Novel thalidomide analogues with potent NFκB and TNF expression inhibition†‡
Sing Yee
Yeung
a,
Sven
Kampmann
a,
Keith A.
Stubbs
a,
Brian W.
Skelton
b,
Belinda J.
Kaskow
a,
Lawrence J.
Abraham
a and
Scott G.
Stewart
*a
aThe School of Biomedical, Biomolecular & Chemical Sciences, The University of Western Australia, Crawley, WA 6009, Australia. E-mail: sgs@cyllene.uwa.edu.au; Fax: +61 (8) 6488 1005; Tel: +61 (8) 6488 3180
bCentre for Microscopy, Characterisation and Analysis, University of Western Australia, Crawley, WA 6009, Australia
First published on 7th September 2011
Abstract
A series of N-phenyl thalidomide analogues have been prepared efficiently through a Buchwald–Hartwig cross coupling reaction as the key step. Several of these compounds have extraordinary TNF expression inhibition compared to both thalidomide and the current pharmaceutical agent Revlimid (Lenalidomide).
The antiemetic agent thalidomide, [(R,S)-2-(2,6-dioxo-3-piperidinyl)-1H-isoindole-1,3(2H)-dione (1) (Fig. 1)] has had a tumultuous history which began when this compound was tragically administered in the 1950′s to pregnant woman as a treatment for insomnia and as an antiemetic agent. This drug was prescribed without a full understanding of the pharmacological profile of both enantiomers and later investigations found that while the R-isomer (at C3′) (R)-1 was responsible for a sedative response the S-isomer (S)-1 had teratogenic properties.1 As a result, in 1962 this commercial drug was withdrawn, although not before 10
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 infants with various limb malformations were born.
000 infants with various limb malformations were born.
 | ||
| Fig. 1 R,S-Thalidomide (1) and Revlimid (Lenalidomide) (2). | ||
Following these early findings, there was a period before this drug was subject to several other biological investigations as well as chemical derivatisation. An example of this recent progress in this area includes the FDA approval to use thalidomide (1), (Thalomid™) for the treatment of erythema nodosum leprosum (ENL). This use was based on results from earlier serendipitous treatment of leprosy patients in 1962 by Jacob Sheskin2 Thus, whilst having side effects, thalidomide (1) has potentially many useful pharmacological properties including anti-angiogenic effects. Work is underway investigating thalidomide and analogues to gain insight into the overall biological processes of this medicinal agent.1d
At present, this reborn drug is being evaluated in the treatment of more than 30 other conditions, including infectious, dermatological and autoimmune diseases.3–7 One of the most promising avenues of these later applications is the use of thalidomide (1) to treat the as yet incurable form of bone marrow cancer, multiple myeloma.1c, 1d
Possibly the largest studied biological processes influenced by thalidomide (1), is the inhibition of the expression of the pro-inflammatory cytokine, tumour necrosis factor (TNF).1cTNF is a central regulator of the inflammatory cascade controlling many effector pathways, including those that are anti-angiogenic, anti-inflammatory and immuno-modulatory. At the molecular level the mode of action of thalidomide (1) in suppressing TNF expression is thought to involve the inhibition of the nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB) signalling pathway and more specifically by inhibiting the activity of the IκB kinase, IKKα.8 However, the direct molecular interaction has yet to be confirmed, and determining the unknown molecular mode of action of thalidomide is considered of high priority for key structural modifications. Some of the early investigations with thalidomide-based molecular probes can be seen in ours and Handa's most recent work.9
