Ostrosky-Wegman, Arsenic-induced alteration in the expression of genes related to type 2 diabetes mellitus, Toxicol. Appl. Pharmacol., 2007, 225, 123–33 CrossRef CAS; (e) A. Navas-Acien, E. K. Silbergeld, R. Pastor-Barriuso and E. Guallar, Arsenic exposure and prevalence of type 2 diabetes in US adults, JAMA, J. Am. Med. Assoc., 2008, 300, 814–822 CrossRef CAS; (f) A. S. Ettinger, A. R. Zota, C. J. Amarasiriwardena, M. R. Hopkins, J. Schwartz, H. Hu and R. O. Wright, Maternal arsenic exposure and impaired glucose tolerance during pregnancy, Environ. Health Perspect., 2009, 117, 1059–1064 CrossRef CAS.
- H.-F. Chiu and C.-Y. Yang, Decreasing trend in renal disease mortality after cessation from arsenic exposure in a previous arseniasis-endemic area in southwestern Taiwan, J. Toxicol. Environ. Health, Part A, 2005, 68, 319–327 Search PubMed.
- W. Lin, S.-L. Wang, H.-J. Wu, K.-H. Chang, P. Yeh, C.-J. Chen and H.-R. Guo, Associations between arsenic in drinking water and pterygium in southwestern Taiwan, Environ. Health Perspect., 2008, 116, 952–955 CrossRef CAS.
- L.-C. See, H.-Y. Chiou, J.-S. Lee, Y.-M. Hsueh, S.-M. Lin, M.-C. Tu, M.-L. Yang and C.-J. Chen, Dose-response relationship between ingested arsenic and cataracts among residents in Southwestern Taiwan, J. Environ. Sci. Health A Tox. Hazard Subst. Environ. Eng., 2007, 42, 1843–1851 Search PubMed.
- B. Krishnapada, R. Akash, M. Lakshmikanta, B. Gautam and T. Amit, Persistent conjunctivitis associated with drinking arsenic-contaminated water, J. Ocul. Pharmacol. Ther., 2006, 22, 208–211 CrossRef.
- (a) A. S. Andrew, D. A. Jewell, R. A. Mason, M. L. Whitfield, J. H. Moore and M. R. Karagas, Drinking-water arsenic exposure modulates gene expression in human lymphocytes from a U.S. population, Environ. Health Perspect., 2008, 116, 524–531 CrossRef CAS; (b) C. D. Kozul, K. H. Ely, R. I. Enelow and J. W. Hamilton, Low-dose arsenic compromises the immune response to influenza A infection in vivo, Environ. Health Perspect., 2009, 117, 1441–1447 CAS; (c) R. Raqib, S. Ahmed, R. Sultana, Y. Wagatsuma, D. Mondal, A. M. W. Hoque, B. Nermell, M. Yunus, S. Roy, L. A. Persson, S. E. Arifeen, S. Moore and M. Vahter, Effects of in utero arsenic exposure on child immunity and morbidity in rural Bangladesh, Toxicol. Lett., 2009, 185, 197–202 CrossRef CAS.
- J. C. Davey, A. P. Nomikos, M. Wungjiranirun, J. R. Sherman, L. Ingram, C. Batki, J. P. Lariviere and J. W. Hamilton, Arsenic as an endocrine disruptor: arsenic disrupts retinoic acid receptor-and thyroid hormone receptor-mediated gene regulation and thyroid hormone-mediated amphibian tail metamorphosis, Environ. Health Perspect., 2008, 116, 165–172 CAS.
- F.-I. Hsieh, T.-S. Hwang, Y.-C. Hsieh, H.-C. Lo, C.-T. Su, H.-S. Hsu, H.-Y. Chiou and C.-J. Chen, Risk of erectile dysfunction induced by arsenic exposure through well water consumption in Taiwan, Environ. Health Perspect., 2008, 116, 532–536 CrossRef CAS.
- C. Castellini, E. Mourvaki, B. Sartini, R. Cardinali, E. Moretti, G. Collodel, S. Fortaner, E. Sabbioni and T. Renieri, In vitro toxic effects of metal compounds on kinetic traits and ultrastructure of rabbit spermatozoa, Reprod. Toxicol., 2009, 27, 46–54 CrossRef CAS.
- S. C. Mukherjee, M. M. Rahman, U. K. Chowdhury, M. K. Sengupta, D. Lodh, C. R. Chanda, K. C. Saha and D. Chakraborti, Neuropathy in arsenic toxicity from groundwater arsenic contamination in West Bengal, India, J. Environ. Sci. Health A Tox. Hazard Subst. Environ. Eng., 2003, 38, 165–183 Search PubMed.
- (a) C. Moon, M. Marlowe, J. Stellern and J. Errera, Main and interaction effects of metallic pollutants on cognitive functioning, J. Learn. Disabil., 1985, 18, 217–221 Search PubMed; (b) G. A. Wasserman, X. Liu, F. Parvez, H. Ahsan, P. Factor-Litvak, A. van Geen, V. Slavkovich, N. J. LoIacono, Z. Cheng, I. Hussain, H. Momotaj and J. H. Graziano, Water arsenic exposure and children's intellectual function in Araihazar, Bangladesh, Environ. Health Perspect., 2004, 112, 1329–1333 CrossRef CAS; (c) J. L. Rosado, D. Ronquillo, K. Kordas, O. Rojas, J. Alatorre, P. Lopez, G. Garcia-Vargas, M. D. C. Caamaño, M. E. Cebrián and R. J. Stoltzfus, Arsenic exposure and cognitive performance in Mexican schoolchildren, Environ. Health Perspect., 2007, 115, 1371–1375 CrossRef CAS.
- (a) A. Aschengrau, S. Zierler and A. Cohen, Quality of community drinking water and the occurrence of spontaneous abortion, Arch. Environ. Health, 1989, 44, 283–290 CrossRef CAS; (b) M. Börzsönyi, A. Bereczky, P. Rudnai, M. Csanady and A. Horvath, Epidemiological studies on human subjects exposed to arsenic in drinking water in southeast Hungary, Arch. Toxicol., 1992, 66, 77–78 CAS; (c) S. A. Ahmad, M. H. Sayed, S. Barua, M. H. Khan, M. H. Faruquee, A. Jalil, S. A. Hadi and H. K. Talukder, Arsenic in drinking water and pregnancy outcomes, Environ. Health Perspect., 2001, 109, 629–631 CrossRef CAS; (d) A. Ashraf, S. M. Mustanzid, S. A. Rahman and B. Sarkar, A community based survey on pregnancy outcome in patients with arsenicosis in rural Bangladesh, Clinical Biochemistry, 2004, 37, 1135; (e) N. Cherry, K. Shaikh, C. McDonald and Z. Chowdhury, Stillbirth in rural Bangladesh: arsenic exposure and other etiological factors: a report from Gonoshasthaya Kendra, Bull. W. H. O., 2008, 86, 172–177 CrossRef.
- M. Vahter, Effects of arsenic on maternal and fetal health, Annu. Rev. Nutr., 2009, 29, 381–399 CrossRef CAS.
- C. Hopenhayn-Rich, S. R. Browning, I. Hertz-Picciotto, C. Ferreccio, C. Peralta and H. Gibb, Chronic arsenic exposure and risk of infant mortality in two areas of Chile, Environ. Health Perspect., 2000, 108, 667–673 CrossRef CAS.
- S.-X. Wang, Z.-H. Wang, X.-T. Cheng, J. Li, Z.-P. Sang, X.-D. Zhang, L.-L. Han, X.-Y. Qiao, Z.-M. Wu and Z.-Q. Wang, Arsenic and fluoride exposure in drinking water: children's IQ and growth in Shanyin county, Shanxi province, China, Environ. Health Perspect., 2007, 115, 643–647 CrossRef CAS.
- (a) J. Shen, J. Liu, Y. Xie, B. A. Diwan and M. P. Waalkes, Fetal onset of aberrant gene expression relevant to pulmonary carcinogenesis in lung adenocarcinoma development induced by in utero arsenic exposure, Toxicol. Sci., 2007, 95, 313–320 CAS; (b) S. Srivastava, S. E. D'Souza, U. Sen and J. C. States, In utero arsenic exposure induces early onset of atherosclerosis in ApoE-/- mice, Reprod. Toxicol., 2007, 23, 449–456 CrossRef CAS; (c) Y. Xie, J. Liu, L. Benbrahim-Tallaa, J. M. Ward, D. Logsdon, B. A. Diwan and M. P. Waalkes, Aberrant DNA methylation and gene expression in livers of newborn mice transplacentally exposed to a hepatocarcinogenic dose of inorganic arsenic, Toxicology, 2007, 236, 7–15 CrossRef CAS.
- M. Vahter, Health effects of early life exposure to arsenic, Basic Clin. Pharmacol. Toxicol., 2008, 102, 204–211 Search PubMed.
- (a) Y. M. Hsueh, H. Y. Chiou, Y. L. Huang, W. L. Wu, C. C. Huang, M. H. Yang, L. C. Lue, G. S. Chen and C. J. Chen, Serum beta-carotene level, arsenic methylation capability, and incidence of skin cancer, Cancer Epidemiol. Biomarkers Prev., 1997, 6, 589–596 Search PubMed; (b) C. Watanabe, T. Inaoka, T. Kadono, M. Nagano, S. Nakamura, K. Ushijima, N. Murayama, K. Miyazaki and R. Ohtsuka, Males in rural Bangladeshi communities are more susceptible to chronic arsenic poisoning than females: analyses based on urinary arsenic, Environ. Health Perspect., 2001, 109, 1265–1270 CrossRef CAS; (c) H. Ahsan, Y. Chen, F. Parvez, L. Zablotska, M. Argos, I. Hussain, H. Momotaj, D. Levy, Z. Cheng, V. Slavkovich, A. van Geen, G. R. Howe and J. H. Graziano, Arsenic exposure from drinking water and risk of premalignant skin lesions in Bangladesh: baseline results from the Health Effects of Arsenic Longitudinal Study, Am. J. Epidemiol., 2006, 163, 1138–1148 CrossRef; (d) Y. Chen, J. H. Graziano, F. Parvez, I. Hussain, H. Momotaj, A. van Geen, G. R. Howe and H. Ahsan, Modification of risk of arsenic-induced skin lesions by sunlight exposure, smoking, and occupational exposures in Bangladesh, Epidemiology, 2006, 17, 459–467 CrossRef; (e) M. Rahman, M. Vahter, N. Sohel, M. Yunus, M. A. Wahed, P. K. Streatfield, E.-C. Ekström and L. A. Persson, Arsenic exposure and age and sex-specific risk for skin lesions: a population-based case-referent study in Bangladesh, Environ. Health Perspect., 2006, 114, 1847–1852 CAS; (f) A.-L. Lindberg, E.-C. Ekström, B. Nermell, M. Rahman, B. Lönnerdal, L.-A. Persson and M. Vahter, Gender and age differences in the metabolism of inorganic arsenic in a highly exposed population in Bangladesh, Environ. Res., 2008, 106, 110–120 CrossRef CAS.
- G. Marshall, C. Ferreccio, Y. Yuan, M. N. Bates, C. Steinmaus, S. Selvin, J. Liaw and A. H. Smith, Fifty-year study of lung and bladder cancer mortality in Chile related to arsenic in drinking water, J. Natl. Cancer Inst., 2007, 99, 920–928 CrossRef CAS.
- (a) C. A. Loffredo, H. V. Aposhian, M. E. Cebrian, H. Yamauchi and E. K. Silbergeld, Variability in human metabolism of arsenic, Environ. Res., 2003, 92, 85–91 CrossRef CAS; (b) M. L. Kile, E. Hoffman, Y.-M. Hsueh, S. Afroz, Q. Quamruzzaman, M. Rahman, G. Mahiuddin, L. Ryan and D. C. Christiani, Variability in biomarkers of arsenic exposure and metabolism in adults over time, Environ. Health Perspect., 2009, 117, 455–460 CAS.
- L. Li, E.-C. Ekström, W. Goessler, B. Lönnerdal, B. Nermell, M. Yunus, A. Rahman, S. E. Arifeen, L. A. Persson and M. Vahter, Nutritional status has marginal influence on the metabolism of inorganic arsenic in pregnant Bangladeshi women, Environ. Health Perspect., 2008, 116, 315–321 CAS.
- A.-L. Lindberg, R. Kumar, W. Goessler, R. Thirumaran, E. Gurzau, K. Koppova, P. Rudnai, G. Leonardi, T. Fletcher and M. Vahter, Metabolism of low-dose inorganic arsenic in a central European population: influence of sex and genetic polymorphisms, Environ. Health Perspect., 2007, 115, 1081–1086 CrossRef CAS.
- T. J. Key, P. N. Appleby, G. K. Reeves, A. Roddam, J. F. Dorgan, C. Longcope, F. Z. Stanczyk, H. E. Stephenson, R. T. Falk, R. Miller, A. Schatzkin, D. S. Allen, I. S. Fentiman, D. Y. Wang, M. Dowsett, H. V. Thomas, S. E. Hankinson, P. Toniolo, A. Akhmedkhanov, K. Koenig, R. E. Shore, A. Zeleniuch-Jacquotte, F. Berrino, P. Muti, A. Micheli, V. Krogh, S. Sieri, V. Pala, E. Venturelli, G. Secreto, E. Barrett-Connor, G. A. Laughlin, M. Kabuto, S. Akiba, R. G. Stevens, K. Neriishi, C. E. Land, J. A. Cauley, L. H. Kuller, S. R. Cummings, K. J. Helzlsouer, A. J. Alberg, T. L. Bush, G. W. Comstock, G. B. Gordon and S. R. Miller, E. H. B. C. C. Group, Body mass index, serum sex hormones, breast cancer risk in postmenopausal women, J. Natl. Cancer Inst., 2003, 95, 1218–1226 CrossRef CAS.
- (a) M. Vahter, G. Concha, B. Nermell, R. Nilsson, F. Dulout and A. T. Natarajan, A unique metabolism of inorganic arsenic in native Andean women, Eur. J. Pharmacol., Environ. Toxicol. Pharmacol. Sect., 1995, 293, 455–462 Search PubMed; (b) M. Vahter, Genetic polymorphism in the biotransformation of inorganic arsenic and its role in toxicity, Toxicol. Lett., 2000, 112–113, 209–217 CrossRef CAS; (c) H. Ahsan, Y. Chen, Q. Wang, V. Slavkovich, J. H. Graziano and R. M. Santella, DNA repair gene XPD and susceptibility to arsenic-induced hyperkeratosis, Toxicol. Lett., 2003, 143, 123–131 CrossRef CAS; (d) M. Meza, A. J. Gandolfi and W. T. Klimecki, Developmental and genetic modulation of arsenic biotransformation: a gene by environment interaction?, Toxicol. Appl. Pharmacol., 2007, 222, 381–387 CrossRef CAS; (e) K. M. Applebaum, M. R. Karagas, D. J. Hunter, P. J. Catalano, S. H. Byler, S. Morris and H. H. Nelson, Polymorphisms in nucleotide excision repair genes, arsenic exposure, and non-melanoma skin cancer in New Hampshire, Environ. Health Perspect., 2007, 115, 1231–1236 CrossRef CAS; (f) S. D. Chaudhuri, M. Kundu, M. Banerjee, J. K. Das, P. Majumdar, S. Basu, S. Roychoudhury, K. K. Singh and A. K. Giri, Arsenic-induced health effects and genetic damage in keratotic individuals: involvement of p53 arginine variant and chromosomal aberrations in arsenic susceptibility, Mutat. Res., Rev. Mutat. Res., 2008, 659, 118–125 CrossRef; (g) C.-J. Chung, C.-J. Huang, Y.-S. Pu, C.-T. Su, Y.-K. Huang, Y.-T. Chen and Y.-M. Hsueh, Polymorphisms in cell cycle regulatory genes, urinary arsenic profile and urothelial carcinoma, Toxicol. Appl. Pharmacol., 2008, 232, 203–209 CrossRef CAS; (h) A. Hernández and R. Marcos, Genetic variations associated with interindividual sensitivity in the response to arsenic exposure, Pharmacogenomics, 2008, 9, 1113–1132 CrossRef CAS; (i) O. L. Valenzuela, Z. Drobná, E. Hernández-Castellanos, L. C. Sánchez-Peña, G. G. García-Vargas, V. H. Borja-Aburto, M. Stýblo and L. M. D. Razo, Association of AS3MT polymorphisms and the risk of premalignant arsenic skin lesions, Toxicol. Appl. Pharmacol., 2009, 239, 200–207 CrossRef CAS.
- S. J. S. Flora, M. Mittal and A. Mehta, Heavy metal induced oxidative stress & its possible reversal by chelation therapy, Indian J. Med. Res., 2008, 128, 501–523 Search PubMed.
- S. H. Frisbie, R. Ortega, D. Maynard and B. Sarkar, in Bangladesh Environment, ed. M. F. Ahmed, S. A. Tanveer and A. B. M. Badruzzaman, BAPA, Dhaka, Bangladesh, 2002, pp. 259–273 Search PubMed.
- J. Pi, H. Yamauchi, G. Sun, T. Yoshida, H. Aikawa, W. Fujimoto, H. Iso, R. Cui, M. P. Waalkes and Y. Kumagai, Vascular dysfunction in patients with chronic arsenicosis can be reversed by reduction of arsenic exposure, Environ. Health Perspect., 2005, 113, 339–341 CAS.
- P. L. Smedley, H. B. Nicolli, D. M. J. Macdonald, A. J. Barros and J. O. Tullio, Hydrogeochemistry of arsenic and other inorganic constituents in groundwaters from La Pampa, Argentina, Applied Geochemistry, 2002, 17, 259–284 Search PubMed.
- (a) G. Sun, X. Li, J. Pi, Y. Sun, B. Li, Y. Jin and Y. Xu, Current research problems of chronic arsenicosis in China, J. Health Popul. Nutr., 2006, 24, 176–181 Search PubMed; (b) G. Yu, D. Sun and Y. Zheng, Health effects of exposure to natural arsenic in groundwater and coal in China: an overview of occurrence, Environ. Health Perspect., 2007, 115, 636–642 CrossRef CAS.
- C. L. Keen and S. Zidenberg-Cherr, in Manganese in Health and Disease, ed. D. J. Klimis-Tavantzis, CRC Press, Boca Raton, FL, 1994, pp. 193–205 Search PubMed.
- N. C. Burton and T. R. Guilarte, Manganese neurotoxicity: lessons learned from longitudinal studies in nonhuman primates, Environ. Health Perspect., 2009, 117, 325–332 CAS.
- G. S. Shukla and R. L. Singhal, The present status of biological effects of toxic metals in the environment: lead, Can. J. Physiol. Pharmacol., 1984, 62, 1015–1031 CrossRef CAS.
