Towards biocompatible nanovalves based on mesoporous silica nanoparticles
Ying-Wei
Yang
*
State Key Laboratory of Supramolecular Structure & Materials, College of Chemistry, Jilin University, Changchun, 130012, P. R. China. E-mail: ywyang@jlu.edu.cn; Fax: +86 431 8515 3812; Tel: +86 431 8515 3810
First published on 30th August 2011
Abstract
Over the past two decades, cancer has ascended to become the number one or two cause of death in many nations worldwide. Encapsulation of anticancer drugs within nanocarriers that selectively target diseased cells promises to increase the effectiveness of conventional chemotherapy and decrease its side effects. Nanoparticles show great potential as superior intelligent drug delivery platforms. Among them, mesoporous silica nanoparticles (MSNs) are particularly interesting candidates for powerful drug carriers because of their unique characteristics and abilities to efficiently and specifically entrap cargo molecules. The biggest challenge in current development is the design and synthesis of controlled biocompatible nanovalves or cap systems on MSNs to realize “zero premature release” of drugs and targeted delivery of anticancer drugs in a controlled fashion for “smart” cancer therapies. This review article evaluates drug delivery systems which comprise MSNs functionalized with well-defined self-assembled layers of biocompatible molecular and supramolecular nanovalves based on (supra)molecular switches, polymers, and biomolecules. Recent research progress on MSN-based smart materials that can simultaneously address targeted delivery of anticancer drugs, ideally “zero premature release”, and controlled release by external physical, chemical and biological stimuli will be highlighted and discussed.
 Ying-Wei Yang | Ying-Wei Yang received his Ph.D. at Nankai University in 2005 (with Professor Yu Liu). He then worked with Professor Fraser Stoddart at the University of California, Los Angeles (UCLA) as a postdoctoral fellow (2006–2008), and conducted research in the field of organic chemistry and material science, with emphasis on biocompatible nanovalves based on mesoporous silica for biomedical applications and mechanically interlocked molecule-based nanomaterials for NEMS. He is now Associate Professor of Chemistry at Jilin University, China. He is the author of more than 30 journal papers. His current research interests include molecular recognition and self-assembly, drug delivery for cancer therapy, molecular machinery, and green chemistry. |
1. Introduction
Mesoporous silica nanoparticles (MSNs) possess many excellent characteristics, such as good biocompatibility, rigidity, chemical stability, optical transparency, high surface areas, large pore volumes, uniform and tunable pore sizes, controllable surface functionalization, and resistance to microbial attack.1–8 In addition, MSNs effectively protect loaded cargo molecules against enzymatic degradation or denaturation induced by external environmental changes because no swelling or porosity changes occur as a response to external stimuli, such as pH and temperature, etc. Furthermore, MSNs can be flexibly surface-modified with a variety of organic functionalities, such as thiols, amines, carboxylic acids, alkoxy groups, and aromatic groups by the post-synthesis grafting or co-condensation (direct synthesis) sol–gel methods, resulting in highly useful organic–inorganic hybrid materials,9–13 which are considered to be promising nanocarriers and ideal scaffolds for targeted drug delivery such as “smart” cancer therapies.14–23On the other hand, macrocyclic synthetic receptors24–27 have proven to be superior candidates in the field of molecular recognition and self-assembly, mimicking the interactions between substrate and enzyme.28–33 Of which, crown ethers and cyclophanes, are very promising in terms of the construction of dynamic nanosystems, such as molecular machines, for different applications.10,34–47 Stoddart, Zink and co-workers have successfully combined these functional organic materials with the above mentioned inorganic nanoparticles and shown a series of supramolecular and molecular nanovalves based on crown ether- and cyclophane-[2]pseudorotaxanes48 and bistable [2]rotaxanes,49,50 which can regulate the release of cargo molecules from nanopores of MSNs, and operate under a range of external stimuli including pH,51,52 competitive binding,52 light,53–55 and redox control,56–58etc. These stimuli-responsive systems mostly rely upon donor–acceptor and hydrogen-bonding interactions between the macrocycle and stalk components.59–62 The nanovalve-based controlled release profile can be applied not only to sustained release but also for rapid release in the case of emergency or targeted delivery and release.