The second generation and more potent thalidomide analogues, Revlimid™ (2) and Actimid™ (Fig. 1) discovered by Celgene10 in 1998 are currently in use or under clinical trials for several disease states. In certain biological assays, these new compounds demonstrated reduced toxicity compared to thalidomide (1).1d, 11,12 Several other groups, including ours, have also reported a range of new thalidomide analogues with potential medicinal properties in a variety of disease states.13 In many cases the large dose required of thalidomide (1) required for beneficial effects has inspired the development of new thalidomide analogues.1b, 14
As part of our ongoing studies into the relationship between thalidomide (1) and TNF expression, we decided to screen new analogues for enhanced ability to specifically inhibit the NFκB pathway. While many studies involving the inhibition of TNF production have been previously reported,13c, 15 they do not specifically address activity at our target NFκB. In this vein we have developed a more specific assay to determine the effects on the NFκB activation pathway, as a measure of immunomodulatory or anti-inflammatory activity.16
To effectively measure inhibition of NFκB pathway signalling by each analogue, a TNF transcriptional reporter cell line was used. The green fluorescent protein (GFP) reporter gene, under the control of the NFκB-responsive human TNF promoter, was inserted into the genome of the human T cell line (Jurkat) to generate the reporter line, FRT-Jurkat TNF, as previously described.9a, 13g, 13h, 16a As a measure of TNF promoter activity, GFP activity was quantified by flow cytometry. This method has the added advantage of being able to easily assess the cellular toxicity of each compound, (by comparing forward- and side-scatter as a measure of cellular size and granularity) during flow cytometry. The cell population in each assay that exhibited low granularity were considered to be not viable. This was confirmed by staining with propidium iodide, a fluorescent DNA-intercalating agent. All thalidomide derivatives were assayed in triplicate at concentrations of 10 μM and percentage inhibition of TNF expression (relative to TNF expression from solvent treated control cells) for each compound measured. Significant solvent effects at 1% dimethylsulfoxide (DMSO) had been previously reported, thus a 0.1% solution was used in this study. At this concentration, the solvent has very little effect on TNF reporter gene expression.
In our previous studies we have examined the aromatic region of thalidomide for the introduction of various functionalities, in many cases greatly improving thalidomide's TNF expression inhibition.9a,13g,13h A series of these new compounds contained the addition of substituted phenyl groups which, at 100 μM concentration, were potent compared to thalidomide (1). Additionally derivatives such as Revlimid (Lenalidomide, 2), and to a lesser extent Actimid, have a common amino functionality attached to the aromatic ring system. Thus, we decided to build analogues that would incorporate both the aforementioned functionalities, the N-phenyl-amino-thalidomides. These novel compounds could then be compared to both thalidomide (1) and Revlimid (Lenalidomide, 2) as indications for improvement in the inhibition TNF expression. Most importantly in this study we wanted to consider the activity of these compounds at low concentrations (10 μM) as some of the previously described analogues had accessed high apoptosis at 100 μM leading to poor cell viability as measured through flow cytometry.
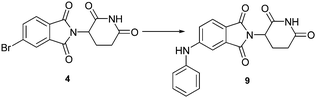