- (a) D. B. Calne, N. S. Chu, C. C. Huang, C. S. Lu and W. Olanow, Manganism and idiopathic parkinsonism: similarities and differences, Neurology, 1994, 44, 1583–1586 CAS; (b) D. G. Barceloux, Manganese, Clin. Toxicol., 1999, 37, 293–307 Search PubMed; (c) A. Beuter, R. Edwards, A. deGeoffroy, D. Mergler and K. Hundnell, Quantification of neuromotor function for detection of the effects of manganese, Neurotoxicology, 1999, 20, 355–366 CAS; (d) M. Aschner, T. R. Guilarte, J. S. Schneider and W. Zheng, Manganese: recent advances in understanding its transport and neurotoxicity, Toxicol. Appl. Pharmacol., 2007, 221, 131–147 CrossRef CAS; (e) J. S. Standridge, A. Bhattacharya, P. Succop, C. Cox and E. Haynes, Effect of chronic low level manganese exposure on postural balance: a pilot study of residents in southern Ohio, J. Occup. Environ. Med., 2008, 50, 1421–1429 CAS.
- I. Hossain, S. N. Islam, M. N. I. Khan, S. Islam and A. Hasnat, Serum level of copper, zinc and manganese in somatization disorder patients, German Journal of Psychiatry, 2007, 10, 13–17 Search PubMed.
- (a) J. Cawte, Accounts of a mystery illness: The Groote Eylandt syndrome, Aust. NZ J. Psychiatry, 1984, 18, 179–187 CrossRef CAS; (b) L. A. Gottschalk, T. Rebello, M. S. Buchsbaum, H. G. Tucker and E. L. Hodges, Abnormalities in hair trace elements as indicators of aberrant behavior, Comprehensive Psychology, 1991, 32, 229–237 Search PubMed.
- (a) P. J. Barlow, A pilot study on the metal levels in the hair of hyperactive children, Med. Hypotheses, 1983, 11, 309–318 CrossRef CAS; (b) A. Woolf, R. Wright, C. Amarasiriwardena and D. Bellinger, A child with chronic manganese exposure from drinking water, Environ. Health Perspect., 2002, 110, 613–616 CrossRef; (c) L. Takser, D. Mergler, S. de Grosbois, A. Smargiassi and J. Lafond, Blood manganese content at birth and cord serum prolactin levels, Neurotoxicol. Teratol., 2004, 26, 811–815 CrossRef CAS.
- P. D. Howe, H. M. Malcolm and S. Dobson, Manganese and its compounds: environmental aspects, Concise International Chemical Assessment Document World Health Organization, Geneva, 2004 Search PubMed.
- G. S. Shukla and S. V. Chandra, Levels of sulfhydryls and sulfhydryl-containing enzymes in brain, liver and testis of manganese treated rats, Arch. Toxicol., 1977, 37, 319–325 CrossRef CAS.
- K. B. Miller, J. S. Caton and J. W. Finley, Manganese depresses rat heart muscle respiration, BioFactors, 2006, 28, 33–46 Search PubMed.
- C. Ma, S. N. Schneider, M. Miller, D. W. Nebert, C. Lind, S. M. Roda, S. E. Afton, J. A. Caruso and M. B. Genter, Manganese accumulation in the mouse ear following systemic exposure, J. Biochem. Mol. Toxicol., 2008, 22, 305–310 CrossRef CAS.
- D. Hafeman, P. Factor-Litvak, Z. Cheng, A. van Geen and H. Ahsan, Association between manganese exposure through drinking water and infant mortality in Bangladesh, Environ. Health Perspect., 2007, 115, 1107–1112 CAS.
- B. Lönnerdal, C. L. Keen and L. S. Hurley, in Manganese in Health and Disease, ed. D. J. Klimis-Tavantzis, CRC Press, Boca Raton, 1994, pp. 175–191 Search PubMed.
- D. C. Dorman, M. F. Struve, D. Vitarella, F. L. Byerly, J. Goetz and R. Miller, Neurotoxicity of manganese chloride in neonatal and adult CD rats following subchronic (21-day) high-dose oral exposure, J. Appl. Toxicol., 2000, 20, 179–187 CrossRef CAS.
- K. A. Cockell, G. Bonacci and B. Belonje, Manganese content of soy or rice beverages is high in comparison to infant formulas, J. Am. Coll. Nutr., 2004, 23, 124–130 CAS.
- P. J. Collipp, S. Y. Chen and S. Maitinsky, Manganese in infant formulas and learning disability, Ann. Nutr. Metab., 1983, 27, 488–494 CrossRef CAS.
- V. Sahni, Y. Léger, L. Panaro, M. Allen, S. Giffin, D. Fury and N. Hamm, Case report: a metabolic disorder presenting as pediatric manganism, Environ. Health Perspect., 2007, 115, 1776–1779 CrossRef.
- V. A. Fitsanakis, C. Au, K. M. Erikson and M. Aschner, The effects of manganese on glutamate, dopamine and gamma-aminobutyric acid regulation, Neurochemistry International, 2006, 48, 426–433 Search PubMed.
- (a) R. H. Kawamura, S. Ikuta, S. Fukuzumi, R. Yamada and S. Tsubaki, Intoxication by manganese in well water, Kitasato Archives of Experimental Medicine, 1941, 18, 145–171 Search PubMed; (b) X. G. Kondakis, N. Makris, M. Leotsinidis, M. Prinou and T. Papapetropoulos, Possible health effects of high manganese concentration in drinking water, Arch. Environ. Health, 1989, 44 Search PubMed; (c) P. Vieregge, B. Heinzow, G. Kork, H. M. Teichert, P. Schleifenbaum and H. U. Mosinger, Long term exposure to manganese in rural well water has no neurological effects, Canadian Journal of Neurological Sciences, 1995, 22, 286–289 Search PubMed.
- M. Baldwin, D. Mergler, F. Larribe, S. Bélanger, R. Tardif, L. Bilodeau and K. Hudnell, Bioindicator and exposure data for a population based study of manganese, Neurotoxicology, 1999, 20, 343–353 CAS.
- A. Khalique, S. Ahmad, T. Anjum, M. Jaffar, M. H. Shah, N. Shaheen, S. R. Tariq and S. Manzoor, A comparative study based on gender and age dependence of selected metals in scalp hair, Environ. Monit. Assess., 2005, 104, 45–57 CrossRef CAS.
- ATSDR, Draft Toxicological Profile for Manganese, US Department of Health and Human Services. Public Health Service. Agency for Toxic Substances and Disease Registry, Atlanta, GA, 2000.
- J. W. Finley, Manganese absorption and retention by young women is associated with serum ferritin concentration, Am. J. Clin. Nutr., 1999, 70, 37–43 CAS.
- K. Watanabe, T. Tanaka, T. Shigemi, Y. Hayashida and K. Maki, Mn and Cu concentrations in mixed saliva of elementary school children in relation to sex, age, and dental caries, J. Trace Elem. Med. Biol., 2009, 23, 93–99 CrossRef CAS.
- (a) D. Mergler, M. Baldwin, S. Bélanger, F. Larribe, A. Beuter, R. Bowler, M. Panisset, R. Edwards, A. de Geoffroy, M. P. Sassine and K. Hudnell, Manganese neurotoxicity, a continuum of dysfunction: results from a community based study, Neurotoxicology, 1999, 20, 327–342 CAS; (b) R. Bowler, D. Mergler, M. P. Sassine, F. Larribe and H. K. Hudnell, Neuropsychiatric effects of manganese on mood, Neurotoxicology, 1999, 20, 367–378 CAS.
- R. T. Brown, W. S. Freeman, J. M. Perrin, M. T. Stein, R. W. Amler, H. M. Feldman, K. Pierce and M. L. Wolraich, Prevalence and assessment of attention-deficit/hyperactivity disorder in primary care settings, Pediatrics, 2001, 107, E43 CrossRef CAS.
- H. K. Hudnell, Effects from environmental Mn exposures: a review of the evidence from non-occupational exposure studies, Neurotoxicology, 1999, 20, 379–397 CAS.
- L. Davidsson, A. Cederblad, B. Lönnerdal and B. Sandström, Manganese retention in man: A method for estimating manganese absorption in man, Am. J. Clin. Nutr., 1989, 49, 170–179 CAS.
- B. B. Williams, D. Li, M. Wegrzynowicz, B. K. Vadodaria, J. G. Anderson, G. F. Kwakye, M. Aschner, K. M. Erikson and A. B. Bowman, Disease-toxicant screen reveals a neuroprotective interaction between Huntington's disease and manganese exposure, J. Neurochem., 2010, 112, 227–237 CrossRef CAS.
- C. C. Huang, N. S. Chu, C. S. Lu, R. S. Chen and D. B. Calne, Long-term progression in chronic manganism: ten years of follow-up, Neurology, 1998, 50, 698–700 CAS.
- H. A. Roels, M. I. O. Eslava, E. Ceulemans, A. Robert and D. Lison, Prospective study on the reversibility of neurobehavioral effects in workers exposed to manganese dioxide, Neurotoxicology, 1999, 20, 255–271 CAS.
- P. L. Diaz Sylvester, R. Lopez, A. M. Ubios and R. L. Cabrini, Exposure to subcutaneously implanted uranium dioxide impairs bone formation, Arch. Environ. Health, 2002, 57, 320–325 CrossRef CAS.
- P. Kurttio, H. Komulainen, A. Leino, L. Salonen, A. Auvinen and H. Saha, Bone as a possible target of chemical toxicity of natural uranium in drinking water, Environ. Health Perspect., 2005, 113, 68–72 CAS.
- (a) M. L. Zamora, B. L. Tracy, J. M. Zielinski, D. P. Meyerhof and M. A. Moss, Chronic ingestion of uranium in drinking water: a study of kidney bioeffects in humans, Toxicol. Sci., 1998, 43, 68–77 CrossRef CAS; (b) M. L. L. Zamora, J. M. Zielinski, G. B. Moodie, R. A. F. Falcomer, W. C. Hunt and K. Capello, Uranium in drinking water: renal effects of long-term ingestion by an aboriginal community, Arch. Environ. Occup. Health, 2009, 64, 228–241 Search PubMed.
- P. Kurttio, A. Harmoinen, H. Saha, L. Salonen, Z. Karpas, H. Kornulainen and A. Anuvinen, Kidney toxicity of ingested uranium from drinking water, Am. J. Kidney Dis., 2006, 47, 972–982 Search PubMed.
- H. Bensoussan, L. Grancolas, B. Dhieux-Lestaevel, O. Delissen, C.-M. Vacher, I. Dublineau, P. Voisin, P. Gourmelon, M. Taouis and P. Lestaevel, Heavy metal uranium affects the brain cholinergic system in rat following sub-chronic and chronic exposure, Toxicology, 2009, 261, 59–67 CrossRef CAS.
- S. Raymond-Whish, L. P. Mayer, T. O'Neal, A. Martinez, M. A. Sellers, P. J. Christian, S. L. Marion, C. Begay, C. R. Propper, P. B. Hoyer and C. A. Dyer, Drinking water with uranium below the U.S. EPA water standard causes estrogen receptor-dependent responses in female mice, Environ. Health Perspect., 2007, 115, 1711–1716 CrossRef CAS.
- (a) E. Arnault, M. Doussau, A. Pesty, B. Gouget, A. V. der Meeren, P. Fouchet and B. Lefèvre, Natural uranium disturbs mouse folliculogenesis in vivo and oocyte meiosis in vitro, Toxicology, 2008, 247, 80–87 CrossRef CAS; (b) M. S. Kundt, C. Martinez-Taibo, M. C. Muhlmann and J. C. Furnari, Uranium in drinking water: effects on mouse oocyte quality, Health Phys., 2009, 96, 568–574 CrossRef CAS.
- M. H. Duncan, C. L. Wiggins, J. M. Samet and C. R. Key, Childhood cancer epidemiology in New Mexico's American Indians, Hispanic whites, and non-Hispanic whites, 1970–82, J. Natl. Cancer Inst., 1986, 76, 1013–1018 CAS.
- (a) R. Bornschein, D. Pearson and L. Reiter, Behavioral effects of moderate lead exposure in children and animal models: part 1, clinical studies, Crit. Rev. Toxicol., 1980, 8, 43–99 CrossRef CAS; (b) L. M. Chiodo, S. W. Jacobson and J. L. Jacobson, Neurodevelopmental effects of postnatal lead exposure at very low levels, Neurotoxicol. Teratol., 2004, 26, 359–371 CrossRef CAS; (c) M. M. Téllez-Rojo, D. C. Bellinger, C. Arroyo-Quiroz, H. Lamadrid-Figueroa, A. Mercado-García, L. Schnaas-Arrieta, R. O. Wright, M. Hernández-Avila and H. Hu, Longitudinal associations between blood lead concentrations lower than 10 microg/dL and neurobehavioral development in environmentally exposed children in Mexico City, Pediatrics, 2006, 118, e323–e330 CrossRef; (d) M. L. Miranda, D. Kim, M. A. O. Galeano, C. J. Paul, A. P. Hull and S. P. Morgan, The relationship between early childhood blood lead levels and performance on end-of-grade tests, Environ. Health Perspect., 2007, 115, 1242–1247 CrossRef CAS; (e) D. C. Bellinger, Very low lead exposures and children's neurodevelopment, Curr. Opin. Pediatr., 2008, 20, 172–177 CrossRef; (f) J. M. Braun, T. E. Froehlich, J. L. Daniels, K. N. Dietrich, R. Hornung, P. Auinger and B. P. Lanphear, Association of environmental toxicants and conduct disorder in U.S. children: NHANES 2001–2004, Environ. Health Perspect., 2008, 116, 956–962 CrossRef; (g) J. T. Nigg, G. M. Knottnerus, M. M. Martel, M. Nikolas, K. Cavanagh, W. Karmaus and M. D. Rappley, Low blood lead levels associated with clinically diagnosed attention-deficit/hyperactivity disorder and mediated by weak cognitive control, Biol. Psychiatry, 2008, 63, 325–331 CrossRef CAS; (h) J. T. Nigg, M. Nikolas, G. M. Knottnerus, K. Cavanagh and K. Friderici, Confirmation and extension of association of blood lead with attention-deficit/hyperactivity disorder (ADHD) and ADHD symptom domains at population-typical exposure, J. Child. Psychol. Psychiatry., 2010, 51, 58–65 CrossRef; (i) G. Wang and B. A. Fowler, Roles of biomarkers in evaluating interactions among mixtures of lead, cadmium and arsenic, Toxicol. Appl. Pharmacol., 2008, 233, 92–99 CrossRef CAS; (j) K. Chandramouli, C. D. Steer, M. Ellis and A. M. Emond, Effects of early childhood lead exposure on academic performance and behaviour of school age children, Arch. Dis. Child., 2009, 94, 844–848 CrossRef CAS; (k) M. Ha, H.-J. Kwon, M.-H. Lim, Y.-K. Jee, Y.-C. Hong, J.-H. Leem, J. Sakong, J.-M. Bae, S.-J. Hong, Y.-M. Roh and S.-J. Jo, Low blood levels of lead and mercury and symptoms of attention deficit hyperactivity in children: a report of the children's health and environment research (CHEER), NeuroToxicology, 2009, 30, 31–36 CrossRef CAS; (l) A. Roy, D. Bellinger, H. Hu, J. Schwartz, A. S. Ettinger, R. O. Wright, M. Bouchard, K. Palaniappan and K. Balakrishnan, Lead exposure and behavior among young children in Chennai, India, Environ. Health Perspect., 2009, 117, 1607–1611 CAS.
- J. Weuve, S. A. Korrick, M. G. Weisskopf, M. A. Weisskopf, L. M. Ryan, J. Schwartz, H. Nie, F. Grodstein and H. Hu, Cumulative exposure to lead in relation to cognitive function in older women, Environ. Health Perspect., 2009, 117, 574–580 CAS.
- A. Rosin, The long-term consequences of exposure to lead, Isr. Med. Assoc. J., 2009, 11, 689–694 Search PubMed.
- (a) M. Vahter, M. Berglund and A. Akesson, Toxic metals and the menopause, J. Br. Menopause Soc., 2004, 10, 60–64 Search PubMed; (b) M. Vahter, A. Akesson, C. Lidén, S. Ceccatelli and M. Berglund, Gender differences in the disposition and toxicity of metals, Environ. Res., 2007, 104, 85–95 CrossRef CAS.
- M. Arora, J. Weuve, M. G. Weisskopf, D. Sparrow, H. Nie, R. I. Garcia and H. Hu, Cumulative lead exposure and tooth loss in men: the normative aging study, Environ. Health Perspect., 2009, 117, 1531–1534 CAS.
- (a) A. Navas-Acien, E. Guallar, E. K. Silbergeld and S. J. Rothenberg, Lead exposure and cardiovascular disease—a systematic review, Environ. Health Perspect., 2007, 115, 472–482 CAS; (b) G. Nagaraj, A. Sukumar, B. Nandlal, S. Vellaichamy, K. Thanasekaran and A. L. Ramanathan, Tooth element levels indicating exposure profiles in diabetic and hypertensive subjects from Mysore, India, Biol. Trace Elem. Res., 2009, 131, 255–262 CrossRef CAS.
- A. A. Berrahal, M. Lasram, N. E. Elj, A. Kerkeni, N. Gharbi and El-Fazâa, Effect of age-dependent exposure to lead on hepatotoxicity and nephrotoxicity in male rats, Environmental Toxicology, 2009 Search PubMed.
- D. C. Bellinger, Teratogen update: lead and pregnancy, Birth Defects Res., Part A, 2005, 73, 409–420 Search PubMed.
- D. A. Schaumberg, F. Mendes, M. Balaram, M. R. Dana, D. Sparrow and H. Hu, Accumulated lead exposure and risk of age-related cataract in men, JAMA, J. Am. Med. Assoc., 2004, 292, 2750–2754 CrossRef CAS.
- T. Golabek, B. Darewicz, M. Borawska, R. Markiewicz, K. Socha and J. Kudelski, Lead concentration in the bladder tissue and blood of patients with bladder cancer, Scandinavian Journal of Urology and Nephrology, 2009, 43, 467–470 Search PubMed.
- T. W. Clarkson, G. F. Nordberg and P. R. Sager, Reproductive and developmental toxicity of metals, Scand. J. Work Environ. Health, 1985, 11, 145–154 CAS.
- M. Lewis, J. Worobey, D. S. Ramsay and M. K. McCormack, Prenatal exposure to heavy metals: effect on childhood cognitive skills and health status, Pediatrics, 1992, 89, 1010–1015 Search PubMed.