Nanovalves are a class of machines constructed from a moving part, which consist of a molecule that slides along a stalk molecule or binds to targets installed around MSN pores. The movable components act as gatekeepers at the entrances and exits to the nano-reservoirs of MSNs, regulating the transport of cargo molecules in and out of these reservoirs. When the movable component is positioned at the pore entrance it blocks cargo molecules from entering or escaping from the interior of the reservoirs, but when it moves off the thread or to a position far away from the nanopores, the cargo molecules can move into or out of the reservoirs.59,60 However most of the early designs of nanovalves are limited largely to use in organic solvents.
Recently, much effort has been dedicated to moving research on nanovalve-based targeted drug delivery systems from abiotic demonstrations to biological uses.18 Biocompatibility of both the nanoparticles and nanovalve components is of primary concern in these delivery systems. It has been proven that the functionalized MSNs with suitable particle sizes have desired properties under physiological conditions, are compatible with cells, and can be taken up by diseased cells.1,14,60 Experimental results have shown that MSNs were able to deliver water-insoluble anticancer drugs—camptothecinetc.—into different types of human cancer cells on demand. But for nanovalves to be viable in biological environments, recognition and binding motifs which operate in biological media have to be identified and tested. In recent years, tremendous progress has been made towards increasing the materials' biocompatibility, as well as the controlled release profile of such nanovalve systems, all witnessing the great potential of being practically useful for therapeutic treatment.1,15–18,60,61 For example, work was carried out with biocompatible stalks/caps in pseudorotaxanes and polymeric/biomolecule gates, which was shown to be a promising and efficient route toward achieving “zero premature release” and establishing functional targeted drug delivery, as compared with bare MSNs. In this article, we highlighted the recent progress and research efforts that have been made on the evolution of the surface functionalization of inorganic MSNs with selected biocompatible valve components, the sophisticated release mechanisms employed to unload cargo molecules from these systems on demand, and the drug delivery applications of the gated organic–inorganic hybrid materials in vitro. Since there are many excellent research accomplishments in the area of MSN-based drug delivery systems, our focus will be from a material construction and cancer treatment point of view even though the trends discussed are of a general nature applicable to any nanomedical and nanomechanical approach.
2. Biocompatible nanovalves based on cucurbit[n]uril (CB[n]) and cyclodextrin (CD)
Water soluble, non-toxic synthetic macrocyclic receptors, such as CB[n]63–66 and CD,67–78 have been of particular interest as a consequence of their highly distinctive range of physical and chemical properties and their ability to form stable inclusion complexes, which can be reversibly dissociated by external stimuli, with a variety of guest molecules in aqueous solutions. These families of synthetic macrocyclic receptors offer several advantages as drug delivery agents, given their additional ability (i) to protect the drug from physical, chemical, and enzymatic degradation and (ii) to enhance cell-membrane permeability. So, they can be employed in aqueous solutions for the controlled release of cargo molecules using nanovalves as the key components on MSNs. These two types of donut-shaped rings are both capable of acting as recognition tori during the template-directed syntheses of mechanically interlocked molecules, including molecular switches and machines. Both macrocycles are soluble in water or aqueous solutions in the presence of salts or acids, and are biologically benign, making them ideal building blocks for incorporation into integrated systems for biological applications.63–74,79CB[n] (n = 5–10) are macrocyclic cation receptors and consist of nglycoluril units joined by pairs of methylene bridges to form a rigid and highly symmetric structure with an annular and hydrophobic cavity and two highly polar carbonyl portals. The nonpolar interior and carbonyl-lined portals of CB[n] receptors could bind tightly and selectively to organic cations and small guests in aqueous solutions by including the hydrophobic portion of the guest within the apolar cavity and stabilizing the cationic group(s) with the portal oxygens.63–65,80 CDs are cyclic oligosaccharides containing six, seven, or eight α-1,4-linked D-glucopyranosyl residues, called α, β, and γ-CD, respectively, which are able to form stable host–guest complexes with hydrophobic molecules given the unique nature imparted by their structures.67–69,74 Both of these gate molecules are capable, in aqueous environments, of blocking the pores of MSNs, thus preventing the cargo molecules from escaping from inside of the MSNs until they are released from the stalks or positioned far away from the pore entrances by sliding under certain external stimuli. In this section, we will highlight some recent advances of (supra)molecular nanovalves based on CB[n] and CDs.