In this instance there were several synthetic approaches to compounds of this type. Either a copper catalysed Ullmann type coupling reaction17 or a palladium mediated Buchwald–Hartwig cross coupling reaction seemed to be the best approach. The latter amination, proposed by Buchwald and Hartwig, relies on a suitably active aryl halide to couple with an amine or amide.18 Thus, in a similar process to our previous reports, we required the halogenated thalidomide analogues (3 and 4). The preparation of such compounds have been described previously (Scheme 1).13g Two of the synthetic precursors for the C–N cross coupling substrates (5 and 6) are commercially available while the iodophthalimide (3) can be prepared in 3 steps from the commercially available 2,3-dimethyliodobenzene.13g, 13h, 19 The condensation partner, amine salt 8, can be prepared through known literature procedures.20
 | ||
| Scheme 1 Reagents and Conditions: (a) phthalic anhydride 8, NEt3, THF reflux, 48h, 66%; (b) 4-iodophthalic anhydride 6, NEt3, 8, THF, reflux, 88%; (c) 5-bromophthalic anhydride, NEt3, 8, THF, reflux, 72h, 68%. | ||
In the early stages of this study we were interested if the introduction of an aniline group was possible through cross coupling procedures or if this group had a dramatic influence on the TNF expression inhibition. Thus, in an initial amination reaction, we trialled catalytic systems including those containing XPhos, Pt-Bu3 and Hermann/Beller catalyst (HB)21 on both of our aryl halides. Even though these conditions were not fully optimised (48 and 7% yield) the reaction produced enough of the two compounds 9 and 10 for an initial TNF expression inhibition assay (Scheme 2). The dramatic improvement in activity for compounds 9 and 10 was revealed with a TNF expression inhibition of 17% and 16% respectively at 100 μM and 6 and 4% for 10 μM respectively. In comparison to that of thalidomide (1) (4% at 100 μM) and the relatively more active Revlimid (2) (6% at 100 μM) we decided to further investigate this dramatic improvement in the structure and prepare a series of other derivatives.
 | ||
| Scheme 2 Reagents and conditions: (a) Pd2(dba-4,4′-OMe)3, XPhos, K3PO4, toluene, 110 °C, 21 h, 48%; (b) Pd2(dba)3 (5 mol%), HP(t-Bu)3BF4 (14 mol%), Cy2NMe, toluene, 110 °C, 16 h, 7%. | ||
Fortunately, as part of this original study, we also were able to obtain crystal structures for both of these compounds (compound 10, Fig. 2). Crystal structures for thalidomide analogues are extremely uncommon in the literature. The structure of 4-phenylaminothalidomide 9 was also confirmed in the solid state with a final R value of 0.050.
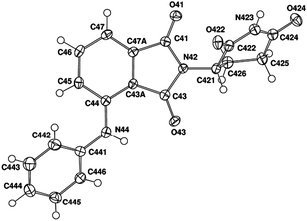 | ||
| Fig. 2 Structure of thalidomide derivative 10. The ellipsoids of the non-hydrogen atoms are drawn at the 50% probability level. | ||
By investigating several Buchwald–Hartwig cross coupling reaction parameters it was possible to generate a suitable general methodology for the coupling of aniline with 5-bromothalidomide (7) (Table 2). Following the trials of several unsuccessful catalysts such as H/B cat,21PdCl2dppf and Pd(OAc)2 with XPhos (Entries 1–3, Table 1), we decided to revert to more traditional C–N cross coupling conditions. The two Buchwald ligands (X-Phos and DavePhos) were explored further as well as the Pd/ligand ratio, catalyst loading, bases, solvent and temperature.
|
|
||||||
|---|---|---|---|---|---|---|
| Entry | Aniline(mol eq) | Catalyst,(mol %) | Phosphine (mol %) | Base (1.9 mol eq) | Solvent Temp | Yield |
| a All reactions were deoxygenated prior to heating to the specified temperature. b Herrmann–Beller catalyst (H/B Cat) is trans-Di(μ-acetato)-bis[o-(di-o-tolylphosphino)benzyl]dipalladium(II). | ||||||
| 1 | 1 | H/B Cat, (5) | — | n-Bu4NOAc | Dioxane | 0% |
| 2 | 1 | PdCl2(dppf) (10) | — | NaOt-Bu | Toluene | 0% |
| 3 | 1 | Pd(OAc)2 (10) | XPhos (10) | K3PO4 | Toluene | 0% |
| 4 | 1.9 | Pd2(dba)3, (4.9) | DavePhos, (9.5) | Cs2CO3 | Toluene, 110 °C | 4% |
| 5 | 1.9 | Pd2(dba)3, (4.9) | Xphos, (9.5) | Cs2CO3 | Toluene, 110 °C | 21% |