- D. C. Bellinger, Children's cognitive health: the influence of environmental chemical exposures, Altern. Ther. Health Med., 2007, 13, S140–S144 Search PubMed.
- W. Jedrychowski, F. Perera, J. Jankowski, D. Mrozek-Budzyn, E. Mroz, E. Flak, S. Edwards, A. Skarupa and I. Lisowska-Miszczyk, Gender specific differences in neurodevelopmental effects of prenatal exposure to very low-lead levels: the prospective cohort study in three-year olds, Early Hum. Dev., 2009, 85, 503–510 CrossRef CAS.
- C. Gundacker, K. J. Wittmann, M. Kukuckova, G. Komarnicki, I. Hikkel and M. Gencik, Genetic background of lead and mercury metabolism in a group of medical students in Austria, Environ. Res., 2009, 109, 786–796 CrossRef CAS.
- (a) M. R. Hopkins, A. S. Ettinger, M. Hernández-Avila, J. Schwartz, M. M. Téllez-Rojo, H. Lamadrid-Figueroa, D. Bellinger, H. Hu and R. O. Wright, Variants in iron metabolism genes predict higher blood lead levels in young children, Environ. Health Perspect., 2008, 116, 1261–1266 CrossRef CAS; (b) S. K. Park, H. Hu, R. O. Wright, J. Schwartz, Y. Cheng, D. Sparrow, P. S. Vokonas and M. G. Weisskopf, Iron metabolism genes, low-level lead exposure and QT interval, Environ. Health Perspect., 2009, 117, 80–85 CAS.
- B. A. Chowdhury and R. K. Chandra, Biological and health implications of toxic heavy metal and essential trace element interactions, Prog. Food Nutr. Sci., 1987, 11, 55–113 Search PubMed.
- M. Loghman-Adham, Renal effects of environmental and occupational lead exposure, Environ. Health Perspect., 1997, 105, 928–938 CrossRef CAS.
- L. Coderre and A. K. Srivastava, Vanadium and the cardiovascular functions, Can. J. Physiol. Pharmacol., 2004, 82, 833–839 CrossRef CAS.
- J. L. Domingo, Vanadium and diabetes. What about vanadium toxicity?, Mol. Cell. Biochem., 2000, 203, 185–187 CrossRef CAS.
- A. Scibior, H. Zaporowska, J. a. Ostrowski and A. Banach, Combined effect of vanadium(V) and chromium(III) on lipid peroxidation in liver and kidney of rats, Chem.-Biol. Interact., 2006, 159, 213–222 CrossRef CAS.
- ATSDR Draft toxicological profile for vanadium; US Department of Health and Human Services. Public Health Service. Agency for Toxic Substances and Disease Registry: 2009.
- E. Stefańska, L. Ostrowska, D. Czapska, J. Karczewski and M. Borawska, [Hair vanadium content and nutritional status of students of the Medical University of Białystok], Rocz Panstw Zakl Hig, 2005, 56, 157–163 Search PubMed.
- (a) O. S. al Attas, N. M. al Dagheri and N. T. Vigo, Vanadate enhances insulin-receptor binding in gestational diabetic human placenta, Cell Biochem. Funct., 1995, 13, 9–14 CrossRef CAS; (b) P. Poucheret, S. Verma, M. D. Grynpas and J. H. McNeill, Vanadium and diabetes, Mol. Cell. Biochem., 1998, 188, 73–80 CrossRef CAS; (c) K. H. Thompson, J. Lichter, C. LeBel, M. C. Scaife, J. H. McNeill and C. Orvig, Vanadium treatment of type 2 diabetes: a view to the future, J. Inorg. Biochem., 2009, 103, 554–558 CrossRef CAS.
- D. A. Barrio and S. B. Etcheverry, Vanadium and bone development: putative signaling pathways, Can. J. Physiol. Pharmacol., 2006, 84, 677–686 CrossRef CAS.
- M. S. Molinuevo, A. M. Cortizo and S. B. Etcheverry, Vanadium(IV) complexes inhibit adhesion, migration and colony formation of UMR106 osteosarcoma cells, Cancer Chemother. Pharmacol., 2008, 61, 767–773 CrossRef CAS.
- T. Akera, K. Temma and K. Takeda, Cardiac actions of vanadium, Fed. Proc., 1983, 42, 2984–2988 Search PubMed.
- (a) H. G. Preuss, S. T. Jarrell, R. Scheckenbach, S. Lieberman and R. A. Anderson, Comparative effects of chromium, vanadium and gymnema sylvestre on sugar-induced blood pressure elevations in SHR, J. Am. Coll. Nutr., 1998, 17, 116–123 CAS; (b) P. Boscolo, M. Carmignani, A. R. Volpe, M. Felaco, G. D. Rosso, G. Porcelli and G. Giuliano, Renal toxicity and arterial hypertension in rats chronically exposed to vanadate, Occup. Environ. Med., 1994, 51, 500–503 CrossRef CAS; (c) S. J. Shi, H. G. Preuss, D. R. Abernethy, X. Li, S. T. Jarrell and N. S. Andrawis, Elevated blood pressure in spontaneously hypertensive rats consuming a high sucrose diet is associated with elevated angiotensin II and is reversed by vanadium, J. Hypertens., 1997, 15, 857–862 CrossRef CAS.
- T. Vodichenska, [An experimental study of the atherogenic effect of vanadium in body uptake with drinking water], Probl. Khig., 1994, 1994, 32–40 Search PubMed.
- O. Jacques-Camarena, M. González-Ortiz, E. Martínez-Abundis, J. F. López-Madrueño and R. Medina-Santillán, Effect of vanadium on insulin sensitivity in patients with impaired glucose tolerance, Ann. Nutr. Metab., 2008, 53, 195–198 CrossRef CAS.
- D. G. Barceloux, Vanadium. Journal of Toxicology: Clinical Toxicology, 1999, 37 Search PubMed.
- H. Afeseh Ngwa, A. Kanthasamy, V. Anantharam, C. Song, T. Witte, R. Houk and A. G. Kanthasamy, Vanadium induces dopaminergic neurotoxicity via protein kinase Cdelta dependent oxidative signaling mechanisms: relevance to etiopathogenesis of Parkinson's disease, Toxicol. Appl. Pharmacol., 2009, 240, 273–285 CrossRef CAS.
- S. S. Soares, F. Henao, M. Aureliano and C. Gutiérrez-Merino, Vanadate induces necrotic death in neonatal rat cardiomyocytes through mitochondrial membrane depolarization, Chem. Res. Toxicol., 2008, 21, 607–618 CrossRef CAS.
- D. Beyersmann and A. Hartwig, Carcinogenic metal compounds: recent insight into molecular and cellular mechanisms, Arch. Toxicol., 2008, 82, 493–512 CrossRef CAS.
- R. S. Ray, M. Basu, B. Ghosh, K. Samanta and M. Chatterjee, Vanadium, a versatile biochemical effector in chemical rat mammary carcinogenesis, Nutr. Cancer, 2005, 51, 184–196 Search PubMed.
- A. Scibior, H. Zaporowska and I. Niedźwiecka, Lipid peroxidation in the liver of rats treated with V and/or Mg in drinking water, J. Appl. Toxicol., 2009, 29, 619–628 CrossRef CAS.
- I. Zwolak and H. Zaparowska, Preliminary studies on the effect of zinc and selenium on vanadium-induced cytotoxicity in vitro, Acta Biol. Hung., 2009, 60, 55–67 Search PubMed.
- E. Denkhaus and K. Salnikow, Nickel essentiality, toxicity, and carcinogenicity, Crit. Rev. Oncol. Hematol., 2002, 42, 35–56 CrossRef CAS.
- M. Costa, K. Salnikow, S. Cosentino, C. B. Klein, X. Huang and Z. Zhuang, Molecular mechanisms of nickel carcinogenesis, Environ. Health Perspect., 1994, 102(Suppl 3), 127–130 CrossRef CAS.
- V. P. Chashschin, G. P. Artunina and T. Norseth, Congenital defects, abortion and other health effects in nickel refinery workers, Sci. Total Environ., 1994, 148, 287–91 CrossRef CAS.
- M. C. Herlant-Peers, H. F. Hildebrand and J.-P. Kerckaert, In vitro and in vivo incorporatino of 63NiCl2 in mice, Ann. Clin. Lab. Sci., 1983, 9, 47–59 Search PubMed.
- E. Szakmáry, V. Morvai, M. Náray and G. Ungváry, Haemodynamic effect of nickel chloride in pregnant rats, Acta Physiol. Hung., 1995, 83, 3–12 Search PubMed.
- M. Anke, L. Angelow, M. Glei, M. Müller and H. Illing, The biological importance of nickel in the food chain, J. Anal. Chem., 1995, 352, 92–96 CAS.
- M. L. Dourson, in Risk assessment of essential elements, ed. W. Mertz, C. O. Abernathy and S. S. Olin, ILSI Press, Washington, DC, 1994, pp. 207–212 Search PubMed.
- C. Pellerin and S. M. Booker, Reflections on hexavalent chromium: health hazards of an industrial heavyweight, Environ. Health Perspect., 2000, 108, A402–A407 CrossRef CAS.
- ATSDR Draft Toxicological Profile for Chromium; US Department of Health and Human Services. Public Health Service. Agency for Toxic Substances and Disease Registry: Atlanta, GA, 2008.
- (a) X. Yang, S.-Y. Li, F. Dong, J. Ren and N. Sreejayan, Insulin-sensitizing and cholesterol-lowering effects of chromium (D-Phenylalanine)3, J. Inorg. Biochem., 2006, 100, 1187–1193 CrossRef CAS; (b) N. Sreejayan, F. Dong, M. R. Kandadi, X. Yang and J. Ren, Chromium alleviates glucose intolerance, insulin resistance, and hepatic ER stress in obese mice, Obesity, 2008, 16, 1331–1337 CrossRef CAS.
- (a) J. K. Speetjens, R. A. Collins, J. B. Vincent and S. A. Woski, The nutrional supplement chromium(III) tris (picolate) cleaves DNA, Chem. Res. Toxicol., 1999, 12, 483–487 CrossRef CAS; (b) A. Levina and P. A. Lay, Chemical properties and toxicity of chromium(III) nutritional supplements, Chem. Res. Toxicol., 2008, 21, 563–571 CrossRef CAS.
- (a) N. S. Raja and B. U. Nair, Chromium(III) complexes inhibit transcription factors binding to DNA and associated gene expression, Toxicology, 2008, 251, 61–65 CrossRef CAS; (b) H. Dai, J. Liu, L. H. Malkas, J. Catalano, S. Alagharu and R. J. Hickey, Chromium reduces the in vitro activity and fidelity of DNA replication mediated by the human cell DNA synthesome, Toxicol. Appl. Pharmacol., 2009, 236, 154–165 CrossRef CAS.
- A. Scibior and H. Zaporowska, Effects of vanadium(V) and/or chromium(III) on L-ascorbic acid and glutathione as well as iron, zinc, and copper levels in rat liver and kidney, J. Toxicol. Environ. Health, Part A, 2007, 70, 696–704 Search PubMed.
- H. Y. Shrivastava, T. Ravikumar, N. Shanmugasundaram, M. Babu and B. U. Nair, Cytotoxicity studies of chromium(III) complexes on human dermal fibroblasts, Free Radical Biol. Med., 2005, 38, 58–69 CrossRef CAS.
- Y. A. Loubieres, A. de Lassence, M. Bernier, A. Viellard-Baron, J. M. Schmitt, B. Page and F. Jardin, Acute, fatal, oral chromic acid poisoning, Clin. Toxicol., 1999, 37, 333–336 Search PubMed.
- A. Elbetieha and M. H. Al-Hamood, Long-term exposure of male and female mice to trivalent and hexavalent chromium compounds: effect on fertility, Toxicology, 1997, 116, 39–47 CrossRef CAS.
- G. Papanikolaou and K. Pantopoulos, Iron metabolism and toxicity, Toxicol. Appl. Pharmacol., 2005, 202, 199–211 CrossRef CAS.
- G. Aamodt, G. Bukholm, J. Jahnsen, B. Moum and M. H. Vatn, I. B. S. E. N. S. Group, The association between water supply and inflammatory bowel disease based on a 1990–1993 cohort study in southeastern Norway, Am. J. Epidemiol., 2008, 168, 1065–1072 CrossRef.
- S. Altamura and M. U. Muckenthaler, Iron toxicity in diseases of aging: Alzheimer's disease, Parkinson's disease and atherosclerosis, J. Alzheimers Dis., 2009, 16, 879–895.
- (a) G. J. Brewer, Risks of copper and iron toxicity during aging in humans, Chem. Res. Toxicol., 2010, 23, 319–326 CrossRef CAS; (b) S. Swaminathan, V. A. Fonseca, M. G. Alam and S. V. Shah, The role of iron in diabetes and its complications, Diabetes Care, 2007, 30, 1926–1933 CrossRef CAS.
- R. S. Britton, G. A. Ramm, J. Olynyk, R. Singh, R. O'Neill and B. R. Bacon, Pathophysiology of iron toxicity, Adv. Exp. Med. Biol., 1994, 356, 239–253 CAS.
- Q. Liu, L. Sun, Y. Tan, G. Wang, X. Lin and L. Cai, Role of iron deficiency and overload in the pathogenesis of diabetes and diabetic complications, Curr. Med. Chem., 2009, 16, 113–129 CrossRef CAS.
- A. Loh, M. Hadziahmetovic and J. L. Dunaief, Iron homeostasis and eye disease, Biochim. Biophys. Acta, Gen. Subj., 2009, 1790, 637–649 CrossRef CAS.
- C. Y. Lok, A. T. Merryweather-Clarke, V. Viprakasit, Y. Chinthammitr, S. Srichairatanakool, C. Limwongse, D. Oleesky, A. J. Robins, J. Hudson, P. Wai, A. Premawardhena, H. J. de Silva, A. Dassanayake, C. McKeown, M. Jackson, R. Gama, N. Khan, W. Newman, G. Banait, A. Chilton, I. Wilson-Morkeh, D. J. Weatherall and K. J. H. Robson, Iron overload in the Asian community, Blood, 2009, 114, 20–25 CrossRef CAS.
- W. H. Kojola, G. R. Brenniman and B. W. Carnow, A review of environmental characteristics and health effects of barium in public water supplies, Rev. Environ. Health, 1979, 3, 79–95 Search PubMed.
- H. M. Perry, S. J. Kopp, E. F. Perry and M. W. Erlanger, Hypertension and associated cardiovascular abnormalities induced by chronic barium feeding, J. Toxicol. Environ. Health, Part A, 1989, 28, 373–388 CAS.
- (a) G. R. Brenniman, W. H. Kojola, P. S. Levy, B. W. Carnow and T. Namekata, High barium levels in public drinking water and its association with elevated blood pressure, Arch. Environ. Health, 1981, 36, 28–32 CAS; (b) R. G. Wones, B. L. Stadler and L. A. Frohman, Lack of effect of drinking water barium on cardiovascular risk factors, Environ. Health Perspect., 1990, 85, 355–359 CrossRef CAS.
- (a) J. A. Zdanowicz, J. D. Featherstone, M. A. Espeland and M. E. Curzon, Inhibitory effect of barium on human caries prevalence, Community Dent. Oral Epidemiol., 1987, 15, 6–9 CrossRef CAS; (b) J. A. Zdanowicz, S. A. Mundorff, J. D. Featherstone and H. M. Proskin, Inhibitory effect of barium on caries formation in rats, Caries Res., 1989, 23, 65–69 CrossRef CAS.
- (a) D. O. Carpenter, K. Arcaro and D. C. Spink, Understanding the human health effects of chemical mixtures, Environ. Health Perspect., 2002, 110(Suppl 1), 25–42 CrossRef CAS; (b) K. Kordas, B. Lönnerdal and R. J. Stoltzfus, Interactions between nutrition and environmental exposures: effects on health outcomes in women and children, J. Nutr., 2007, 137, 2794–2797 CAS.
- Z. Cheng, Y. Zheng, R. Mortlock and A. V. Geen, Rapid multi-element analysis of groundwater by high-resolution inductively coupled plasma mass spectrometry, Anal. Bioanal. Chem., 2004, 379, 512–518 CrossRef CAS.
- (a) M. Marlowe, J. Stellern, J. Errera and C. Moon, Main and interaction effects of metal pollutants on visual-motor performance, Arch. Environ. Health, 1985, 40, 221–225 CAS; (b) G. Samanta, R. Sharma, T. Roychowdhury and D. Chakraborti, Arsenic and other elements in hair, nails, and skin-scales of arsenic victims in West Bengal, India, Sci. Total Environ., 2004, 326, 33–47 CrossRef CAS; (c) L. Carrizales, I. Razo, J. L. Tellez-Hernández, R. Torres-Nerio, A. Torres, L. E. Batres, A. C. Cubillas and F. Díaz-Barriga, Exposure to arsenic and lead of children living near a copper-smelter in San Luis Potosi, Mexico: importance of soil contamination for exposure of children, Environ. Res., 2006, 101, 1–10 CrossRef CAS; (d) M. M. Diawara, J. S. Litt, D. Unis, N. Alfonso, L. Martínez, J. G. Crock, D. Smith and J. Carsella, Arsenic, cadmium, lead, and mercury in surface soils, Pueblo, Colorado: implications for population health risk, Environ. Geochem. Health, 2006, 28, 297–315 Search PubMed.
- (a) C. G. Elinder, L. Friberg, B. Lind and M. Jawaid, Lead and cadmium levels in blood samples from the general population of Sweden, Environ. Res., 1983, 30, 233–253 CrossRef CAS; (b) A. Sukumar and R. Subramanian, Elements in hair and nails of residents from a village adjacent to New Delhi. Influence of place of occupation and smoking habits, Biol. Trace Elem. Res., 1992, 34, 99–105 CrossRef CAS; (c) N. Fréry, C. Nessmann, F. Girard, J. Lafond, T. Moreau, P. Blot, J. Lellouch and G. Huel, Environmental exposure to cadmium and human birthweight, Toxicology, 1993, 79, 109–118 CrossRef CAS; (d) A. Sukumar and R. Subramanian, Relative element levels in the paired samples of scalp hair and fingernails of patients from New Delhi, Sci. Total Environ., 2007, 372, 474–479 CrossRef CAS; (e) D. Dhaware, A. Deshpande, R. N. Khandekar and R. Chowgule, Determination of toxic metals in Indian smokeless tobacco products, Scientific World Journal, 2009, 9, 1140–1147 Search PubMed; (f) L. Järup and A. Akesson, Current status of cadmium as an environmental health problem, Toxicol. Appl. Pharmacol., 2009, 238, 201–208 CrossRef; (g) P. A. Richter, E. E. Bishop, J. Wang and M. H. Swahn, Tobacco smoke exposure and levels of urinary metals in the U.S. youth and adult population: the National Health and Nutrition Examination Survey (NHANES) 1999–2004, Int. J. Environ. Res. Public Health, 2009, 6, 1930–1946 Search PubMed.