2.1. CB[6]-based nanovalves
CB[6] is a six-membered pumpkin-shaped polymacrocycle, with D6h symmetry, in the CB[n] family of biocompatible, synthetic receptors.81,82 Of particular interest in the field of supramolecular chemistry and the materials field is the ability of CB[6] to form stable 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 inclusion complexes with a variety of polymethylene derivatives, especially protonated diaminoalkanes.83,84 The pH-dependent complexation/decomplexation behavior of CB[6] with diaminoalkanes enabled the preparation of dynamic supramolecular entities which can be controlled by pH.63,66,85–87
1 inclusion complexes with a variety of polymethylene derivatives, especially protonated diaminoalkanes.83,84 The pH-dependent complexation/decomplexation behavior of CB[6] with diaminoalkanes enabled the preparation of dynamic supramolecular entities which can be controlled by pH.63,66,85–87
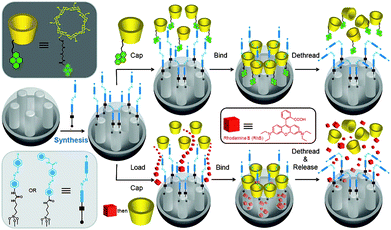
Taking advantage of the particular ability88–90 of CB[6] to catalyze 1,3-dipolar cycloadditions,63,91–97 Stoddart and we firstly constructed a pH-responsive nanovalve system based on [2]pseudorotaxanes consisting of bisammonium stalks and CB[6] rings on the surface of MSNs by the reaction between an azide-substituted ammonium ion and an alkyne-containing ammonium ion (Fig. 1).98 The pH-dependent binding of the bisammonium stalks and CB[6] was exploited to control the release of guest molecules, i.e., Rhodamine B (RhB), from the pores of the silica nanoparticles. At neutral and acidic pH values, the CB[6] rings encircle the bisammonium stalks tightly, thereby blocking the pores of MSNs efficiently when employing tethers of suitable lengths. Consequently, the cargo molecules (RhB) are released, as evidenced by fluorescence spectroscopy, upon deprotonation of the ammonium stalks at pH > 10. These biocompatible nanovalve systems rely on ion–dipole interactions between the CB[6] rings and the bisammonium stalks on MSNs and can be operated quite simply by raising and lowering the pH value of the system.
![pH-responsive supramolecular nanovalves based on CB[6]/bisammonium [2]pseudorotaxanes. The alkyne-functionalized MSNs are loaded with RhB molecules, and then capped with CB[6]-based [2]pseudorotaxanes by a “click” reaction under neutral to acidic pH. Upon deprotonation of the –CH2NH2+CH2– centers at pH >10, the CB[6] rings dethread from the bisammonium stalks and the RhB molecules are released. Copyright 2008 Wiley. Used with permission from ref. 98.](/image/article/2011/MD/c1md00158b/c1md00158b-f1.gif) | ||
| Fig. 1 pH-responsive supramolecular nanovalves based on CB[6]/bisammonium [2]pseudorotaxanes. The alkyne-functionalized MSNs are loaded with RhB molecules, and then capped with CB[6]-based [2]pseudorotaxanes by a “click” reaction under neutral to acidic pH. Upon deprotonation of the –CH2NH2+CH2– centers at pH >10, the CB[6] rings dethread from the bisammonium stalks and the RhB molecules are released. Copyright 2008 Wiley. Used with permission from ref. 98. | ||
Later on, we put a lot of efforts into increasing the biocompatibility of the above CB[6]/bisalkylammonium [2]pseudorotaxane and developed tunable pH-operable nanovalves based on MSNs, in which biologically relevant pH changes are used to trigger the release of cargo molecules. This enables the development of CB[6]-based nanovalves for in vivo applications using the natural variations in pH that exist within healthy and diseased cells in living systems. In a typical design (Fig. 2),99 we developed nanovalves consisting of bistable CB[6]/trisammonium pseudorotaxanes that are attached to MSN surfaces and operate to trap propidium iodide (PI) cargo molecules at neutral pH and then release the cargos under mildly acidic conditions. The opening and closing of this novel nanovalve system relies on the shuttling of the CB[6] rings on the stalks upon lowering the pH. This is due to the difference in the binding affinity of CB[6] with NH3+(CH2)6NH3+ and NH3+(CH2)4NH3+, where the former is an order of magnitude greater than the latter. Since the six-carbon spacer is a better match with the inner cavity of the CB[6] ring, it allows stronger ion–dipole interactions between the CB[6] ring and the ammonium center. More importantly, the release rate of cargo molecules can also be adjusted by the modification of the pKa of the anilinium nitrogen by changing the aniline groups with p-anisidines. For the nanovalves with aniline, only 13% of the entrapped cargo molecules are released after 600 s, compared to 50% for that with p-anisidines. The operation of this type of nanovalves based on CB[6] demonstrates that the pH sensitivity of these nanovalves can be fine-tuned by chemical modification of the stalks. In addition, these nanovalves can also be operated by changing the pH to a high value (>10), at which the two alkylammonium centers are deprotonated and the ion–dipole interactions between the stalks and CB[6] rings are disrupted, and thus realize the de-threading of the rings, the opening of the nanopores and the release of the cargo molecules.