| 6 | 1.9 | Pd2(dba)3, (9.8) | XPhos,(19.0) | Cs2CO3 | Toluene, 110 °C | 18% |
| 7 | 1.9 | Pd2(dba)3, (4.9) | XPhos,(9.5) | Cs2CO3 | Toluene, 90 °C | 5% |
| 8 | 1.9 | Pd2(dba)3, (4.9) | XPhos, (9.5) | Cs2CO3 | Dioxane, 90 °C | 22% |
| 9 | 1.9 | Pd2(dba)3, (4.9) | XPhos, (9.5) | Cs2CO3 | Dioxane, 100 °C | 28% |
| 10 | 1.9 | Pd2(dba)3, (4.9) | XPhos, (19.0) | Cs2CO3 | Dioxane, 100 °C | 34% |
| 11 | 1.5 | Pd2(dba)3, (4.9) | XPhos, (19.0) | Cs2CO3 | Dioxane, 100 °C | 20% |
| 12 | 1.5 | Pd2(dba)3, (4.9) | XPhos, (19.0) | K2CO3 | Dioxane, 100 °C | 7 |
| 13 | 1.5 | Pd2(dba)3, (4.9) | XPhos, (19.0) | K3PO4 | Dioxane, 100 °C | 49% |
| 14 | 1.5 | Pd2(dba)3, (4.9) | XPhos, (19.0) | K2CO3 | Dioxane, 65 °C | 99% |
| 15 | 1.5 | Pd2(dba)3, (4.9) | XPhos, (19.0) | K2CO3 | THF, 65 °C | 95% |
| 16 | 1.5 | Pd2(dba)3, (4.9) | XPhos, (19.0) | K2CO3 | Toluene, 65 °C | 38% |
| 17 | 1.5 | Pd2(dba)3, (2.0) | XPhos,.(7.8) | K2CO3 | Dioxane, 90 °C | 97% |
A fivefold improvement in yield was observed by replacing the ligand DavePhos with XPhos (Entry 4 to 5, Table 1). The critical balance between the oxidative addition and reductive elimination to ensure effective catalytic turnover was explored22 by trialing catalyst, phosphine and temperature (Entries 3–7) and the alterations indicated that XPhos at the higher temperature was optimum. Following this, both dioxane (Entry 5 and 11, Table 1) and THF (Entry 12, Table 1) were trialled as solvents and consistently produced a three to fourfold enhancement in yield, as compared to toluene. Latter trials involving the use of K2CO3 with a reduced catalyst loading (Entry 17, Table 1), gave the desired amine 9 in an excellent 97% yield.23
Once the optimum conditions for the coupling of aniline and aryl bromide 4 were obtained (Pd2(dba)3, XPhos, K2CO3 and dioxane) these conditions were used for the coupling of a series of aniline derivatives. The yield for the Buchwald–Hartwig amination process in these electronically diverse coupling partners was exceptional (72–99%). These yields are exemplary, considering the other functionalities contained within the thalidomide N-heterocyclic skeleton. Each of these new derivatives was assayed in our TNF transcriptional reporter Jurkat cell line. The derivatives were initially selected based on possible receptor interactions, although several derivatives were assumed to vary water solubility or hydrophobicity. The earlier prepared derivative 9 was more potent than thalidomide (1) by approximately two fold at 10 μM however, this result fell just outside the (p < 0.05) confidence interval. The series of derivatives containing a p-substituent considered to be a linear extension away from the thalidomide core. Replacing a carbon with a nitrogen atom in the ring system, i.e. a 2-pyridyl substituent had no significant improvement in TNF expression activity. Likewise, adding both an N-methyl or simple substituents like a methyl ketone or hydroxyl group in the p-position of the aromatic ring attachment (Entries 5, 6 and 8, Table 2) had no significant improvement in activity. In each of these examples a hydrogen bond acceptor or donor was added to the N-phenyl thalidomide scaffold to potentially increase protein binding pocket interactions. The methyl ether 14 proved to be very active at 10 μM, however the additional activity could not be attributed to this electron donating bond acceptor characteristic because the corresponding phenol 15 was not active.
| Entry | Compound | R | TNF expression inhibitionc |
|---|---|---|---|
| a Each TNF expression inhibition assay was carried out in triplicate. b Unusually these compounds initiate an increase in the production of TNF. c p values confidence interval are indicated in brackets and are compared to thalidomide. | |||
| 1 | Thalidomide (1) | R = H | 2 |
| 2 | Revlimid (2) | — | 4 |
| 3 | 9 |