- N. Lal, P. K. Sharma, K. K. Nagpaul, D. Behera and S. K. Malik, Radioactive uranium in various Indian tobaccos and consumable products (snuff, chutta, bidi and cigarette), Int. J. Clin. Pharmacol. Ther. Toxicol., 1987, 25, 36–37 Search PubMed.
- A. K. Mitra, A. Haque, M. Islam and S. A. M. K. Bashar, Lead poisoning: an alarming public health problem in Bangladesh, Int. J. Environ. Res. Public Health, 2009, 6, 84–95 Search PubMed.
- (a) H. Kreppel, J. Liu, Y. Liu, F. X. Reichl and C. D. Klaassen, Zinc-induced arsenite tolerance in mice, Fundam. Appl. Toxicol., 1994, 23, 32–37 CrossRef CAS; (b) O. Ramos, L. Carrizales, L. Yáñez, J. Mejía, L. Batres, D. Ortíz and F. Díaz-Barriga, Arsenic increased lipid peroxidation in rat tissues by a mechanism independent of glutathione levels, Environmental Health Perspectives, 1995, 103, 85–88 CAS.
- (a) H. G. Petering, Some observations on the interaction of zinc, copper, and iron metabolism in lead and cadmium toxicity, Environ. Health Perspect., 1978, 25, 141–145 CrossRef CAS; (b) F. L. Cerklewski and R. M. Forbes, Influence of dietary zinc on lead toxicity in the rat, Journal of Nutrition, 1976, 106, 689–696 Search PubMed.
- P. R. Flanagan, J. Haist and L. S. Valberg, Comparative effects of iron deficiency induced by bleeding and a low-iron diet on the intestinal absorptive interactions of iron, cobalt, manganese, zinc, lead and cadmium, J. Nutr., 1980, 110, 1754–1763 CAS.
- (a) L. L. Hopkins and K. Schwartz, Chromium(III) binding to serum proteins, specifically siderophilin, Biochim. Biophys. Acta, Gen. Subj., 1964, 90, 484–491 CrossRef CAS; (b) C. J. Hahn and G. W. Evans, Absorption of trace metals in the zinc-deficient rat, Am. J. Physiol., 1975, 228, 1020–1023 Search PubMed.
- C. T. Walsh, H. H. Sandstead, A. S. Prasad, P. M. Newberne and P. J. Fraker, Zinc: health effects and research priorities for the 1990s, Environmental Health Perspectives, 1994, S2, 5–46.
- F. T. Wieringa, J. Berger, M. A. Dijkhuizen, A. Hidayat, N. X. Ninh, B. Utomo, E. Wasantwisut and P. Winichagoon, Z. for the Seamtizi Study Group, Combined iron and zinc supplementation in infants improved iron and zinc status, but interactions reduced efficacy in a multicountry trial in southeast Asia, J. Nutr., 2007, 137, 466–471 CAS.
- (a) J. E. Spallholz, L. M. Boylan and M. M. Rhaman, Environmental hypothesis: is poor dietary selenium intake an underlying factor for arsenicosis and cancer in Bangladesh and West Bengal, India?, Sci. Total Environ., 2004, 323, 21–32 CrossRef CAS; (b) W. J. Christian, C. Hopenhayn, J. A. Centeno and T. Todorov, Distribution of urinary selenium and arsenic among pregnant women exposed to arsenic in drinking water, Environ. Res., 2006, 100, 115–122 CrossRef CAS; (c) O. A. Levander and C. A. Baumann, Selenium metabolism. VI: Effect of arsenic on the excretion of selenium in the bile, Toxicol. Appl. Pharmacol., 1966, 9, 106–115 CrossRef CAS.
- (a) H. A. Schroeder, J. J. Balassa and I. H. Tipton, Essential trace metals in man: Manganese. A study in homeostasis, J. Chronic Dis., 1966, 19, 545–571 CrossRef CAS; (b) S. D. McDermott and C. Kies, in Nutritional Bioavailability of Manganese, ed. C. Kies, American Chemical Society,Washington, D.C., 1987, pp. 146–151 Search PubMed; (c) L. Davidsson, A. Cederblad, B. Lönnerdal and B. Sandström, The effect of individual dietary components on manganese absorption in humans, Am. J. Clin. Nutr., 1991, 54, 1065–1070 CAS; (d) J. H. Freeland-Graves and P. H. Lin, Plasma uptake of manganese as affected by oral loads of manganese, calcium, milk, phosphorus, copper, and zinc, J. Am. Coll. Nutr., 1991, 10, 38–43 CAS; (e) T. A. Lutz, A. Schroff and E. Scharrer, Effect of calcium and sugars on intestinal manganese absorption, Biol. Trace Elem. Res., 1993, 39, 221–227 CrossRef CAS.
- M. E. Markowitz, M. Sinnett and J. F. Rosen, A randomized trial of calcium supplementation for childhood lead poisoning, Pediatrics, 2004, 113, 34–39.
- R. A. Anderson, M. M. Polansky and N. A. Bryden, Stability and absorption of chromium and absorption of chromium histidinate complexes by humans, Biol. Trace Elem. Res., 2004, 101, 211–218 CrossRef CAS.
- L. Hallberg, L. Rossander-Hultén, M. Brune and A. Gleerup, Calcium and iron absorption: mechanism of action and nutritional importance, Eur. J. Clin. Nutr., 1992, 46, 317–327 CAS.
- A. Wise, Phytate and zinc bioavailability, Int. J. Food Sci. Nutr., 1995, 46, 53–63 CrossRef CAS.
- M. Modi, R. K. Kaul, G. M. Kannan and S. J. S. Flora, Co-administration of zinc and n-acetylcysteine prevents arsenic-induced tissue oxidative stress in male rats, J. Trace Elem. Med. Biol., 2006, 20, 197–204 CrossRef CAS.
- M. B. Zimmermann, S. Muthayya, D. Moretti, A. Kurpad and R. F. Hurrell, Iron fortification reduces blood lead levels in children in Bangalore, India, Pediatrics, 2006, 117, 2014–21 CrossRef.
- M. Berglund, A. Åkesson, B. Nermell and M. Vahter, Intestinal absorption of dietary cadmium in women is dependent on body iron stores and fiber intake, Environ. Health Perspect., 1994, 102, 1058–66 CrossRef CAS.
- R. A. Anderson, Chromium metabolism and its role in disease processes in man, Clin. Physiol. Biochem., 1986, 4, 31–41 Search PubMed.
- H. Spencer, L. Kramer, C. Norris and E. Wiatrowski, Effect of aluminum hydroxide on plasma fluoride and fluoride excretion during a high fluoride intake in man, Toxicol. Appl. Pharmacol., 1981, 58, 140–141 CrossRef CAS.
- A. Trüpschuh, M. Anke, M. Müller, M. Illing-Günther and E. Hartmann, in Mengen und Spurenelemente, ed. M. Anke, W. Arnhold, H. Bergmann, R. Bitsch, W. Dorn, G. Flachowsky, M. Glei, B. Groppel, M. Grün, H. Gürtler, G. Jahreis, I. Lombeck, B. Luckas, D. Meißner, W. Merbach, M. Müller, R. Müller and H.-J. Schneider, Verlag Harald Schubert, Leipzig, 1997, pp. 699–705 Search PubMed.
- K. K. Das, S. N. Das and S. A. Dhundasi, Nickel, its adverse health effects & oxidative stress, Indian J. Med. Res., 2008, 128, 412–425 Search PubMed.
- J. Bingham-Smith, L. Smith, V. Pijuan, Y. Zhuang and Y. C. Chen, Transmembrane signals and protooncogene induction evoked by carcinogenic metals and prevented by zinc, Environmental Health Perspectives, 1994, 102, 181–189.
- K. Salnikow and K. S. Kasprzak, Ascorbate depletion: a critical step in nickel carcinogenesis?, Environ. Health Perspect., 2005, 113, 577–584 CrossRef CAS.
- (a) L. Rossander-Hultén, M. Brune, B. Sandström, B. Lonnerdal and L. Hallberg, Competitive inhibition of iron absorption by manganese and zinc in humans, Amer. J. Clin. Nutr., 1991, 54, 152–156 Search PubMed; (b) M. M. A. Boojar, F. Goodarzi and M. A. Basedaghat, Long-term follow-up of workplace and well water manganese effects on iron status indexes in manganese miners, Arch. Environ. Health, 2002, 57, 519–528 CrossRef CAS; (c) D. G. Ellingsen, E. Haug, R. J. Ulvik and Y. Thomassen, Iron status in manganese alloy production workers, J. Appl. Toxicol., 2003, 23, 239–247 CrossRef CAS.
- B. Elsenhans, G. Schmolke, K. Kolb, J. Stokes and W. Forth, Metal–metal interactions among dietary toxic and essential trace metals in the rat, Ecotoxicol. Environ. Saf., 1987, 14, 275–287 CrossRef CAS.
- W. Mertz, C. O. Abernathy and S. S. Olin, Risk Assessment of Essential Elements, ILSI Press, Washington, D.C., 1994 Search PubMed.
- Z. Li, J.-Y. Gu, X.-W. Wang, Q.-H. Fan, Y.-X. Geng, Z.-X. Jiao, Y.-P. Hou and W.-S. Wu, Effects of cadmium on absorption, excretion, and distribution of nickel in rats, Biol. Trace Elem. Res., 2010, 135, 211–219 CrossRef CAS.
- M. Abdulla and J. Chmielnicka, New aspects on the distribution and metabolism of essential trace elements after dietary exposure to toxic metals, Biol. Trace Elem. Res., 1990, 23, 25–53.
- M. R. Fox, Nutritional influences on metal toxicity: cadmium as a model toxic element, Environ. Health Perspect., 1979, 29, 95–104 CrossRef CAS.
- B. Lönnerdal, Dietary factors influencing zinc absorption, J. Nutr., 2000, 130, 1378S–1383S CAS.
- (a) T. Jin, G. Nordberg, T. Ye, M. Bo, H. Wang, G. Zhu, Q. Kong and A. Bernard, Osteoporosis and renal dysfunction in a general population exposed to cadmium in China, Environ. Res., 2004, 96, 353–359 CrossRef CAS; (b) M. Kippler, W. Goessler, B. Nermell, E. C. Ekström, B. Lönnerdal, S. E. Arifeen and M. Vahter, Factors influencing intestinal cadmium uptake in pregnant Bangladeshi women—a prospective cohort study, Environ. Res., 2009, 109, 914–921 CrossRef CAS.
- P. Christian and K. P. West, Interactions between zinc and vitamin A: an update, Am. J. Clin. Nutr., 1998, 68, 435S–441S CAS.
- K. T. Suzuki, H. Tamagawa, K. Takahashi and N. Shimojo, Pregnancy-induced mobilization of copper and zinc bound to renal metallothionein in cadmium loaded rats, Toxicology, 1990, 60, 199–210 CrossRef CAS.
- B. Teucher, M. Olivares and H. Cori, Enhancers of iron absorption: ascorbic acid and other organic acids, Int. J. Vitam. Nutr. Res., 2004, 74, 403–419 CrossRef CAS.
- A. J. Roodenburg, C. E. West, R. Hovenier and A. C. Beynen, Supplemental vitamin A enhances the recovery from iron deficiency in rats with chronic vitamin A deficiency, Br. J. Nutr., 1996, 75, 623–636 CrossRef CAS.
- S. Tuntipopipat, K. Judprasong, C. Zeder, E. Wasantwisut, P. Winichagoon, S. Charoenkiatkul, R. Hurrell and T. Walczyk, Chili, but not turmeric, inhibits iron absorption in young women from an iron-fortified composite meal, J. Nutr., 2006, 136, 2970–2974 CAS.
- I. M. Zijp, O. Korver and L. B. Tijburg, Effect of tea and other dietary factors on iron absorption, Crit. Rev. Food Sci. Nutr., 2000, 40, 371–398 CrossRef CAS.
- R. A. Anderson, in Trace Elements in Human and Animal Nutrition, ed. W. Mertz, Academic Press, San Diego, CA, 1994, pp. 225–244 Search PubMed.
- C. Kies, K. D. Aldrich, J. M. Johnson, C. Creps, C. Kowalski and R. H. Wang, in Nutritional Bioavailability of Manganese, ed. C. Kies, American Chemical Society, Washington, DC, 1987, pp. 136–145 Search PubMed.
- (a) M. Aschner, Manganese: brain transport and emerging research needs, Environ. Health Perspect., 2000, 108(Suppl 3), 429–432 CrossRef CAS; (b) S. V. Chandra and G. S. Shukla, Role of iron deficiency in inducing susceptibility to manganese toxicity, Arch. Toxicol., 1976, 35, 319–323 CrossRef CAS; (c) A. Spencer, Whole blood manganese levels in pregnancy and the neonate, Nutrition, 1999, 15, 731–734 CrossRef CAS; (d) S. L. Hansen, N. Trakooljul, H.-C. Liu, A. J. Moeser and J. W. Spears, Iron transporters are differentially regulated by dietary iron, and modifications are associated with changes in manganese metabolism in young pigs, J. Nutr., 2009, 139, 1474–1479 CrossRef CAS.
- P. C. Paul, M. Misbahuddin, A. N. Ahmed, Z. F. Dewan and M. A. Mannan, Accumulation of arsenic in tissues of iron-deficient rats, Toxicol. Lett., 2002, 135, 193–197 CrossRef CAS.
- S. Turgut, A. Polat, M. Inan, G. Turgot, G. Emmungil, M. Bican, T. Y. Karakus and O. Genc, Interaction between anemia and blood levels of iron, zinc, copper, cadmium and lead in children, Indian J. Pediatr., 2007, 74, 827–830 Search PubMed.
- (a) M. Piscator and S. E. Larsson, in Trace Element Metabolism in Animals-2, ed. W. G. Hoekstra, H. E. Suttle, H. E. Ganther and W. Mertz, University Park Press, Baltimore, 1974, p. 687 Search PubMed; (b) E. Bárány, I. A. Bergdahl, L. E. Bratteby, T. Lundh, G. Samuelson, S. Skerfving and A. Oskarsson, Iron status influences trace element levels in human blood and serum, Environ. Res., 2005, 98, 215–223 CrossRef CAS; (c) M. Kippler, E.-C. Ekström, B. Lönnerdal, W. Goessler, A. Akesson, S. E. Arifeen, L.-A. Persson and M. Vahter, Influence of iron and zinc status on cadmium accumulation in Bangladeshi women, Toxicol. Appl. Pharmacol., 2007, 222, 221–226 CrossRef CAS.
- (a) J. Quarterman and J. N. Morrison, The effects of dietary calcium and phosphorus on the retention and excretion of lead in rats, Br. J. Nutr., 1975, 34, 351–362 CAS; (b) J. C. Barton, M. E. Conrad, L. Harrison and S. Nuby, Effects of calcium on the absorption and retention of lead, J. Lab. Clin. Med., 1978, 91, 366–376 CAS; (c) R. A. Goyer, Nutrition and metal toxicity, Am. J. Clin. Nutr., 1995, 61, 646S–650S CAS.
- (a) I.-M. Olsson, I. Bensryd, T. Lundh, H. Ottosson, S. Skerfving and A. Oskarsson, Cadmium in blood and urine—impact of sex, age, dietary intake, iron status, and former smoking—association of renal effects, Environ. Health Perspect., 2002, 110, 1185–1190 CrossRef CAS; (b) M. Vahter, M. Berglund, A. Akesson and C. Lidén, Metals and women's health, Environ. Res., 2002, 88, 145–155 CrossRef CAS.
- C. C. Bridges and R. K. Zalups, Molecular and ionic mimicry and the transport of toxic metals, Toxicol. Appl. Pharmacol., 2005, 204, 274–308 CrossRef CAS.
- D. J. Svensgaard and R. C. Hertzberg, in Toxicology of Chemical Mixtures, ed. R. S. H. Yang, Academic Press, San Diego, CA, 1994, pp. 599–640 Search PubMed.
- D. S. Bae, C. Gennings, W. H. Carter, R. S. Yang and J. A. Campain, Toxicological interactions among arsenic, cadmium, chromium, and lead in human keratinocytes, Toxicol. Sci., 2001, 63, 132–142 CrossRef CAS.
- D. Beyersmann, Interactions in metal carcinogenicity, Toxicol. Lett., 1994, 72, 333–338 CrossRef CAS.
- (a) J. P. Berry and P. Galle, Selenium–arsenic interaction in renal cells: role of lysosomes. Electron microprobe study, J. Submiscors. Cytol. Pathol., 1994, 26, 203–210 Search PubMed; (b) S. Satarug, M. Nishijo, P. Ujjin, Y. Vanavanitkun, J. R. Baker and M. R. Moore, Evidence for concurrent effects of exposure to environmental cadmium and lead on hepatic CYP2A6 phenotype and renal function biomarkers in nonsmokers, Environ. Health Perspect., 2004, 112, 1512–1518 CrossRef CAS.
- M. Valko, H. Morris and M. T. D. Cronin, Metals, toxicity and oxidative stress, Curr. Med. Chem., 2005, 12, 1161–1208 CrossRef CAS.
- H. H. Sanstead, Understanding zinc: recent observations and interpretations, J. Lab. Clin. Med., 1994, 124, 322–327.
- J. J. Chisholm, Heavy metal exposures: Toxicity from metal–metal interactions, and behavioral effects, Pediatrics, 1974, 53, 841–843 Search PubMed.
- M. A. Peraza, F. Ayala-Fierro, D. S. Barber, E. Casarez and L. T. Rael, Effects of micronutrients on metal toxicity, Environ. Health Perspect., 1998, 106(Suppl 1), 203–216 CrossRef CAS.
- J. Pi, H. Yamauchi, Y. Kumagai, G. Sun, T. Yoshida, H. Aikawa, C. Hopenhayn-Rich and N. Shimojo, Evidence for induction of oxidative stress caused by chronic exposure of Chinese residents to arsenic contained in drinking water, Environ. Health Perspect., 2002, 110, 331–336 CAS.
- R. R. Engel, C. Hopenhayn-Rich, O. Receveur and A. H. Smith, Vascular effects of chronic arsenic exposure: a review, Epidemiol. Rev., 1994, 16, 184–209 Search PubMed.
- X.-J. Qin, L. G. Hudson, W. Liu, W. Ding, K. L. Cooper and K. J. Liu, Dual actions involved in arsenite-induced oxidative DNA damage, Chem. Res. Toxicol., 2008, 21, 1806–1813 CrossRef CAS.