![Acid-responsive supramolecular nanovalves based on bistable CB[6]/trisammonium [2]pseudorotaxanes. These nanovalves operate by encapsulating PI molecules inside the pores of MSNs at neutral pH (b), but release them when the pH is either lowered (a) or elevated (c). (a) Upon lowering the pH, the anilinium nitrogen is protonated and the CB[6] rings shuttle to the upper and middle ammonium centres (the distal hexamethylenediammonium station). (b) At neutral pH, the CB[6] rings remain on the lower ammonium centres (the tetramethylenediammonium station), blocking the nanopores. (c) These nanovalves can also operate upon elevating the pH to deprotonate all the nitrogen centers on the stalk to result in dethreading of the CB[6] rings from the trisamine stalks. Reprinted with permission from ref. 99. Copyright 2009 American Chemical Society.](/image/article/2011/MD/c1md00158b/c1md00158b-f2.gif) | ||
| Fig. 2 Acid-responsive supramolecular nanovalves based on bistable CB[6]/trisammonium [2]pseudorotaxanes. These nanovalves operate by encapsulating PI molecules inside the pores of MSNs at neutral pH (b), but release them when the pH is either lowered (a) or elevated (c). (a) Upon lowering the pH, the anilinium nitrogen is protonated and the CB[6] rings shuttle to the upper and middle ammonium centres (the distal hexamethylenediammonium station). (b) At neutral pH, the CB[6] rings remain on the lower ammonium centres (the tetramethylenediammonium station), blocking the nanopores. (c) These nanovalves can also operate upon elevating the pH to deprotonate all the nitrogen centers on the stalk to result in dethreading of the CB[6] rings from the trisamine stalks. Reprinted with permission from ref. 99. Copyright 2009 American Chemical Society. | ||
For drug-delivery applications, Stoddart, Zink and we recently reported two dual-controlled systems, which introduce a method for attaining even more sophisticated levels of controlled release.100 Two different types of molecular machines, i.e., azobenzene-based photo-responsive nanoimpellers and pH-responsive nanovalves, which function independently as controlled release mechanisms, are tethered to the inner pore walls and around the nanopores of MSNs. Only when both machines are activated in tandem to one another, the cargo molecules are released (Fig. 3). Two different nanovalves are employed in these drug-delivery systems, based on pH-switchable [2]pseudorotaxanes in which CB[6] rings encircle the bis-ammonium stalks which are tethered to the outer rim of the nanopores. One of the systems is base-responsive which consists of CB[6]/bisalkylammonium [2]pseudorotaxanes; the other one is acid-responsive which consists of bistable CB[6]/tris-ammonium [2]pseudorotaxanes. Both dual-controlled MSN systems behave as AND logic gates. Positive output is obtained only when the dynamic wagging motion of the azobenzenes of the nanoimpellers—resulting from the cis–transisomerization of the azobenzenes—is activated by a 448 nm excitation beam and followed by a pH change, whereupon the CB[6] rings dethread from the bis-ammonium stalks, thus open the nanopores and release the cargo molecules. By having one release mechanism operate in the presence of a specific biological trigger and having another release mechanism externally controlled, it might be possible to manually regulate the dosage delivered to a specific region.