|
6 (1.4 × 10−2) |
| 4 | 11 |
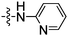
|
9 (3.6 × 10−3) |
| 5 | 12 |
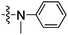
|
-2b (0.12) |
| 6 | 13 |

|
-2b (6.2 × 10−2) |
| 7 | 14 |

|
29 (1.3 × 10−5) |
| 8 | 15 |

|
4 (0.26) |
| 9 | 16 |

|
5 (0.12) |
| 10 | 17 |
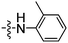
|
2 (0.69) |
| 11 | 18 |
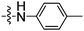
|
5 (2.2 × 10−2) |
| 12 | 19 |
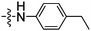
|
5 (6.0 × 10−2) |
| 13 | 20 |

|
11 (1.8 × 10−3) |
| 14 | 21 |

|
17 (1.3 × 10−2) |
| 15 | 22 |

|
10 (2.2 × 10−2) |
| 16 | 23 |

|
12 (1.8 × 10−2) |
As we observed improvements in thalidomide derivatives bearing hydrophobic groups in previous studies we thought a hydrophobic binding pocket in thalidomide's ultimate target was possible. In perusing this theory concerning hydrophobic groups in this portion of the molecule we targeted such compounds synthetically.13g Initially it was found that the p-position of the aromatic ring was more favourable than the o-position (Entries 10 and 11, Table 2). Following this, increasing the hydrophobicity of the attachment through each iteration (Entries 12 to 16, Table 2) revealed a similar trend in the TNF expression inhibition. This improvement was highly encouraging because a trend was developing even at these low concentrations (10 μM). In the case of both the t-butyl and the n-propyl N-phenyl derivatives (Entries 14 and 15, Table 2) we observed a 4 and 2.5 fold increase in the inhibition respectively, without any effect on cell viability. In general the cell viability for the compounds at 10 μM, as measure through flow cytometry, was consistently between 87 and 94%. Therefore, from these series of compounds, it is clear that a hydrophobic tether on the N-phenyl group will bring about a clear improvement in activity. Compounds (18–23) are more lipophilic and this issue that cannot be discounted when considering their mode of action. In an effort to determine if the TNF expression inhibition activity was also more pronounced at higher concentrations in the more active analogues, compounds 21 and 23 were tested at 100 μM. At this concentration both analogues were highly potent (89 and 93% respectively) however, poorer cell viability accompanied this potency. As both thalidomide derivatives Revlimid (2) and Actimid have their amino functionalities in the C4 position a series of derivatives with an amine in this position was also prepared. Additionally, this would also determine if the position of the hydrophobic group was essential for their effectiveness. This would potentially validate if a potential hydrophobic effect with a receptor site or if the overall polarity of the molecule was the major influence in potency.
Reaction optimisation for the generation of C4 analogues was slightly more complex given the more steric nature of this position. The optimum reaction conditions for this position (Pd2(dba)3 (5 mol %), Xphos (20 mol %), K2CO3, dioxane, 90 °C) allowed for a clean C–N bond formation in yields ranging from 35–92%, depending on the bulk of the aniline (Table 3).
| Entry | Compound | R | TNF expression inhibitionb |
|---|---|---|---|
| a Each TNF expression inhibition assay was carried out in triplicate. b p values confidence interval are indicated in brackets and are compared to thalidomide. | |||
| 1 | Thalidomide (1) | R = H | 2 |
| 2 | Revlimid (2) | — | 4 |
| 3 | 10 |
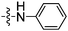
|
4 0.42 |
| 4 | 24 |