- P. Sidhu, M. L. Garg and D. K. Dhawan, Protective role of zinc in nickel induced hepatotoxicity in rats, Chem.-Biol. Interact., 2004, 150, 199–209 CrossRef CAS.
- O. A. Levander, Metabolic interactions between metals and metalloids, Environ. Health Perspect., 1978, 25, 77–80 CrossRef CAS.
- (a) H. Babich, N. Martin-Alguacil and E. Borenfreund, Arsenic–selenium interactions determined with cultured fish cells, Toxicol. Lett., 1989, 45, 157–164 CrossRef CAS; (b) S. Biswas, G. Talukder and A. Sharma, Prevention of cytotoxic effects of arsenic by short-term dietary supplementation with selenium in mice in vivo, Mutat. Res., Genet. Toxicol. Environ. Mutagen., 1999, 441, 155–160 CrossRef CAS; (c) C. D. Davis, E. O. Uthus and J. W. Finley, Dietary selenium and arsenic affect DNA methylation in vitro in caco-2 cells and in vivo in rat liver and colon, J. Nutr., 2000, 130, 2903–2909 CAS; (d) W. Wang, L. Yang, S. Hou, S. Tan and H. Li, Effects of selenium supplementation on arsenism: an intervention trial in Inner Mongolia, Environ. Geochem. Health, 2002, 24, 359–374 Search PubMed.
- S. L. Wagner, J. S. Maliner, W. E. Morton and R. S. Braman, Skin cancer and arsenical intoxication from well water, Arch. Dermatol., 1979, 115, 1205–1207 CAS.
- J. Gailer, Reactive selenium metabolites as targets of toxic metals/metalloids in mammals: a molecular toxicological perspective, Appl. Organomet. Chem., 2002, 16, 701–707 CrossRef CAS.
- (a) A. B. Kar, R. P. Das and B. Mukerji, Prevention of cadmium-induced changes in the gonads of rats by zinc and selenium. A study in antagonism between metals in the biological system, Proc. Natl. Inst. Sci. India, Part B Suppl., 1960, 26B, 40 Search PubMed; (b) A. N. Uddin, F. J. Burns and T. G. Rossman, Vitamin E and organoselenium prevent the cocarcinogenic activity of arsenite with solar UVR in mouse skin, Carcinogenesis, 2005, 26, 2179–2186 CrossRef CAS.
- F. L. Cerklewski and R. M. Forbes, Influence of dietary selenium on lead toxicity in the rat, J. Nutr., 1976, 106, 778–783 CAS.
- C. A. Northrop-Clewes and D. I. Thurnham, Monitoring micronutrients in cigarette smokers, Clin. Chim. Acta, 2007, 377, 14–38 CrossRef CAS.
- (a) E. Marafante and M. Vahter, The effect of methyltransferase inhibition on the metabolism of [74As]arsenite in mice and rabbits, Chem.-Biol. Interact., 1994, 50, 49–57; (b) M. Vahter, Mechanisms of arsenic biotransformation, Toxicology, 2002, 181, 211–217 CrossRef; (c) T. Hayakawa, Y. Kobayashi, X. Cui and S. Hirano, A new metabolic pathway of arsenite: arsenic–glutathione complexes are substrates for human arsenic methyltransferase Cyt19, Arch. Toxicol., 2005, 79, 183–191 CrossRef CAS.
- (a) J. De Kimpe, R. Cornelis and R. Vanholder, In vitro methylation of arsenite by rabbit liver cytosol: effect of metal ions, metal chelating agents, methyltransferase inhibitors and uremic toxins, Drug Chem. Toxicol., 1999, 22, 613–628 CrossRef CAS; (b) K. H. Hong, C. L. Keen, Y. Mizuno, K. E. Johnston and T. Tamura, Effects of dietary zinc deficiency on homocysteine and folate metabolism in rats, J. Nutr. Biochem., 2000, 11, 165–169 CrossRef CAS; (c) F. S. Walton, S. B. Waters, S. L. Jolley, E. L. LeCluyse, D. J. thomas and M. Styblo, Selenium compounds modulate the activity of recombinant rat AsIII-methyltransferase and the methylation of arsenite by rat and human hepatocytes, Chem. Res. Toxicol., 2003, 16, 261–265 CrossRef CAS.
- L. M. Fischer, K. A. daCosta, L. Kwock, P. W. Stewart, T. S. Lu, S. P. Stabler, R. H. Allen and S. H. Zeisel, Sex and menopausal status influence human dietary requirements for the nutrient choline, Am. J. Clin. Nutr., 2007, 85, 1275–1285 CAS.
- T. Gebel, Suppression of arsenic-induced chromosome mutagenicity by antimony, Mutat. Res., Genet. Toxicol. Environ. Mutagen., 1998, 412, 213–218 CrossRef CAS.
- C. de Burbure, J.-P. Buchet, A. Leroyer, C. Nisse, J.-M. Haguenoer, A. Mutti, Z. Smerhovsky, M. Cikrt, M. Trzcinka-Ochocka, G. Razniewska, M. Jakubowski and A. Bernard, Renal and neurologic effects of cadmium, lead, mercury, and arsenic in children: evidence of early effects and multiple interactions at environmental exposure levels, Environ. Health Perspect., 2006, 114, 584–590 CAS.
- (a) F. Hong, T. Jin and A. Zhang, Risk assessment on renal dysfunction caused by co-exposure to arsenic and cadmium using benchmark dose calculation in a Chinese population, BioMetals, 2004, 17, 573–580 Search PubMed; (b) G. F. Nordberg, T. Jin, F. Hong, A. Zhang, J.-P. Buchet and A. M. Bernard, Biomarkers of cadmium and arsenic interactions, Toxicol. Appl. Pharmacol., 2005, 206, 191–197 CrossRef CAS.
- S. C. Frost, R. A. Kohanski and M. D. Lane, Effect of phenylarsine oxide on insulin-dependent protein phosphorylation and glucose transport in 3T3-L1 adipocytes, J. Biol. Chem., 1987, 262, 9872–9876 CAS.
- Z. Merali and R. L. Singhal, Protective effect of selenium on certain hepatotoxic and pancreotoxic manifestations of subacute cadmium administration, J. Pharmacol. Exp. Ther., 1975, 195, 58–66 CAS.
- G. S. Shukla and S. V. Chandra, Effect of interation of Mn2+ with Zn2+, Hg2+ and Cd2+ on some neurochemicals in rat, Toxicol. Lett., 1981, 10, 163–168.
- T. Baba, T. Shimizu, Y.-I. Suzuki, M. Ogawara, K.-I. Isono, H. Koseki, H. Kurosawa and T. Shirasawa, Estrogen, insulin, and dietary signals cooperatively regulate longevity signals to enhance resistance to oxidative stress in mice, J. Biol. Chem., 2005, 280, 16417–16426 CrossRef CAS.
- C. Byrne, S. D. Divekar, G. B. Storchan, D. A. Parodi and M. B. Martin, Cadmium—a metallohormone?, Toxicol. Appl. Pharmacol., 2009, 238, 266–271 CrossRef CAS.
- S.-Y. Choe, S.-J. Kim, H.-G. Kim, J. H. Lee, Y. Choi, H. Lee and Y. Kim, Evaluation of estrogenicity of major heavy metals, Sci. Total Environ., 2003, 312, 15–21 CrossRef CAS.
- (a) J. H. Li and T. G. Rossman, Mechanism of comutagenesis of sodium arsenite with n-methyl-n-nitrosourea, Biol. Trace Elem. Res., 1989, 21, 373–381 CrossRef CAS; (b) T. C. Wang, J. S. Huang, V. C. Yang, H. J. Lan, C. J. Lin and K. Y. Jan, Delay of the excision of UV light-induced DNA adducts is involved in the coclastogenicity of UV light plus arsenite, Int. J. Radiat. Biol., 1994, 66, 367–372 CrossRef CAS; (c) M. J. Mass and L. Wang, Arsenic alters cytosine methylation patterns of the promoter of the tumor suppressor gene p53 in human lung cells: a model for a mechanism of carcinogenesis, Mutat. Res., Rev. Mutat. Res., 1997, 386, 263–277 CrossRef CAS; (d) A. Basu, J. Mahata, S. Gupta and A. K. Giri, Genetic toxicology of a paradoxical human carcinogen, arsenic: a review, Mutat. Res., Rev. Mutat. Res., 2001, 488, 171–194 CrossRef CAS; (e) S. H. Lamm, A. Engel, C. A. Penn, R. Chen and M. Feinleib, Arsenic cancer risk confounder in southwest Taiwan data set, Environ. Health Perspect., 2006, 114, 1077–1082 CrossRef CAS; (f) T. G. Rossman, A. N. Uddin and F. J. Burns, Evidence that arsenite acts as a cocarcinogen in skin cancer, Toxicol. Appl. Pharmacol., 2004, 198, 394–404 CrossRef CAS; (g) D. R. Germolec, J. Judson Spalding, H. S. Yu, J. S. Chen, P. P. Simeonova, P. C. Humble, A. Bruccoleri, G. A. Boorman, J. F. Foley, T. Yoshida and M. I. Luster, Arsenic enhancement of skin neoplasia by chronic stimulation of growth factors, Am. J. Pathol., 1998, 153, 1175–1785; (h) F. J. Burns, A. N. Uddin, F. Wu, A. Nádas and T. G. Rossman, Arsenic-induced enhancement of ultraviolet radiation carcinogenesis in mouse skin: a dose-response study, Environ. Health Perspect., 2004, 112, 599–603 CAS.
- K. R. Mahaffey, S. G. Capar, B. C. Gladen and B. A. Fowler, Concurrent exposure to lead, cadmium, and arsenic. Effects on toxicity and tissue metal concentrations in the rat, J. Lab. Clin. Med., 1981, 98, 463–481 CAS.
- (a) C.-L. Chen, L.-I. Hsu, H.-Y. Chiou, Y.-M. Hsueh, S.-Y. Chen, M.-M. Wu and C.-J. Chen, B. D. S. Group, Ingested arsenic, cigarette smoking, lung cancer risk: a follow-up study in arseniasis-endemic areas in Taiwan, JAMA, J. Am. Med. Assoc., 2004, 292, 2984–2990 CrossRef CAS; (b) K. M. McCarty, E. A. Houseman, Q. Quamruzzaman, M. Rahman, G. Mahiuddin, T. Smith, L. Ryan and D. C. Christiani, The impact of diet and betel nut use on skin lesions associated with drinking-water arsenic in Pabna, Environ. Health Perspect., 2006, 114, 334–340 CAS.
- C.-L. Chen, H.-Y. Chiou, L.-I. Hsu, Y.-M. Hsueh, M.-M. Wu and C.-J. Chen, Ingested arsenic, characteristics of well water consumption and risk of different histological types of lung cancer in northeastern Taiwan, Environ. Res., 2009 Search PubMed.
- (a) C. J. Chen, C. W. Chen, M. M. Wu and T. L. Kuo, Cancer potential in liver, lung, bladder and kidney due to ingested inorganic arsenic in drinking water, Br. J. Cancer, 1992, 66, 888–892 CAS; (b) M. N. Bates, A. H. Smith and K. P. Cantor, Case-control study of bladder cancer and arsenic in drinking water, Am. J. Epidemiol., 1995, 141, 523–530 CAS; (c) P. Kurttio, E. Pukkala, H. Kahelin, A. Auvinen and J. Pekkanen, Arsenic concentrations in well water and risk of bladder and kidney cancer in Finland, Environ. Health Perspect., 1999, 107, 705–710 CrossRef CAS; (d) M. R. Karagas, T. D. Tosteson, J. S. Morris, E. Demidenko, L. A. Mott, J. Heaney and A. Schned, Incidence of transitional cell carcinoma of the bladder and arsenic exposure in New Hampshire, Cancer, Causes Control, 2004, 15, 465–472 CrossRef; (e) C. Ferreccio, C. Gonzalez, V. Milosavjlevic, G. Marshall, A. M. Sancha and A. H. Smith, Lung cancer and arsenic concentrations in drinking water in Chile, Epidemiology, 2000, 11, 673–679 CrossRef CAS; (f) C. Steinmaus, Y. Yuan, M. N. Bates and A. H. Smith, Case-control study of bladder cancer and drinking water arsenic in the western United States, Am. J. Epidemiol., 2003, 158, 1193–1201 CrossRef; (g) L. M. Knobeloch, K. M. Zierold and H. A. Anderson, Association of arsenic-contaminated drinking-water with prevalence of skin cancer in Wisconsin's Fox River Valley, J. Health Popul. Nutr., 2006, 24, 206–213 Search PubMed.
- A. Basu, P. Ghosh, J. K. Das, A. Banerjee, K. Ray and A. K. Giri, Micronuclei as biomarkers of carcinogen exposure in populations exposed to arsenic through drinking water in West Bengal, India: a comparative study in three cell types, Cancer Epidemiol. Biomarkers Prev., 2004, 13, 820–827 CAS.
- A. S. Andrew, J. L. Burgess, M. M. Meza, E. Demidenko, M. G. Waugh, J. W. Hamilton and M. R. Karagas, Arsenic exposure is associated with decreased DNA repair in vitro and in individuals exposed to drinking water arsenic, Environ. Health Perspect., 2006, 114, 1193–1198 CrossRef CAS.
- T. G. Rossman, A. N. Uddin, F. J. Burns and M. C. Bosland, Arsenite is a cocarcinogen with solar ultraviolet radiation for mouse skin: an animal model for arsenic carcinogenesis, Toxicol. Appl. Pharmacol., 2001, 176, 64–71 CrossRef CAS.
- (a) C.-H. Lee, C.-L. Yu, W.-T. Liao, Y.-H. Kao, C.-Y. Chai, G.-S. Chen and H.-S. Yu, Effects and interactions of low doses of arsenic and UVB on keratinocyte apoptosis, Chem. Res. Toxicol., 2004, 17, 1199–1205 CrossRef CAS; (b) P.-H. Chen, C.-C. E. Lan, M.-H. Chiou, M.-C. Hsieh and G.-S. Chen, Effects of arsenic and UVB on normal human cultured keratinocytes: impact on apoptosis and implication on photocarcinogenesis, Chem. Res. Toxicol., 2005, 18, 139–144 CrossRef CAS; (c) X.-J. Qin, L. G. Hudson, W. Liu, G. S. Timmins and K. J. Liu, Low concentration of arsenite exacerbates UVR-induced DNA strand breaks by inhibiting PARP-1 activity, Toxicol. Appl. Pharmacol., 2008, 232, 41–50 CrossRef CAS; (d) W. Ding, W. Liu, K. L. Cooper, X.-J. Qin, P. L. de Souza Bergo, L. G. Hudson and K. J. Liu, Inhibition of poly(ADP-ribose) polymerase-1 by arsenite interferes with repair of oxidative DNA damage., J. Biol. Chem., 2009, 284, 6809–6817 CAS.
- F. J. Burns, T. Rossman, K. Vega, A. Uddin, S. Vogt, B. Lai and R. J. Reeder, Mechanism of selenium-induced inhibition of arsenic-enhanced UVR carcinogenesis in mice, Environ. Health Perspect., 2008, 116, 703–708 CrossRef CAS.
- L. B. Zablotska, Y. Chen, J. H. Graziano, F. Parvez, A. van Geen, G. R. Howe and H. Ahsan, Protective effects of B vitamins and antioxidants on the risk of arsenic-related skin lesions in Bangladesh, Environ. Health Perspect., 2008, 116, 1056–1062 CrossRef CAS.
- G. E. Arteel, L. Guo, T. Schlierf, J. I. Beier, J. P. Kaiser, T. S. Chen, M. Liu, D. J. Conklin, H. L. Miller, C. von Montfort and J. C. States, Subhepatotoxic exposure to arsenic enhances lipopolysaccharide-induced liver injury in mice, Toxicol. Appl. Pharmacol., 2008, 226, 128–139 CrossRef CAS.
- A. N. Uddin, F. J. Burns, T. G. Rossman, H. Chen, T. Kluz and M. Costa, Dietary chromium and nickel enhance UV-carcinogenesis in skin of hairless mice, Toxicol. Appl. Pharmacol., 2007, 221, 329–338 CrossRef CAS.
- S. Ivankovic and R. Preussman, Absence of toxic and carcinogenic effects after administration of high doses of chromic oxide pigment in subacute and long-term feeding experiments in rats, Food Cosmet. Toxicol., 1975, 13, 347–351 CrossRef CAS.
- (a) D. M. Stearns, J. P. S. Wise, S. R. Patierno and K. E. Wetterhahn, Chromium(III) picolinate produces chromosome damage in Chinese hamster ovary cells, FASEB J., 1995, 9, 1643–1649 CAS; (b) D. Bagchi, M. Bagchi, J. Balmoori, X. Ye and S. J. Stohs, Comparative induction of oxidative stress in cultured J774A.1 macrophage cells by chromium picolinate and chromium nicotinate, Res. Comm. Mol. Pathol, Pharmaco., 1997, 97, 335–346 Search PubMed.
- V. H. Ferm, The synteratogenic effect of lead and cadmium, Experientia, 1969, 25, 56 CAS.
- ATSDR Interaction profile for: arsenic, cadmium, chromium, and lead; US Department of Health and Human Services. Public Health Service. Agency for Toxic Substances and Disease Registry: Atlanta, GA, 2004.
- H. Choudhury and A. Mudipalli, Potential considerations & concerns in the risk characterization for the interaction profiles of metals, Indian J. Med. Res., 2008, 128, 462–483 Search PubMed.
- Y. Kim, B.-N. Kim, Y.-C. Hong, M.-S. Shin, H.-J. Yoo, J.-W. Kim, S.-Y. Bhang and S.-C. Cho, Co-exposure to environmental lead and manganese affects the intelligence of school-aged children, NeuroToxicology, 2009, 30, 564–571 CrossRef CAS.
- T. Gebel, Arsenic and antimony: comparative approach on mechanistic toxicology, Chem.-Biol. Interact., 1997, 107, 131–144 CrossRef CAS.
- (a) A. G. Schauss, Comparative hair mineral analysis results of 21 elements in a random selected behaviorally normal 19–59 year old population and violent adult criminal offenders, International Journal of Biosocial and Medical Research, 1981, 1, 21–41 Search PubMed; (b) H. Hu, Knowledge of diagnosis and reproductive history among survivors of childhood plumbism, Am. J. Public Health, 1991, 81, 1070–1072 Search PubMed.
- K. M. Erikson, K. Thompson, J. Aschner and M. Aschner, Manganese neurotoxicity: a focus on the neonate, Pharmacol. Ther., 2007, 113, 369–377 CrossRef CAS.