 | ||
| Fig. 3 Dual-controlled MSN systems composed of both nanoimpellers and pH-responsive nanovalves, exhibiting AND logic. These systems operate only when both molecular machines operate in tandem to one another. Two different types of systems were developed to operate at either acidic (left-hand side) or basic (right-hand side) pH. Reprinted with permission from ref. 100, Copyright 2009 American Chemical Society, and ref. 60, Copyright 2009—Reproduced by permission of The Royal Society of Chemistry. | ||
Stoddart and Zink et al.101 reported another pH-responsive nanovalve system gated by semi-rotaxanes of CB[6] and a viologen thread containing terminal carboxylic acid. The MSN-based nanovalve system operates by pH changes in aqueous medium, whereby CB[6] used as capping agents can either encase (pH < 5) or release (pH > 5) the cargo molecules. The large increase in emission intensity, upon adjusting an initially neutral aqueous solution containing these nanovalves to pH 4, clearly indicates that RhB molecules are released from the nanopores of the MSNs. Upfield chemical shifts in the 1H NMR spectrum of the α- and β-protons of the viologen unit further supports the de-threading of CB[6] rings upon acid activation of the nanovalve system.
Controlled release of cargo molecules based on CB[6]-based nanovalve systems is also shown under the influence of a typical biological trigger—reductive chemistry.102 This system incorporated the disulfide bond into the stalks of CB[6]-based rotaxanes on top of the mesoporous MCM-41, providing an excellent cleavage handle for intracellular delivery due to the significantly higher concentration of glutathione present within cells (∼10 mM) compared to that in the blood stream (∼2 μM). Various gates instead of CB[6] are also employed in aqueous solutions for the controlled release of cargos in a similar system, which will be further reviewed in the following sections.
Alternatively, magnetic hyperthermia was successfully used to selectively release drug molecules. Cheon and Zink et al.103 loaded MSNs with doxorubicin (DOX) and superparamagnetic iron oxide nanoparticles and employed a simple heat labile nanovalve based on CB[6]-bisammonium pseudorotaxanes as gatekeepers to control the targeted release of the included cargo molecules (Fig. 4). At room temperature, no drug release was observed due to the tight capping of the pores of the magnetic-core MSNs by nanovalves, but at high temperatures induced by oscillating magnetic field, valve opening occurs and most of the drug molecules were released from the pores. This controlled release generates ca. 8-times higher cancer cell kill than without hyperthermic release in vitro. Varying the length of the magnetic actuation could balance the hyperthermic and apoptotic effects on targeted drug release, providing a significant advantage over current chemotherapies in clinical settings.
![Oscillating magnetic field operated nanovalve based on CB[6]/bisammonium pseudorotaxanes. Superparamagnetic iron oxide nanoparticles (1) are synthetically positioned at the core of the MSNs (2). Bisammonium stalks of the molecular machine are then linked to the nanoparticle outer surface (3). Drug is loaded into the particle and capped with CB[6] (4) to complete the drug delivery system. Release can then be realized by magnetic hyperthermia (5). Reprinted with permission from ref. 103. Copyright 2010 American Chemical Society.](/image/article/2011/MD/c1md00158b/c1md00158b-f4.gif) | ||
| Fig. 4 Oscillating magnetic field operated nanovalve based on CB[6]/bisammonium pseudorotaxanes. Superparamagnetic iron oxide nanoparticles (1) are synthetically positioned at the core of the MSNs (2). Bisammonium stalks of the molecular machine are then linked to the nanoparticle outer surface (3). Drug is loaded into the particle and capped with CB[6] (4) to complete the drug delivery system. Release can then be realized by magnetic hyperthermia (5). Reprinted with permission from ref. 103. Copyright 2010 American Chemical Society. | ||
2.2. CB[7]-based nanovalves
CB[7] is the seven-membered macrocycle, which possess a larger cavity compared to CB[6] and has attracted considerable attention owing to its superior solubility (20–30 mM) in pure water and strong binding ability to cationic ferrocene derivatives, viologen, ammonium ion forms of amino acids, and etc.63–65As shown in a recent paper, Stoddart and Zink et al.104 employed CB[7] and ferrocene carboxylate in the construction of base-activated release systems (Fig. 5). It has been demonstrated that CB[7] forms very stable inclusion complexes (association constants between 109 and 1011 M−1) with ferrocene derivatives. However, anionic ferrocene carboxylates do not bind to CB[7] because of the electrostatic repulsion between the carboxylate groups and the carbonyl groups of CB[7]. The dependence of pH on CB[7] binding towards ferrocene dicarboxylic acid was investigated by 1H NMR, which shows that CB[7] binds its neutral form at pH 2 as illustrated by an upfield shift of the resonances for ferrocene protons. By changing the pH to 10, the ferrocene guest forms a deprotonated dianion, which does not bind to the electron-rich interior of CB[7]. Furthermore, fluorescence spectra confirmed the release of the cargo molecules from MSNs upon changing the pH of the solution from 3 to 10.