|
1 (0.33) |
| 5 | 25 |

|
2 (0.88) |
| 6 | 26 |

|
13 (3.1 × 10−4) |
| 7 | 27 |

|
4 (0.21) |
| 8 | 28 |
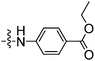
|
10 (0.069) |
| 9 | 29 |

|
7 (3.8 × 10−2) |
| 10 | 30 |

|
9 (1.2 × 10−3) |
| 11 | 31 |
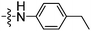
|
17 (4.2 × 10−4) |
| 12 | 32 |

|
19 (3.8 × 10−2) |
| 13 | 33 |

|
36 (1.3 × 10−3) |
| 14 | 34 |

|
23 (1.8 × 10−3) |
| 15 | 35 |

|
23(6.4 × 10−5) |
The TNF expression inhibition of the pyridine derivative and the compounds containing para-substituted polar functional groups were mixed (Entries 4 to 8, Table 3). The hydrogen bond acceptors, para-methoxyphenyl 26 and the ester 28, both generated good TNF expression inhibition. The potency of compounds containing hydrophobic tethers followed the same trends as in the C5 series, however in this series TNF expression inhibition was more pronounced. Substitution at the para-position was once again more favoured (Entries 9 and 10, Table 3) in the case of a methyl substituent. Increasing the length and the shape of the non polar side chain also brought about highly favourable results, for example adding an additional methylene unit (Entry 11, Table 3) provided a derivative with 17% TNF expression inhibition, almost double that of the phenylmethyl derivative 30.
Again, increasing the hydrophobicity and size of the p-substituent increases the TNF expression inhibition (Entries 12 and 13, Table 2). Significantly, the derivative 33 containing a t-butyl group had a nine fold increase in TNF expression inhibition to that of Revlimid (2). The final two compounds 34 and 35 (Entries 14 and 15, Table 3) have also retained the activity seen in this hydrophobic series of compounds further strengthening our hypothesis for this alkyl group requirement. This new series of C4 substituted compounds is far more active in our expression assays than both thalidomide (1), Revlimid (2) and many of the other previously prepared compounds within this group. Again the cell viability of these compounds was excellent at 10 μM (89–94%) Additionally, in these examples each C4 derivative is more active than their C5 counterpart, except compound 26, which suggests that the position of the hydrophobic substitution is important. Interestingly, it is obvious that the decreased water solubility in compounds containing a hydrophobic tail is not adversely influencing the TNF expression inhibition. The mode of action or the molecular interactions of these derivatives is speculated to be enhanced through a hydrophobic binding pocket in an unknown active site. Furthermore the improved lipophilicity of this series of compounds opens up a potentially more efficient transport pathway.
Conclusions