- J. G. Anderson, S. C. Fordahl, P. T. Cooney, T. L. Weaver, C. L. Colyer and K. M. Erikson, Extracellular norepinephrine, norepinephrine receptor and transporter protein and mRNA levels are differentially altered in the developing rat brain due to dietary iron deficiency and manganese exposure, Brain Res., 2009, 1281, 1–14 CrossRef CAS.
- K. M. Jamil, A. S. Rahman, P. K. Bardhan, A. I. Khan, F. Chowdhury, S. A. Sarker, A. M. Khan and T. Ahmed, Micronutrients and anaemia, J. Health Popul. Nutr., 2008, 26, 340–355 Search PubMed.
- (a) H. A. Ruff, M. E. Markowitz, P. E. Bijur and J. F. Rosen, Relationships among blood lead levels, iron deficiency, and cognitive development in two-year-old children, Environ. Health Perspect., 1996, 104, 180–185 CAS; (b) H. Sachdev, T. Gera and P. Nestel, Effect of iron supplementation on mental and motor development in children: systematic review of randomised controlled trials, Public Health Nutr., 2005, 8, 117–132 Search PubMed; (c) J. Y. Yager and D. S. Hartfield, Neurologic manifestations of iron deficiency in childhood, Pediatr. Neurol., 2002, 27, 85–92 CrossRef.
- (a) E. S. Halas and M. J. Eberhardt, A behavioral review of trace element deficiencies in animals and humans, Nutr. Behav., 1987, 3, 257–271 Search PubMed; (b) C. J. Frederickson, Neurobiology of zinc and zinc-containing neurons, Int. Rev. Neurobiol., 1989, 31, 145–238 CAS.
- R. Amani, S. Saeidi, Z. Nazari and S. Nematpour, Correlation Between Dietary Zinc Intakes and Its Serum Levels with Depression Scales in Young Female Students, Biol. Trace Elem. Res., 2009 Search PubMed.
- (a) B. Rimland and G. E. Larson, Hair mineral analysis and behavior: an analysis of 51 studies, J. Learn. Disabil., 1983, 16, 279–285 Search PubMed; (b) S. Y. Tsai, H. Y. Chou, H. W. The, C. M. Chen and C. J. Chen, The effects of chronic arsenic exposure from drinking water on the neurobehavioral development in adolescence, NeuroToxicology, 2003, 24, 747–753 CrossRef CAS.
- R. O. Pihl and M. Parkes, Hair element content in learning disabled children, Science, 1977, 198, 204–206 CrossRef CAS.
- (a) E. F. Madden and B. A. Fowler, Mechanisms of nephrotoxicity from metal combinations: a review, Drug Chem. Toxicol., 2000, 23, 1–12 CrossRef CAS; (b) O. Barbier, G. Jacquillet, M. Tauc, M. Cougnon and P. Poujeol, Effect of heavy metals on, and handling by, the kidney, Nephron Physiol., 2005, 99, 105–110; (c) I. Saboli, Common mechanisms in nephropathy induced by toxic metals, Nephron Physiol., 2006, 104, p107–p114 CrossRef.
- J. Liu, Y. Liu, S. M. Habeebu, M. P. Waalkes and C. D. Klaassen, Chronic combined exposure to cadmium and arsenic exacerbates nephrotoxicity, particularly in metallothionein-I/II null mice, Toxicology, 2000, 147, 157–166 CrossRef CAS.
- M. Föller, M. Sopjani, H. Mahmud and F. Lang, Vanadate-induced suicidal erythrocyte death, Kidney Blood Pressure Res., 2008, 31, 87–93 Search PubMed.
- M. Donnadieu-Claraz, M. Bonnehorgne, B. Dhieux, C. Maubert, M. Cheynet, F. Paquet and P. Gourmelon, Chronic exposure to uranium leads to iron accumulation in rat kidney cells, Radiat. Res., 2007, 167, 454–464 CrossRef CAS.
- P. Kurttio, A. Auvinen, L. Salonen, H. Saha, J. Pekkanen, I. Mäkeläinen, S. B. Väisänen, I. M. Penttilä and H. Komulainen, Renal effects of uranium in drinking water, Environ. Health Perspect., 2002, 110, 337–342 CrossRef CAS.
- (a) B. Fristedt, R. Lindqvist, A. Schutz and P. Ovrum, Survival in a case of acute chromic acid poisoning with acute renal failure treated by haemodialysis, Acta Med. Scand., 1965, 177, 153 Search PubMed; (b) World Health Organization (WHO) Principles and methods for the assessment of nephrotoxicity associated with exposure to chemicals, Environmental Health Criteria World Health Organization: Geneva, 1991; (c) J. Fadrowski, A. Navas-Acien, M. Tellez-Plaza, E. Guallar, V. Weaver and S. Furth, Blood lead level and kidney function in US adolescents, Arch. Intern. Med., 2010, 170, 75–82 CrossRef CAS.
- I. o. M. Food and Nutrition Board, in Dietary reference intakes for vitamin A, vitamin K, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc, National Academy Press, Washington, DC, 2001, pp. 197–223 Search PubMed.
- A. S. Prasad, Essential and toxic trace elements in human health and disease, ed. A. S. Prasad and A. Alan R. Liss, New York, 1988, pp. 3–53 Search PubMed.
- (a) H. Malluche, Renal bone disease—an ongoing challenge to the nephrologist, Clin Nephrol, 1995, 44, S38–S41 Search PubMed; (b) H. Malluche and M. C. Faugere, Renal bone disease 1990: an unmet challenge for the nephrologist, Kidney Int., 1990, 38, 193–211 CrossRef CAS.
- (a) T. Kido, K. Nogawa, R. Honda, I. Tsuritani, M. Ishizaki, Y. Yamada and H. Nakagawa, The association between renal dysfunction and osteopenia in environmental cadmium-exposed subjects, Environ. Res., 1990, 51, 71–82 CrossRef CAS; (b) K. Aoshima, J. Fan, Y. Cai, T. Katoh, H. Teranishi and M. Kasuya, Assessment of bone metabolism in cadmium-induced renal tubular dysfunction by measurements of biochemical markers, Toxicol. Lett., 2003, 136, 183–192 CrossRef CAS.
- M. Ikeda, T. Ezaki, T. Tsukahara and J. Moriguchi, Dietary cadmium intake in polluted and non-polluted areas in Japan in the past and in the present, Int. Arch. Occup. Environ. Health, 2004, 77, 227–234 CrossRef CAS.
- (a) D. M. Medeiros, A. Plattner, D. Jennings and B. Stoecker, Bone morphology, strength and density are compromised in iron-deficient rats and exacerbated by calcium restriction, J. Nutr., 2002, 132, 3135–3141 CAS; (b) D. M. Medeiros, B. Stoecker, A. Plattner, D. Jennings and M. Haub, Iron deficiency negatively affects vertebrae and femurs of rats independently of energy intake and body weight, J. Nutr., 2004, 134, 3061–3067 CAS.
- M. Rahman, M. Tondel, S. A. Ahmad, I. A. Chowdhury, M. H. Faruquee and O. Axelson, Hypertension and arsenic exposure in Bangladesh, Hypertension, 1999, 33, 74–78 CAS.
- M. S. Lubanski and P. A. McCullough, Kidney's role in hypertension, Minerva Cardioangiol, 2009, 57, 743–759 Search PubMed.
- L. Järup, M. Berglund, C. G. Elinder, G. Nordberg and M. Vahter, Health effects of cadmium exposure—a review of the literature and a risk estimate, Scand. J. Work Environ. Health, 1998, 24(Suppl 1), 1–51.
- J. Liu, Y. Xie, R. Cooper, D. M. K. Ducharme, R. Tennant, B. A. Diwan and M. P. Waalkes, Transplacental exposure to inorganic arsenic at a hepatocarcinogenic dose induces fetal gene expression changes in mice indicative of aberrant estrogen signaling and disrupted steroid metabolism, Toxicol. Appl. Pharmacol., 2007, 220, 284–91 CrossRef CAS.
- S. V. Chandra, D. K. Saxena and M. Z. Hasan, Effect of zinc on manganese induced testicular injury in rats, Ind. Health, 1975, 13, 51–56 CrossRef CAS.
- (a) M. Takiguchi and S. i. Yoshihara, New aspects of cadmium as endocrine disruptor, Environ Sci, 2006, 13, 107–116 Search PubMed; (b) W. H. Watson and J. D. Yager, Arsenic: extension of its endocrine disruption potential to interference with estrogen receptor-mediated signaling, Toxicol. Sci., 2007, 98, 1–4 CrossRef CAS.
- G. S. Prins, Endocrine disruptors and prostate cancer risk, Endocr. Relat. Cancer, 2008, 15, 649–656 CrossRef CAS.
- J. D. Meeker, M. G. Rossano, B. Protas, M. P. Diamond, E. Puscheck, D. Daly, N. Paneth and J. J. Wirth, Cadmium, lead, and other metals in relation to semen quality: human evidence for molybdenum as a male reproductive toxicant, Environ. Health Perspect., 2008, 116, 1473–1479 CrossRef CAS.
- A. H. Milton, A. H. Smith, A. Rahman, Z. Hasan, U. Kulsum, K. Dear, M. Rakibuddin and A. Ali, Chronic arsenic exposure and adverse preganancy outcomes in Bangladesh, Epidemiology, 2005, 16, 82–86 CrossRef.
- N. W. Flodin, Micronutrient supplements: toxicity and drug interactions, Prog. Food Nutr. Sci., 1990, 14, 277–331 Search PubMed.
- B. Haglund, K. Ryckenberg, O. Selinus and G. Dahlquist, Evidence of a relationship between childhood-onset type I diabetes and low groundwater concentration of zinc, Diabetes Care, 1996, 19, 873–875 CrossRef CAS.
- R. R. Bell, J. L. Early, V. K. Nonavinakere and Z. Mallory, Effect of cadmium on blood glucose level in the rat, Toxicol. Lett., 1990, 54, 199–205 CrossRef CAS.
- A. W. Dobson, K. M. Erikson and M. Aschner, Manganese neurotoxicity, Ann. N. Y. Acad. Sci., 2004, 1012, 115–128 CrossRef CAS.
- N. Hasgekar, J. P. Beck, H. Dunkelberg, K. I. Hirsch-Ernst and T. W. Gebel, Influence of antimonite, selenite, and mercury on the toxicity of arsenite in primary rat hepatocytes, Biol. Trace Elem. Res., 2006, 111, 167–83 CrossRef CAS.
- M. S. Bhuiyan and K. Fukunaga, Cardioprotection by vanadium compounds targeting Akt-mediated signaling, J. Pharmacol. Sci., 2009, 110, 1–13 CAS.
- L. Yáñez, L. Carrizales, M. T. Zanatta, J. J. Mejía, L. Batres and F. Díaz-Barriga, Arsenic-cadmium interaction in rats: toxic effects in the heart and tissue metal shifts, Toxicology, 1991, 67, 227–234 CrossRef CAS.
- A. A. Meharg, E. Lombi, P. N. Williams, K. G. Scheckel, J. Feldmann, A. Raab, Y. Zhu and R. Islam, Speciation and Localization of Arsenic in White and Brown Rice, Environ. Sci. Technol., 2008, 42, 1051–1057 CrossRef CAS.
- P. N. Williams, A. H. Price, A. Raab, A. Hossain, J. Feldmann and A. A. Meharg, Variation in arsenic speciation and concentration in paddy rice related to dietary exposure, Environ. Sci. Technol., 2005, 39, 5531–5540 CrossRef CAS.
- (a) T. Roychowdhury, H. Tokunaga and M. Ando, Survey of arsenic and other heavy metals in food composites and drinking water and estimation of dietary intake by the villagers from an arsenic-affected area of West Bengal, India, Sci. Total Environ., 2003, 308, 15–35 CrossRef CAS; (b) R. Ortega, S. H. Frisbie, D. Alber, G. Devès, B. Stanik, D. Maynard and B. Sarkar, Presented in part at Nineteenth Annaul Conference on Contaminated Soils, University of Massachusetts, Amherst, MA, Yea Search PubMed; (c) S. W. A. Rmalli, P. I. Haris, C. F. Harrington and M. Ayub, A survey of arsenic in foodstuffs on sale in the United Kingdom and imported from Bangladesh, Sci. Total Environ., 2005, 337, 23–30 CrossRef CAS; (d) A. Heikens, G. M. Panaullah and A. A. Meharg, Arsenic behaviour from groundwater and soil to crops: impacts on agriculture and food safety, Rev. Environ. Contam. Toxicol., 2007, 189, 43–87 CrossRef CAS; (e) F. Queirolo, S. Stegen, M. F. Restovic, M. Paz, P. Ostapczuk, M. J. Schwuger and L. Muñoz, Total arsenic level, lead, and cadmium in vegetables cultivated at the Andean villages of northern Chile, Sci. Total Environ., 2000, 255, 75–84 CrossRef CAS; (f) N. M. Smith, R. Lee, D. T. Heitkemper, K. D. Cafferky, A. Haque and A. K. Henderson, Inorganic arsenic in cooked rice and vegetables from Bangladeshi households, Sci. Total Environ., 2006, 370, 294–301 CrossRef; (g) T. Uchino, T. Roychowdhury, M. Ando and H. Tokunaga, Intake of arsenic from water, food composites and excretion through urine, hair from a studied population in West Bengal, India, Food Chem. Toxicol., 2006, 44, 455–461 CrossRef CAS; (h) M. L. Kile, E. A. Houseman, C. V. Breton, T. Smith, Q. Quamruzzaman, M. Rahman, G. Mahiuddin and D. C. Christiani, Dietary arsenic exposure in bangladesh, Environ. Health Perspect., 2007, 115, 889–893 CrossRef CAS; (i) B. K. Mandal, K. T. Suzuki and K. Anzai, Impact of arsenic in foodstuffs on the people living in the arsenic-affected areas of West Bengal, India, J. Environ. Sci. Health A Tox. Hazard Subst. Environ. Eng., 2007, 42, 1741–1752 Search PubMed; (j) A. A. Carbonell-Barrachina, A. J. Signes-Pastor, L. Vázquez-Araújo, F. Burló and B. Sengupta, Presence of arsenic in agricultural products from arsenic-endemic areas and strategies to reduce arsenic intake in rural villages, Mol. Nutr. Food Res., 2009, 53, 531–541 CrossRef CAS; (k) M. M. Rahman, G. Owens and R. Naidu, Arsenic levels in rice grain and assessment of daily dietary intake of arsenic from rice in arsenic-contaminated regions of Bangladesh—implications to groundwater irrigation, Environ. Geochem. Health, 2009, 31(Suppl 1), 179–187 Search PubMed.
- (a) T. Roychowdhury, T. Uchino, H. Tokunaga and M. Ando, Survey of arsenic in food composites from an arsenic-affected area of West Bengal, India, Food Chem. Toxicol., 2002, 40, 1611–1621 CrossRef CAS; (b) M. L. Kile, E. A. Houseman, C. V. Breton, Q. Quamruzzaman, M. Rahman, G. Mahiuddin and D. C. Christiani, Association between total ingested arsenic and toenail arsenic concentrations, J. Environ. Sci. Health A Tox. Hazard Subst. Environ. Eng., 2007, 42, 1827–1834 Search PubMed.
- C. D. Kozul, A. P. Nomikos, T. H. Hampton, L. A. Warnke, J. A. Gosse, J. C. Davey, J. E. Thorpe, B. P. Jackson, M. A. Ihnat and J. W. Hamilton, Laboratory diet profoundly alters gene expression and confounds genomic analysis in mouse liver and lung, Chem.-Biol. Interact., 2008, 173, 129–140 CrossRef CAS.
- J. A. T. Pennington, B. E. Young, D. B. Wilson, R. D. Johnson and J. E. Vanderveen, Mineral content of foods and total diets: the selected minerals in foods survey, 1982–1984, J. Am. Diet. Assoc., 1986, 86, 876–891 CAS.
- S. F. Velazquez and J. T. Du, in Risk Assessment of Essential elements, ed. W. Mertz, C. O. Abernathy and S. S. Olin, ILSI Press, Washington, D.C., 1994, pp. 253–266 Search PubMed.
- K. Ljung and M. Vahter, Time to re-evaluate the guideline value for manganese in drinking water?, Environ. Health Perspect., 2007, 115, 1533–1538 CrossRef CAS.
- R. W. Wenlock, D. H. Buss and E. J. Dixon, Trace nutrients. 2. Manganese in British food, Br. J. Nutr., 1979, 41, 253–261 CrossRef CAS.
- ATSDR Toxicological profile for cadmium; Agency for Toxic Substances and Disease Registry: Atlanta, GA, 1999.
- R. W. Simmons, P. Pongsakul, D. Saiyasitpanich and S. Klinphoklap, Elevated levels of cadmium and zinc in paddy soils and elevated levels of cadmium in rice grain downstream of a zinc mineralized area in Thailand: implications for public health, Environ. Geochem. Health, 2005, 27, 501–511 Search PubMed.
- (a) I. Mariam, S. Iqbal and S. A. Nagra, Distribution of some trace and macrominerals in beef, mutton and poultry, International Journal of Agriculture and Biology, 2004, 6, 816–820 Search PubMed; (b) D. Santhi, V. B. Balakrishnan, A. Kalaikanna and K. T. Radhakrishnan, Presence of heavy metals in pork products in Chennai (India), Am. J. Food Technol., 2008, 3, 192–199 Search PubMed.
- S. O. Welsh and R. M. Marston, Zinc levels of the US food supply 1909–1980, Food Technol., 1982, 36, 70–76 CAS.
- D. G. Barceloux, Zinc, Clin. Toxicol., 1999, 37, 279–292 Search PubMed.
- (a) M. B. Kristensen, O. Hels, C. M. Morberg, J. Marving, S. Bügel and I. Tetens, Total zinc absorption in young women, but not fractional zinc absorption, differs between vegetarian and meat-based diets with equal phytic acid content, Br. J. Nutr., 2006, 95, 963–967 CrossRef CAS; (b) A. L. Rauma and H. Mykkänen, Antioxidant status in vegetarians versus omnivores, Nutrition, 2000, 16, 111–119 CrossRef CAS.
- T. Roychowdhury, T. Uchino, H. Tokunaga and M. Ando, Arsenic and other heavy metals in soils from an arsenic-affected area of West Bengal, India, Chemosphere, 2002, 49, 605–618 CrossRef CAS.