![pH-responsive CB[7]/ferrocene pseudorotaxane-based nanovalve (top route). The cargo is released by acidifying the solution in which MSNs are dispersed, the ferrocene carboxylic acid is deprotonated and the CB[7] dethreads from the stalk due to the strong electrostatic repulsion between the carboxylate anion of the guest and the carbonyl oxygen atoms of CB[7]. The release profile of RhB from CB[7]-capped MSNs was shown in the bottom right. Upon adjusting the pH to 10, the emission intensity of RhB increases rapidly and then asymptotically approachs the maximum with a time-to-half maximum release of ca. 100 min. Copyright 2009 Wiley. Used with permission from ref. 104.](/image/article/2011/MD/c1md00158b/c1md00158b-f5.gif) | ||
| Fig. 5 pH-responsive CB[7]/ferrocene pseudorotaxane-based nanovalve (top route). The cargo is released by acidifying the solution in which MSNs are dispersed, the ferrocene carboxylic acid is deprotonated and the CB[7] dethreads from the stalk due to the strong electrostatic repulsion between the carboxylate anion of the guest and the carbonyl oxygen atoms of CB[7]. The release profile of RhB from CB[7]-capped MSNs was shown in the bottom right. Upon adjusting the pH to 10, the emission intensity of RhB increases rapidly and then asymptotically approachs the maximum with a time-to-half maximum release of ca. 100 min. Copyright 2009 Wiley. Used with permission from ref. 104. | ||
Besides the pH-driven nanovalves based on CB[7] pseudorotaxanes, Du et al.105 also reported a new controlled release mechanism, i.e., competitive binding-driven nanovalves. The threading of CB[7] onto the protonated 1,4-butanediammonium stalks anchored on the surfaces of the pore interiors and around the pore entrances of MSNs resulted in the tight closing of the nanopores filled with cargo molecules. These nanovalves could then be opened by deprotonation of the stalks and/or competitive binding of cetyltrimethylammonium bromide (CTAB) and 1,6-hexanediamine with CB[7] in aqueous solutions, releasing the cargo calcein molecules from the inside of the nanopores into bulk solution, as observed from the changes in fluorescence intensity of calcein and in color of suspended solutions. The increases in binding affinity and concentration of the competitors gave rise to an increase of the cargo release rate and efficiency. It is possible that the targeted release of drug molecules through these CB[7]-based nanovalves can be realized within some tissues and organs in basic environments or in the presence of biologically relevant cationic species under physiological conditions.
Taking advantage of this competitive binding motif, the same research group recently combined bio-stimuli to operate CB[7]-based nanovalves by using biocompatible magnetic MSNs instead of traditional MSNs.106 This is the first report on the enzyme-inspired controlled release of CB[n] nanovalves by using magnetic MSNs in near-neutral aqueous solutions. In this system, the same CB[7]-based pseudorotaxanes were employed to be tethered to the external surfaces of superparamagnetic Fe3O4-embedded MSNs, leading to tight blocking of the nanopores. The nanovalves can be operated by the enzymatic decarboxylation product of lysine, cadaverine in its protonated form, which has a higher affinity to CB[7]. The use of superparamagnetic Fe3O4-embedded MSNs offers both site-targeted delivery and easy separation and recovery. Lysine had a low affinity to CB[7] and could not activate the nanovalve. But, in the presence of lysine decarboxylase, the enzymatic decarboxylation product, protonated cadaverine, has a very high binding affinity to CB[7] to realize the continuous dethreading of CB[7] rings from the 1,4-butanediammonium stalks through competitive binding and thus the releasing of the cargo calcein molecules from the MSNs. In addition, the release of the targeted delivery system could be controlled in small portions on command. The released cargo molecules can act not only on sensing probes for levels of the polyamines and enzymes but also on efficacious drugs for regulation of polyamine synthesis.