In this article we have described the synthesis of a series of thalidomide-derived compounds containing an amino phenyl group. This series of compounds has been prepared through a highly efficient Buchwald–Hartwig amination of the respective halogenated thalidomides. A group of these compounds, bearing a p-hydrophobic group, have been found to provide highly potent inhibitors of TNF expression. The most active derivative compound 33 is nine-fold more active than current therapeutical agent Revlimid (2) and eighteen fold more than thalidomide itself. The use of our described assay provides an excellent guide into NFκB inhibition and corresponding TNF expression inhibition. We are currently investigating the use of several of these compounds in other cell lines including multiple myeloma.References and notes
- (a) R. Brynner, T. Stephens, Dark Remedy, Perseus, New York, 2001 Search PubMed; (b) D. Ribatti and A. Vacca, Leukemia, 2005, 19, 1525 CrossRef CAS; (c) J. B. Bartlett, K. Dredge and A. G. Dalgleish, Nat. Rev. Cancer, 2004, 4, 314 CrossRef CAS; (d) M. Melchert and A. List, Int. J. Biochem. Cell Biol., 2007, 39, 1489 CrossRef CAS.
- J. Sheskin, Clin. Pharmacol. Ther., 1962, 6, 303.
- O. Gutierrez-Rodriguez, Arthritis Rheum., 1984, 27, 1118 CrossRef CAS.
- M. H. Hamza, Clin. Rheumatol., 1986, 5, 365 CrossRef CAS.
- M. E. Franks, G. R. Macpherson and W. D. Figg, Lancet, 2004, 363, 1802 CrossRef CAS.
- P. Baas, W. Boogerd, O. Dalesio, A. Haringhuizen, F. Custers and N.v. Zandwijk, Lung Cancer, 2005, 48, 291 CrossRef.
- I. Zidi, S. Mestiri, A. Bartegi and N. Amor, Med. Pediatr. Oncol., 2010, 27, 185 CAS.
- (a) E. J. Carcache de-Blanco, B. Pandit, Z. Hu, J. Shi, A. Lewis and P.-K. Li, Bioorg. Med. Chem. Lett., 2007, 17, 6031 CrossRef CAS; (b) J. A. Keifer, D. C. Guttridge, B. P. Ashburner and A. S. Baldwin, J. Biol. Chem., 2001, 276, 22382 CrossRef CAS.
- (a) S. G. Stewart, C. J. Braun, M. E. Polomska, M. Karimi, L. J. Abraham and K. A. Stubbs, Org. Biomol. Chem., 2010, 8, 4059 RSC; (b) T. Ito, H. Ando and H. Handa, Cell. Mol. Life Sci., 2011, 68, 1569 CrossRef CAS.
- Celgene Corporation, http://www.celgene.com/.
- G. W. Muller, R. Chen, S.-Y. Huang, L. G. Corral, L. M. Wong, R. T. Patterson, Y. Chen, G. Kaplan and D. I. Stirling, Bioorg. Med. Chem. Lett., 1999, 9, 1625 CrossRef CAS.
- (a) J. B. Bartlett, K. Dredge and A. G. Dalgleish, Nat. Rev. Cancer, 2004, 4, 314 CrossRef CAS; (b) F. A. Luzzio and W. D. Figg, Expert Opin. Ther. Pat., 2004, 14, 215 Search PubMed.
- (a) M. Li, W. Sun, Y.-p. Yang, B. Xu, W.-y. Yi, Y.-x. Ma, Z.-j. Li and J.-r. Cui, Acta Pharmacol. Sin., 2008, 30, 134 Search PubMed; (b) H. Sano, T. Noguchi, A. Tanatani, H. Miyachi and T. Hashimoto, Chem. Pharm. Bull., 2004, 52, 1021 CrossRef CAS; (c) A. L. Machado, L. M. Lima, J. X. Araújo-Jr, C. A. M. Fraga, V. L. Gonçalves Koatz and E. J. Barreiro, Bioorg. Med. Chem. Lett., 2005, 15, 1169 CrossRef CAS; (d) L. M. Lima, C. A. M. Fraga, V. L. G. Koatz and E. J. Barreiro, Curr. Med. Chem. Anti Inflamm. Anti Allergy Agents, 2006, 5, 79 Search PubMed; (e) S. Inatsuki, T. Noguchi, H. Miyachi, S. Oda, T. Iguchi, M. Kizaki, Y. Hashimoto and H. Kobayashi, Bioorg. Med. Chem. Lett., 2005, 15, 321 CrossRef CAS; (f) H. Miyachi, A. Ogasawara, A. Azuma and Y. Hashimoto, Bioorg. Med. Chem., 1997, 5, 2095 CrossRef CAS; (g) S. G. Stewart, C. J. Braun, S.-L. Ng, M. E. Polomska, M. Karimi and L. J. Abraham, Bioorg. Med. Chem., 2010, 18, 650–662 CrossRef CAS; (h) S. G. Stewart, D. Spagnolo, M. E. Polomska, M. Sin, M. Karimi and L. J. Abraham, Bioorg. Med. Chem. Lett., 2007, 17, 5819 CrossRef CAS.