- (a) M. J. Abedin, M. S. Cresser, A. A. Meharg, J. Feldmann and J. Cotter-Howells, Arsenic accumulation and metabolism in rice (Oryza sativa L.), Environ. Sci. Technol., 2002, 36, 962–968 CrossRef CAS; (b) M. G. M. Alam, E. T. Snow and A. Tanaka, Arsenic and heavy metal contamination of vegetables grown in Samta village, Bangladesh, Sci. Total Environ., 2003, 308, 83–96 CrossRef CAS; (c) J. M. Duxbury, A. B. Mayer, J. G. Lauren and N. Hassan, Food chain aspects of arsenic contamination in Bangladesh: effects on quality and productivity of rice, J. Environ. Sci. Health A Tox. Hazard Subst. Environ. Eng., 2003, 38, 61–69 Search PubMed; (d) A. A. Meharg and M. Rahman, Arsenic contamination of Bangladesh paddy field soils: implications for rice contribution to arsenic consumption, Environ. Sci. Technol., 2003, 37, 229–234 CrossRef CAS; (e) S. Norra, Z. A. Berner, P. Agarwala, F. Wagner, D. Chandrasekharam and D. Stuben, Impact of irrigation with As rich groundwater on soil and crops: a geochemical case study in West Bengal Delta Plain, India, Appl. Geochem., 2005, 20, 1890–1906 CrossRef CAS; (f) S. M. I. Huq, J. C. Joardar, S. Parvin, R. Correll and R. Naidu, Arsenic contamination in food-chain: transfer of arsenic into food materials through groundwater irrigation, J. Health Popul. Nutr., 2006, 24, 305–316 Search PubMed.
- M. B. Arain, T. G. Kazi, J. A. Baig, M. K. Jamali, H. I. Afridi, N. Jalbani, R. A. Sarfraz, A. Q. Shah and G. A. Khandro, Respiratory effects in people exposed to arsenic via the drinking water and tobacco smoking in southern part of Pakistan, Sci. Total Environ., 2009, 407, 5524–5530 CrossRef CAS.
- P. Saipan and S. Ruangwises, Health risk assessment of inorganic arsenic intake of Ronphibun residents via duplicate diet study, J. Med. Assoc. Thai., 2009, 92, 849–855 Search PubMed.
- R. A. Karim, S. M. Hossain, M. M. H. Miah, K. Nehar and M. S. H. Mubin, Arsenic and heavy metal concentrations in surface soils and vegetables of Feni district in Bangladesh, Environ. Monit. Assess., 2008, 145, 417–425 CrossRef CAS.
- J. R. Peralta-Videa, M. L. Lopez, M. Narayan, G. Saupe and J. Gardea-Torresdey, The biochemistry of environmental heavy metal uptake by plants: implications for the food chain, Int. J. Biochem. Cell Biol., 2009, 41, 1665–1677 CrossRef CAS.
- P. Zaprjanova, L. Dospatliev, V. Angelova and K. Ivanov, Correlation between soil characteristics and lead and cadmium content in the aboveground biomass of Virginia tobacco, Environ. Monit. Assess., 2010, 163, 253–261 CrossRef CAS.
- N. Waegeneers, H. D. Steur, L. D. Temmerman, S. V. Steenwinkel, X. Gellynck and J. Viaene, Transfer of soil contaminants to home-produced eggs and preventitive measures to reduce contamination, Sci. Total Environ., 2009, 407, 4438–4446 CrossRef CAS.
- V. Devesa, D. Vélez and R. Montoro, Effect of thermal treatments on arsenic species contents in food, Food Chem. Toxicol., 2008, 46, 1–8 CrossRef CAS.
- M. Bae, C. Watanabe, T. Inaoka, M. Sekiyama, N. Sudo, M. H. Bokul and R. Ohtsuka, Arsenic in cooked rice in Bangladesh, Lancet, 2002, 360, 1839–1840 CrossRef.
- M. M. Rahman, B. K. Mandal, T. R. Chowdhury, M. K. Sengupta, U. K. Chowdhury, D. Lodh, C. R. Chanda, G. K. Basu, S. C. Mukherjee, K. C. Saha and D. Chakraborti, Arsenic groundwater contamination and sufferings of people in North 24-Parganas, one of the nine arsenic affected districts of West Bengal, India, J. Environ. Sci. Health A Tox. Hazard Subst. Environ. Eng., 2003, 38, 25–59 Search PubMed.
- O. P. Díaz, I. Leyton, O. Muñoz, N. Nuñez, V. Devesa, M. A. Suñer, V. Dinoraz and R. Montoro, Contribution of water, bread, and vegetables (raw and cooked) to dietary intake of inorganic arsenic in a rural village of northern Chile, J. Agric. Food Chem., 2004, 52, 1773–1779 CrossRef CAS.
- D. G. Barceloux, Nickel, Clin. Toxicol., 1999, 37, 239–258 Search PubMed.
- (a) M. Ajmal, A. Khan, A. A. Nomani and S. Ahmed, Heavy metals: leaching from glazed surfaces of tea mugs, Sci. Total Environ., 1997, 207, 49–54 CrossRef CAS; (b) R. Raghunath and K. S. Nambi, Lead leaching from pressure cookers, Sci. Total Environ., 1998, 224, 143–148 CrossRef CAS; (c) P. K. Sinha, S. Mandal and D. Kundu, Leaching of lead and cadmium from glass dinnerware, J. Environ. Sci. Eng., 2007, 49, 58–61 Search PubMed.
- N. Shivapurkar and L. A. Poirer, Tissue levels of S-adenosylmethio-nine and S-adenosylhomocysteine in rats fed methyl-deficient, amino acid-defined diets for one to five weeks, Carcinogenesis, 1983, 4, 1051–1057 CrossRef CAS.
- (a) J. Kristiansen, J. M. Christensen, B. S. Iversen and E. Sabbioni, Toxic trace element reference levels in blood and urine: influence of gender and lifestyle factors, Sci. Total Environ., 1997, 204, 147–160 CrossRef CAS; (b) P. L. Goering, H. V. Aposhian, M. J. Mass, M. Cebrian, B. D. Beck and M. P. Waalkes, The enigma of arsenic carcinogenesis: role of metabolism, Toxicol. Sci., 1999, 49, 5–14 CrossRef CAS; (c) S. R. Mitra, D. N. G. Mazumder, A. Basu, G. Block, R. Haque, S. Samanta, N. Ghosh, M. M. H. Smith, O. S. von Ehrenstein and A. H. Smith, Nutritional factors and susceptibility to arsenic-caused skin lesions in West Bengal, India, Environ. Health Perspect., 2004, 112, 1104–1109 CrossRef CAS; (d) C. Steinmaus, K. Carrigan, D. Kalman, R. Atallah, Y. Yuan and A. H. Smith, Dietary intake and arsenic methylation in a U.S. population, Environ. Health Perspect., 2005, 113, 1153–1159 CrossRef CAS.
- O. A. Levander, Lead toxicity and nutritional deficiencies, Environ. Health Perspect., 1979, 29, 115–125 CrossRef CAS.
- M. V. Gamble, X. Liu, H. Ahsan, R. Pislner, V. Ilievski, V. Slavkovich, F. Parvez, D. Levy, P. Factor-Litvak and J. Graziano, Folate, homocysteine, and arsenic metabolism in arsenic-exposed individuals in Bangladesh, Environ. Health Perspect., 2005, 113, 1683–1688 CrossRef CAS.
- (a) M. V. Gamble, X. Liu, H. Ahsan, J. R. Pilsner, V. Ilievski, V. Slavkovich, F. Parvez, Y. Chen, D. Levy, P. Factor-Litvak and J. H. Graziano, Folate and arsenic metabolism: a double-blind, placebo-controlled folic acid-supplementation trial in Bangladesh, Am. J. Clin. Nutr., 2006, 84, 1093–1101 CAS; (b) M. V. Gamble, X. Liu, V. Slavkovich, J. R. Pilsner, V. Ilievski, P. Factor-Litvak, D. Levy, S. Alam, M. Islam, F. Parvez, H. Ahsan and J. H. Graziano, Folic acid supplementation lowers blood arsenic, Am. J. Clin. Nutr., 2007, 86, 1202–1209 CAS; (c) M. L. Kile and A. G. Ronnenberg, Can folate intake reduce arsenic toxicity?, Nutr. Rev., 2008, 66, 349–353 Search PubMed.
- Y.-K. Huang, Y.-S. Pu, C.-J. Chung, H.-S. Shiue, M.-H. Yang, C.-J. Chen and Y.-M. Hsueh, Plasma folate level, urinary arsenic methylation profiles, and urothelial carcinoma susceptibility, Food Chem. Toxicol., 2008, 46, 929–938 CrossRef CAS.
- J. R. Pilsner, X. Liu, H. Ahsan, V. Ilievski, V. Slavkovich, D. Levy, P. Factor-Litvak, J. H. Graziano and M. V. Gamble, Folate deficiency, hyperhomocysteinemia, low urinary creatinine, and hypomethylation of leukocyte DNA are risk factors for arsenic-induced skin lesions, Environ. Health Perspect., 2009, 117, 254–260 CAS.
- P. N. Baker, S. J. Wheeler, T. A. Sanders, J. E. Thomas, C. J. Hutchinson, K. Clarke, J. L. Berry, R. L. Jones, P. T. Seed and L. Poston, A prospective study of micronutrient status in adolescent pregnancy, Am. J. Clin. Nutr., 2009, 89, 1114–1124 CrossRef CAS.
- (a) A. Alberg, The influence of cigarette smoking on circulating concentrations of antioxidant micronutrients, Toxicology, 2002, 180, 121–137 CrossRef CAS; (b) S. K. Bashar and A. K. Mitra, Effect of smoking on vitamin A, vitamin E, and other trace elements in patients with cardiovascular disease in Bangladesh: a cross-sectional study, Nutr. J., 2004, 3, 18 CrossRef; (c) P. Wallström, E. Wirfält, P. H. Lahmann, B. Gullberg, L. Janzon and G. Berglund, Serum concentrations of beta-carotene and alpha-tocopherol are associated with diet, smoking, and general and central adiposity, Am. J. Clin. Nutr., 2001, 73, 777–785 CAS.
- O. Müller and M. Krawinkel, Malnutrition and health in developing countries, Can. Med. Assoc. J., 2005, 173, 279–286 CrossRef.
- (a) F. Ahmed, Vitamin A deficiency in Bangladesh: a review and recommendations for improvement, Public Health Nutr., 1999, 2, 1–14 Search PubMed; (b) F. Ahmed, Anaemia in Bangladesh: a review of prevalence and aetiology, Public Health Nutr., 2000, 3, 385–393 Search PubMed; (c) F. Ahmed, M. R. Khan, C. P. Banu, M. R. Qazi and M. Akhtaruzzaman, The coexistence of other micronutrient deficiencies in anaemic adolescent schoolgirls in rural Bangladesh, Eur. J. Clin. Nutr., 2008, 62, 365–372 CrossRef CAS; (d) V. Persson, F. Ahmed, M. Gebre-Medhin and T. Greiner, Relationships between vitamin A, iron status and helminthiasis in Bangladeshi school children, Public Health Nutr., 2000, 3, 83–89 Search PubMed; (e) M. Z. Islam, C. Lamberg-Allardt, M. A. Bhuyan and Q. Salamatullah, Iron status of premenopausal women in two regions of Bangladesh: prevalence of deficiency in high and low socio-economic groups, Eur. J. Clin. Nutr., 2001, 55, 598–604 CrossRef CAS; (f) S. M. Z. Hyder, L.-A. Persson, M. Chowdhury, B. O. Lönnerdal and E.-C. Ekström, Anaemia and iron deficiency during pregnancy in rural Bangladesh, Public Health Nutr., 2004, 7, 1065–1070 Search PubMed; (g) A. S. G. Faruque, A. I. Khan, M. A. Malek, S. Huq, M. A. Wahed, M. A. Salam, G. J. Fuchs and M. A. Khaled, Childhood anemia and vitamin a deficiency in rural Bangladesh, Southeast Asian J. Trop Med. Public Health, 2006, 37, 771–777 Search PubMed; (h) A. Khambalia, D. L. O'Connor and S. Zlotkin, Periconceptional iron and folate status is inadequate among married, nulliparous women in rural Bangladesh, J. Nutr., 2009, 139, 1179–1184 CrossRef CAS; (i) M. H. Or-Rashid, U. F. Khatun, Y. Yoshida, S. Morita, N. Chowdhury and J. Sakamoto, Iron and iodine deficiencies among under-2 children, adolescent girls, and pregnant women of Bangladesh: association with common diseases, Nagoya J. Med. Sci., 2009, 71, 39–49 Search PubMed.
- (a) A. Misra, N. K. Vikram, R. M. Pandey, M. Dwivedi, F. U. Ahmad, K. Luthra, K. Jain, N. Khanna, J. R. Devi, R. Sharma and R. Guleria, Hyperhomocysteinemia and low intakes of folic acid and vitamin B12 in urban North India, Eur. J. Nutr., 2002, 41, 68–77 CrossRef CAS; (b) P. Pathak, U. Kapil, S. K. Kapoor, R. Saxena, A. Kumar, N. Gupta, S. N. Dwivedi, R. Singh and P. Singh, Prevalence of multiple micronutrient deficiencies amongst pregnant women in a rural area of Haryana, Indian J. Pediatr., 2004, 71, 1007–1014 Search PubMed; (c) P. Pathak, U. Kapil, C. S. Yajnik, S. K. Kapoor, S. N. Dwivedi and R. Singh, Iron, folate, and vitamin B12 stores among pregnant women in a rural area of Haryana State, India, Food Nutr. Bull., 2007, 28, 435–438 Search PubMed.
- T. Jiang, P. Christian, S. K. Khatry, L. Wu and K. P. West, Micronutrient deficiencies in early pregnancy are common, concurrent, and vary by season among rural Nepali pregnant women, J. Nutr., 2005, 135, 1106–1112 CAS.
- V. P. Anderson, S. Jack, D. Monchy, N. Hem, P. Hok, K. B. Bailey and R. S. Gibson, Co-existing micronutrient deficiencies among stunted Cambodian infants and toddlers, Asia Pac. J. Clin. Nutr., 2008, 17, 72–79 CAS.
- N. V. Nhien, N. C. Khan, N. X. Ninh, P. V. Huan, L. T. Hop, N. T. Lam, F. Ota, T. Yabutani, V. Q. Hoa, J. Motonaka, T. Nishikawa and Y. Nakaya, Micronutrient deficiencies and anemia among preschool children in rural Vietnam, Asia Pac. J. Clin. Nutr., 2008, 17, 48–55.
- R. A. Thurlow, P. Winichagoon, T. Pongcharoen, S. Gowachirapant, A. Boonpraderm, M. S. Manger, K. B. Bailey, E. Wasantwisut and R. S. Gibson, Risk of zinc, iodine, and other micronutrient deficiences among school children in North East Thailand, Eur. J. Clin. Nutr., 2006, 60, 623–632 CrossRef CAS.
- R. L. Lander, T. Enkhjargal, J. Batjargal, K. B. Bailey, S. Diouf, T. J. Green, C. M. Skeaff and R. S. Gibson, Multiple micronutrient deficiencies persist during early childhood in Mongolia, Asia Pac. J. Clin. Nutr., 2008, 17, 429–440 CAS.
- P. Galan, F. E. Viteri, S. Bertrais, S. Czernichow, H. Faure, J. Arnaud, D. Ruffieux, S. Chenal, N. Arnault, A. Favier, A. M. Roussel and S. Hercberg, Serum concentrations of beta-carotene, vitamins C and E, zinc and selenium are influenced by sex, age, diet, smoking status, alcohol consumption and corpulence in a general French adult population, Eur. J. Clin. Nutr., 2005, 59, 1181–1190 CrossRef CAS.
- T. G. Kazi, S. K. Wadhwa, H. I. Afridi, N. Kazi, G. A. Kandhro, J. A. Baig, A. Q. Shah, N. F. Kolachi and M. B. Arain, Interaction of cadmium and zinc in biological samples of smokers and chewing tobacco female mouth cancer patients, J. Hazard. Mater., 2010, 176, 985–991 CrossRef CAS.
- Y. M. Hseuh, C. S. Cheng, M. M. Wu, T. L. Kuo and C. J. Chen, Multiple risk factors associatecd with arsenic-induced skin cancers: effects of chronic liver disease and malnutrition, Br. J. Cancer, 1995, 71, 109–114 CrossRef.
- J. I. Anetor, H. Wanibuchi and S. Fukushima, Arsenic exposure and its health effects and risk of cancer in developing countries: micronutrients as host defence, Asian Pac. J. Cancer Prev., 2007, 8, 13–23 Search PubMed.
- U. K. Chowdhury, M. M. Rahman, M. K. Sengupta, D. Lodh, C. R. Chanda, S. Roy, Q. Quamruzzaman, H. Tokunaga, M. Ando and D. Chakraborti, Pattern of excretion of arsenic compounds [arsenite, arsenate, MMA(V), DMA(V)] in urine of children compared to adults from an arsenic exposed area in Bangladesh, J. Environ. Sci. Health A Tox. Hazard Subst. Environ. Eng., 2003, 38, 87–113 Search PubMed.
- J. Calderon, M. E. Navarro, M. E. Jimenez-Capdeville, M. A. Santos-Diaz, A. Golden, I. Rodriguez-Leyva, V. H. Borja-Aburto and F. Diaz-Barriga, Exposure to arsenic and lead and neuropsychological development in Mexican children, Environ. Res., 2001, 85, 69–76 CrossRef CAS.
- G. V. Iyengar and H. Kawamura, Dietary intakes of seven elements of importance in radiological protection by Asian population: comparison with ICRP data, Health Phys., 2004, 86, 557–564 CrossRef CAS.
- A. Jain, B. K. Agrawal, M. Varma and A. A. Jadhav, Antioxidant status and smoking habits: relationship with diet, Singapore Med. J., 2009, 50, 624–627 Search PubMed.
- S. Wani, Ahmad, F. Ahmad, S. A. Zargar, A. Pervaiz and H. Tak, Prevalence of intestinal parasites and associated risk factors among schoolchildren in Srinagar City, Kashmir, India, J. Parasitol., 2007, 93, 1541–1543 CrossRef.
- S. A. Sarker, L. Davidsson, H. Mahmud, T. Walczyk, R. F. Hurrell, N. Gyr and G. J. Fuchs, Helicobacter pylori infection, iron absorption, and gastric acid secretion in Bangladeshi children, Am. J. Clin. Nutr., 2004, 80, 149–153 CAS.
- E. I. Brima, R. O. Jenkins, P. R. Lythgoe, A. G. Gault, D. A. Polya and P. I. Haris, Effect of fasting on the pattern of urinary arsenic excretion, J. Environ. Monit., 2007, 9, 98–103 RSC.
- (a) D. Brugge, J. L. de Lemos and B. Oldmixon, Exposure pathways and health effects associated with chemical and radiological toxicity of natural uranium: a review, Reviews on Environmental Health, 2005, 20, 177–193 Search PubMed; (b) R. W. Leggett and J. D. Harrison, Fractional absorption of ingested uranium in humans, Health Phys., 1995, 68, 484–498 CrossRef CAS.
- M. Z. Islam, C. Lamberg-Allardt, M. Kärkkäinen and S. M. K. Ali, Dietary calcium intake in premenopausal Bangladeshi women: do socio-economic or physiological factors play a role?, Eur. J. Clin. Nutr., 2003, 57, 674–680 CrossRef CAS.
- J. E. D. de Oliveira, J. B. Ferreira, V. P. Vasconcellos and J. S. Marchini, Drinking water as an iron carrier to control anemia in preschool children in a day-care center, J. Am. Coll. Nutr., 1994, 13, 198–202.
- M. Z. Islam, C. Lamberg-Allardt, M. Kärkkäinen, T. Outila, Q. Salamatullah and A. A. Shamim, Vitamin D deficiency: a concern in premenopausal Bangladeshi women of two socio-economic groups in rural and urban region, Eur. J. Clin. Nutr., 2002, 56, 51–56 CrossRef CAS.
- Bangladesh Bureau of Statistics Local Estimation of Poverty and Malnutrition in Bangladesh; United Nations World Food Program: 2004.
- (a) S. M. Ahmed, A. Adams, A. M. Chowdhury and A. Bhuiya, Chronic energy deficiency in women from rural Bangladesh: some socioeconomic determinants, J. Biosoc. Sci., 1998, 30, 349–358 CrossRef CAS; (b) Y. Balarajan and E. Villamor, Nationally representative surveys show recent increases in the prevalence of overweight and obesity among women of reproductive age in Bangladesh, Nepal, and India, J. Nutr., 2009, 139, 2139–2144 CrossRef CAS; (c) M. Z. Islam, M. Akhtaruzzaman and C. Lamberg-Allardt, Nutritional status of women in Bangladesh: comparison of energy intake and nutritional status of a low income rural group with a high income urban group, Asia Pac. J. Clin. Nutr., 2004, 13, 61–68; (d) B. L. Pierce, T. Kalra, M. Argos, F. Parvez, Y. Chen, T. Islam, A. Ahmed, R. Hasan, M. Rakibuz-Zaman, J. Graziano, P. J. Rathouz and H. Ahsan, A prospective study of body mass index and mortality in Bangladesh, Int. J. Epidemiol., 2009 Search PubMed; (e) S. Shafique, N. Akhter, G. Stallkamp, S. de Pee, D. Panagides and M. W. Bloem, Trends of under- and overweight among rural and urban poor women indicate the double burden of malnutrition in Bangladesh, Int. J. Epidemiol., 2007, 36, 449–457 Search PubMed.
- D. N. Guha Mazumder, R. Haque, N. Ghosh, B. K. De, A. Santra and D. Chakraborty, Arsenic levels in drinking water and the prevalence of skin lesions in West Bengal, India, Int. J. Epidemiol., 1998, 27, 871–877 Search PubMed.
- (a) M. S. Pednekar, P. C. Gupta, H. C. Shukla and J. R. Hebert, Association between tobacco use and body mass index in urban Indian population: implications for public health in India, BMC Public Health, 2006, 6, 70 CrossRef; (b) T. Rastogi, P. Jha, K. S. Reddy, D. Prabhakaran, D. Spiegelman, M. J. Stampfer, W. C. Willett and A. Ascherio, Bidi and cigarette smoking and risk of acute myocardial infarction among males in urban India, Tob. Control, 2005, 14, 356–358 CrossRef CAS; (c) H. C. Shukla, P. C. Gupta, H. C. Mehta and J. R. Hebert, Descriptive epidemiology of body mass index of an urban adult population in western India, J. Epidemiol. Community Health, 2002, 56, 876–880 CrossRef CAS.
- S. V. Subramanian and G. D. Smith, Patterns, distribution, and determinants of under- and overnutrition: a population-based study of women in India, Am. J. Clin. Nutr., 2006, 84, 633–640 CAS.
- G. A. Wasserman, X. Liu, P. Factor-Litvak, J. M. Gardner and J. H. Graziano, Developmental impacts of heavy metals and undernutrition, Basic Clin. Pharmacol. Toxicol., 2008, 102, 212–217 Search PubMed.
- C. Watanabe, T. Matsui, T. Inaoka, T. Kadono, K. Miyazaki, M.-J. Bae, T. Ono, R. Ohtsuka and A. T. M. M. H. Bokul, Dermatological and nutritional/growth effects among children living in arsenic-contaminated communities in rural Bangladesh, J. Environ. Sci. Health A Tox. Hazard Subst. Environ. Eng., 2007, 42, 1835–1841 Search PubMed.
- (a) M. Argos, F. Parvez, Y. Chen, A. Z. M. I. Hussain, H. Momotaj, G. R. Howe, J. H. Graziano and H. Ahsan, Socioeconomic status and risk for arsenic-related skin lesions in Bangladesh, Am. J. Public Health, 2007, 97, 825–831 Search PubMed; (b) J. Brinkel, M. H. Khan and A. Kraemer, A systematic review of arsenic exposure and its social and mental health effects with special reference to Bangladesh, Int. J. Environ. Res. Public Health, 2009, 6, 1609–1619 Search PubMed; (c) A. Hadi and R. Parveen, Arsenicosis in Bangladesh: prevalence and socio-economic correlates, Public Health, 2004, 118, 559–564 CrossRef CAS.
- R. A. Lynch, D. T. Boatright and S. K. Moss, Lead-contaminated imported tamarind candy and children's blood lead levels, Public Health Rep., 2000, 115, 537–543 Search PubMed.
- (a) J. F. Kauffman, B. J. Westenberger, J. D. Robertson, J. Guthrie, A. Jacobs and S. K. Cummins, Lead in pharmaceutical products and dietary supplements, Regul. Toxicol. Pharmacol., 2007, 48, 128–134 CrossRef CAS; (b) W. R. Mindak, J. Cheng, B. J. Canas and P. M. Bolger, Lead in women's and children's vitamins, J. Agric. Food Chem., 2008, 56, 6892–6896 CrossRef CAS.
- J. K. Nduka and O. E. Orisakwe, Heavy metal hazards of pediatric syrup administration in Nigeria: a look at chromium, nickel and manganese, Int. J. Environ. Res. Public Health, 2009, 6, 1972–1979 Search PubMed.
- G. B. van der Voet, A. Sarafanov, T. I. Todorov, J. A. Centeno, W. B. Jonas, J. A. Ives and F. G. Mullick, Clinical and analytical toxicology of dietary supplements: a case study and a review of the literature, Biol. Trace Elem. Res., 2008, 125, 1–12 CrossRef CAS.
- (a) A. A. Abou-Arab and M. A. A. Donia, Heavy metals in Egyptian spices and medicinal plants and the effect of processing on their levels, J. Agric. Food Chem., 2000, 48, 2300–2304 CrossRef CAS; (b) S. Dey, A. Saxena, A. Dan and D. Swarup, Indian medicinal herb: a source of lead and cadmium for humans and animals, Arch. Environ. Occup. Health, 2009, 64, 164–167 Search PubMed.
- (a) J. G. Ayenimo, A. M. Yusuf, A. S. Adekunle and O. W. Makinde, Heavy metal exposure from personal care products, Bull. Environ. Contam. Toxicol., 2010, 84, 8–14 CrossRef CAS; (b) N. Z. Janjua, E. Delzell, R. R. Larson, S. Meleth, E. K. Kabagambe, S. Kristensen and N. Sathiakumar, Maternal nutritional status during pregnancy and surma use determine cord lead levels in Karachi, Pakistan, Environ. Res., 2008, 108, 69–79 CrossRef CAS; (c) C. Parry and J. Eaton, Kohl: a lead-hazardous eye makeup from the Third World to the First World, Environ. Health Perspect., 1991, 94, 121–123 CrossRef CAS.
- S. Oshikawa, A. Geater, V. Chongsuvivatwong and D. Chakraborti, Arsenic contamination of Ronphibun residents associated with uses of arsenic-contaminated shallow-well water other than drinking, J. Environ. Sci. Health A Tox. Hazard Subst. Environ. Eng., 2007, 42, 1753–1761 Search PubMed.
- B. A. Fowler and M. J. Sexton, in Heavy Metals in the Environment, ed. B. Sarkar, Marcel Dekker, Inc., New York, 2002, pp. 631–645 Search PubMed.
- (a) M. Vahter, Methylation of inorganic arsenic in different mammalian species and population groups, Sci. Prog., 1999, 82(Pt 1), 69–88 CAS; (b) D. S. Paul, A. Hernández-Zavala, F. S. Walton, B. M. Adair, J. Dedina, T. Matousek and M. Stýblo, Examination of the effects of arsenic on glucose homeostasis in cell culture and animal studies: development of a mouse model for arsenic-induced diabetes, Toxicol. Appl. Pharmacol., 2007, 222, 305–314 CrossRef CAS.
- World Health Organization (WHO) Manganese in drinking water. Background document for development of WHO Guide for drinking water quality; World Health Organization: 1994.
- W. Mertz, Risk assessment of essential trace elements: new approaches to setting recommended dietary allowances and safety limits, Nutr. Rev., 1995, 53, 179–185 Search PubMed.
- C. F. Wilkinson, G. R. Christoph, E. Julien, J. M. Kelley, J. Kronenberg, J. McCarthy and R. Reiss, Assessing the risks of exposures to multiple chemicals with a common mechanism of toxicity: how to cumulate?, Regul. Toxicol. Pharmacol., 2000, 31, 30–43 CrossRef CAS.
- (a) M. A. Callahan and K. Sexton, If cumulative risk assessment is the answer, Environ. Health Perspect., 2007, 115, 799–806 CrossRef CAS; (b) A. Kortenkamp, M. Faust, M. Scholze and T. Backhaus, Low-level exposure to multiple chemicals: reason for human health concerns?, Environ. Health Perspect., 2007, 115(Suppl 1), 106–114; (c) E. A. C. Hubal, J. Moya and S. G. Selevan, A lifestage approach to assessing children's exposure, Birth Defects Res., Part B, 2008, 83, 522–529 Search PubMed; (d) J. I. Levy, Is epidemiology the key to cumulative risk assessment?, Risk Anal., 2008, 28, 1507–1513 Search PubMed; (e) G. Rice, M. MacDonell, R. C. Hertzberg, L. Teuschler, K. Picel, J. Butler, Y.-S. Chang and H. Hartmann, An approach for assessing human exposures to chemical mixtures in the environment, Toxicol. Appl. Pharmacol., 2008, 233, 126–136 CrossRef CAS; (f) S. J. Ryker and M. J. Small, Combining occurrence and toxicity information to identify priorities for drinking-water mixture research, Risk Anal., 2008, 28, 653–666 Search PubMed.
- (a) G. W. Chance, Environmental contaminants and children's health: Cause for concern, time for action, Paediatr Child Health, 2001, 6, 731–743 Search PubMed; (b) C. Watanabe, T. Inaoka, T. Matsui, K. Ishigaki, N. Murayama and R. Ohtsuka, Effects of arsenic on younger generations, J. Environ. Sci. Health A Tox. Hazard Subst. Environ. Eng., 2003, 38, 129–139 Search PubMed.
- E. A. C. Hubal, L. S. Sheldon, J. M. Burke, T. R. McCurdy, M. R. Berry, M. L. Rigas, V. G. Zartarian and N. C. Freeman, Children's exposure assessment: a review of factors influencing Children's exposure, and the data available to characterize and assess that exposure, Environ. Health Perspect., 2000, 108, 475–486 CrossRef CAS.
- G. Daston, E. Faustman, G. Ginsberg, P. Fenner-Crisp, S. Olin, B. Sonawane, J. Bruckner, W. Breslin and T. J. McLaughlin, A framework for assessing risks to children from exposure to environmental agents, Environ. Health Perspect., 2004, 112, 238–256 CrossRef CAS.
- (a) D. Rice and S. J. Barone, Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models, Environ. Health Perspect., 2007, 108, 511S–533S; (b) R. O. Wright and A. Baccarelli, Metals and neurotoxicology, J. Nutr., 2007, 137, 2809–2812 CAS.
- (a) J. Liu, Y. Xie, J. M. Ward, B. A. Diwan and M. P. Waalkes, Toxicogenomic analysis of aberrant gene expression in liver tumors and nontumorous livers of adult mice exposed in utero to inorganic arsenic, Toxicol. Sci., 2004, 77, 249–257 CrossRef CAS; (b) M. P. Waalkes, J. Liu, J. M. Ward and B. A. Diwan, Enhanced urinary bladder and liver carcinogenesis in male CD1 mice exposed to transplacental inorganic arsenic and postnatal diethylstilbestrol or tamoxifen, Toxicol. Appl. Pharmacol., 2006, 215, 295–305 CrossRef CAS.
- A. Makri, M. Goveia, J. Balbus and R. Parkin, Children's susceptibility to chemicals: a review by developmental stage, J. Toxicol. Environ. Health B Crit. Rev., 2004, 7, 417–435 CrossRef CAS.
- J. A. Moreno, E. C. Yeomans, K. M. Streifel, B. L. Brattin, R. J. Taylor and R. B. Tjalkens, Age-dependent susceptibility to manganese-induced neurological dysfunction, Toxicol. Sci., 2009, 112, 394–404 CrossRef CAS.
- J. M. Borgoño, P. Vicent, H. Venturino and A. Infante, Arsenic in the drinking water of the city of Antofagasta: epidemiological and clinical study before and after the installation of a treatment plant, Environ. Health Perspect., 1977, 19, 103–105 CAS.
- G. Concha, B. Nermell and M. V. Vahter, Metabolism of inorganic arsenic in children with chronic high arsenic exposure in northern Argentina, Environ. Health Perspect., 1998, 106, 355–359 CrossRef CAS.
- D. N. G. Mazumder, Effect of drinking arsenic contaminated water in children, Indian Pediatr, 2007, 44, 925–927 Search PubMed.
- American Public Health Association, Standard Method for the Examination of Water and Wastewater, American Public Health Association, 21 edn, 2005.
- K. R. Neubauer, R. E. Wolf, 2004.
- (a) A. van Geen, K. M. Ahmed, A. A. Seddique and M. Shamsudduha, Community wells to mitigate the arsenic crisis in Bangladesh, Bull. World Health Organ., 2003, 81, 632–638; (b) N. Shibasaki, P. Lei and A. Kamata, Evaluation of deep groundwater development for arsenic mitigation in western Bangladesh, J. Environ. Sci. Health A Tox. Hazard Subst. Environ. Eng., 2007, 42, 1919–1932 Search PubMed.
- (a) S. H. Frisbie, E. J. Mitchell, A. Z. Yusuf, M. Y. Siddiq, R. E. Sanchez, R. Ortega, D. M. Maynard and B. Sarkar, The development and use of an innovative laboratory method for measuring arsenic in drinking water from western Bangladesh, Environ. Health Perspect., 2005, 113, 1196–1204 CrossRef CAS; (b) F. Parvez, Y. Chen, M. Argos, A. Z. M. I. Hussain, H. Momotaj, R. Dhar, A. van Geen, J. H. Graziano and H. Ahsan, Prevalence of arsenic exposure from drinking water and awareness of its health risks in a Bangladeshi population: results from a large population-based study, Environ. Health Perspect., 2006, 114, 355–359 CAS.
- M. M. Hira-Smith, Y. Yuan, X. Savarimuthu, J. Liaw, A. Hira, C. Green, T. Hore, P. Chakraborty, O. S. von Ehrenstein and A. H. Smith, Arsenic concentrations and bacterial contamination in a pilot shallow dugwell program in West Bengal, India, J. Environ. Sci. Health A Tox. Hazard Subst. Environ. Eng., 2007, 42, 89–95 Search PubMed.
- K. M. Lokuge, W. Smith, B. Caldwell, K. Dear and A. H. Milton, The effect of arsenic mitigation interventions on disease burden in Bangladesh, Environ. Health Perspect., 2004, 112, 1172–1177 CrossRef CAS.
- (a) M. R. Islam, P. W. Lahermo, R. Salminen, S. Rojstaczer and V. Peuraniemi, Lake and reservoir water quality affects by metals leaching from tropical soils, Environ. Geol., 2000, 39, 1083–1089 CrossRef CAS; (b) M. R. Islam, R. Salminen and P. W. Lahermo, Arsenic and other toxic elemental contamination of groundwater, surface water and soil in Bangladesh and its possible effects on human health, Environ. Geochem. Health, 2000, 22, 33–53 Search PubMed; (c) H. B. Bennett, A. Shantz, G. Shin, M. L. Sampson and J. S. Meschke, Characterisation of the water quality from open and rope-pump shallow wells in rural Cambodia, Water Sci. Technol., 2010, 61, 473–479 Search PubMed.
- K. Gopal, S. S. Tripathy, J. L. Bersillon and S. P. Dubey, Chlorination byproducts, their toxicodynamics and removal from drinking water, J. Hazard. Mater., 2007, 140, 1–6 CrossRef CAS.
- A. Mukherjee, A. Fryar and P. D. Howell, Regional hydrostratigraphy and groundwater flow modeling in the arenic-affected areas of the western Bengal basin, West Bengla, India, Hydrogeol. J., 2007, 15, 1397–1418 CrossRef CAS.
- S. J. Hug, O. X. Leupin and M. Berg, Bangladesh and Vietnam: different groundwater compositions require different approaches to arsenic mitigation, Environ. Sci. Technol., 2008, 42, 6318–6323 CrossRef CAS.
- L. Wang and J. Huang, in Arsenic in the Environment. Part II: Human Health and Ecosystem Effects, ed. J. O. Nriagu, Wiley, New York, 1994, pp. 159–172 Search PubMed.
- (a) D. Sutherland, P. M. Swash, A. C. Macqueen, L. E. McWilliam, D. J. Ross and S. C. Wood, A field based evaluation of household arsenic removal technologies for the treatment of drinking water, Environ. Technol., 2002, 23, 1385–1403 CrossRef CAS; (b) M. F. Ahmed .
- D. Mohan and C. U. Pittman, Arsenic removal from water/wastewater using adsorbents—A critical review, J. Hazard. Mater., 2007, 142, 1–53 CrossRef CAS.
- G. L. Amy, H.-W. Chen, A. Drizo and P. Brandhuber, Adsorbent Treatment Technologies for Arsenic Removal, Awwa Research Foundation, Denver, CO, 2005 Search PubMed.
- M. A. Hossain, M. K. Sengupta, S. Ahamed, M. M. Rahman, D. Mondal, D. Lodh, B. Das, B. Nayak, B. K. Roy, A. Mukherjee and D. Chakraborti, Ineffectiveness and poor reliability of arsenic removal plants in West Bengal, India, Environ. Sci. Technol., 2005, 39, 4300–4306 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2011 |