- Y. Hashimoto, Arch. Pharm., 2008, 341, 536 CrossRef CAS.
- (a) H. Miyachi, A. Azuma, A. Ogasawara, E. Uchimura, N. Watanabe, Y. Kobayashi, F. Kato, M. Kato and Y. Hashimoto, J. Med. Chem., 1997, 40, 2858 CrossRef CAS; (b) T. Nakamura, T. Noguchi, H. Kobayashi, H. Miyachi and Y. Hashimoto, Chem. Pharm. Bull., 2006, 54, 1709 CrossRef CAS; (c) C. Chaulet, C. Croix, D. Alagille, S. Normand, A. Delwail, L. Favot, J.-C. Lecron and M.-C. Viaud-Massuard, Bioorg. Med. Chem. Lett., 2011, 21, 1019 CrossRef CAS.
- (a) M. Karimi, L. C. Goldie, D. Ulgiati and L. J. Abraham, BioTechniques, 2007, 42, 217 CrossRef CAS; (b) Z. Yan, D. Lei-Butters, J. Engelhardt and G. Leno, BMC Biol., 2009, 7, 1 CrossRef.
- F. Monnier and M. Taillefer, Angew. Chem., Int. Ed., 2009, 48, 6954 CrossRef CAS.
- D. S. Surry and S. L. Buchwald, Angew. Chem., Int. Ed., 2008, 47, 6338 CrossRef CAS.
- C. Soucy, D. Favreau and M. M. Kayser, J. Org. Chem., 1987, 52, 129 CrossRef CAS.
- (a) S. M. Capitosti, T. P. Hansen and M. L. Brown, Org. Lett., 2003, 5, 2865 CrossRef CAS; (b) G. W. Muller, W. E. Konnecke, A. M. Smith and V. D. Khetani, Org. Process Res. Dev., 1999, 3, 139 Search PubMed.
- W. A. Herrmann, C. Brossmer, K. Öfele, C. P. Reisinger, T. Priermeier, M. Beller and H. Fischer, Angew. Chem., Int. Ed. Engl., 1995, 34, 1844 CrossRef CAS.
- V. T. Abaev and O. V. Serdyuk, Russ. Chem. Rev., 2008, 77, 177 Search PubMed.
- General Procedure for C–N cross coupling: Bromothalidomide 4 (0.59 mmol), Pd2(dba3)·CHCl3 (0.012 mmol), 2-dicyclohexylphosphino-2′,4′,6′-triisopropylbiphenyl (0.046 mmol), K2CO3 (1.1 mmol) were added into an oven dried schlenk tube with a magnetic stirrer and evacuated plus backfilled using argon 3 times. The corresponding aniline (0.89 mmol) and 1,4-dioxane (2 mL, anhydrous) were added to the reaction mixture and the ensuing mixture was left to stir at 90 °C for 18 h. The resulting reaction mixture was fused onto silica and purified using flash column chromatography using EtOAc/hexane as described in the supplemantary infomation. The resulting thalidomide analogue was subsequently recrystalised from EtOAc. Note: if the required substituted aniline is a solid this coupling parner was added in the early stages with compound 4.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental details. CCDC reference numbers 834073 and 834074. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c1md00184a |
| ‡ Crystal data: Compound 9. C19H15N3O4, M = 349.34, monoclinic, space groupP21/c, a = 8.0529(6), b = 16.0380(8), c = 12.8344(7) Å, β = 101.335(6)°, V = 1625.26(17) Å3, Z = 4, T = 100 K, μ = 0.103 mm−1, reflections measured = 17482, independent reflections = 5757 (R(int) = 0.0447), R1(I > 2σ(I)) = 0.0495, wR2 (all data) = 0.117. CCDC 834074 |
| This journal is © The Royal Society of Chemistry 2011 |