2.3. CD-based nanovalves
One of the simplest methods to control molecular recognition between a stalk component and a CD cap is by pH changes, thereby making it a decent release mechanism for controlled drug delivery. Kim et al.107 reported one of the first pH-responsive nanovalve systems based on polyethyleneimine(PEI)/CD polypseudorotaxanes and MSNs. In this case, cargo molecules—calcein—are entrapped in the nanopores of MSNs which are then blocked by a layer of surface-attached pH-responsive PEI/CD polypseudorotaxanes. Two systems, one employing α-CD and the other γ-CD, were tested. A series of macrocyclic α-CD and γ-CD could accommodate one and two PEI polymer threads, respectively, and therefore form a binary 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and a ternary 2
1 and a ternary 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex. In both instances, calcein molecules are tightly entrapped in the pores of MSNs as long as the PEI polymer remains deprotonated (pH ≥ 11). Upon lowering the pH, the CD rings de-thread from the PEI chains as a result of weak interaction between the protonated PEI and hydrophobic cavity of CDs (Fig. 6). An immediate increase in fluorescence intensity of the solutions of calcein-loaded CD/PEI MSNs, upon decreasing the pH, clearly indicates the release of calcein from MSNs.
1 complex. In both instances, calcein molecules are tightly entrapped in the pores of MSNs as long as the PEI polymer remains deprotonated (pH ≥ 11). Upon lowering the pH, the CD rings de-thread from the PEI chains as a result of weak interaction between the protonated PEI and hydrophobic cavity of CDs (Fig. 6). An immediate increase in fluorescence intensity of the solutions of calcein-loaded CD/PEI MSNs, upon decreasing the pH, clearly indicates the release of calcein from MSNs.
 | ||
Fig. 6 pH-responsive nanovalves based on PEI/CD polypseudorotaxanes. It consists of three biocompatible components, namely, MSNs, PEI and CD. With α-CD, PEI forms a binary 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex and with γ-CD, the polymers form a ternary 2 1 complex and with γ-CD, the polymers form a ternary 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex. Upon raising the pH of the solution, the CD rings dethread from the PEI polymers, releasing the entrapped calcein molecules. Copyright 2007 Wiley. Used with permission from ref. 107. 1 complex. Upon raising the pH of the solution, the CD rings dethread from the PEI polymers, releasing the entrapped calcein molecules. Copyright 2007 Wiley. Used with permission from ref. 107. | ||
Recently, Stoddart, Zink and we reported the so-called snap-top version of molecular nanovalves, one of the stoppers of a rotaxane, the other one being the MSN itself, is cleaved enzymatically or reductively, allowing the gate component (CD) to escape from its stalk.102,108 The modular synthesis of the snap-top systems can lead to a high degree of variation in the separate components, i.e., the solid support, the linear stalk, the gatekeeper, and the bulky stopper, of the integrated system. A versatile system based on esterase hydrolysis,108 that is capable of entrapment and controlled release of cargo molecules, was firstly described (Fig. 7). In order to test the viability of our enzyme-responsive snap-top nanovalve motif, stoppers that could be hydrolyzed by porcine liver esterase (PLE) were carefully introduced into the nanovalves where the ring components are α-CDs. MSNs loaded with luminescent cargo molecules (RhB) were capped with the ester-linked adamantyl stoppers. PLE catalyzes the hydrolysis of the adamantyl ester stopper, resulting in de-threading of the α-CD tori, and release of the luminescent dye molecules from the nanopores. As a control experiment, MSNs were also prepared using an adamantyl amide stopper analog, but which does not undergo hydrolysis by PLE. Luminescence spectra show the enzyme-triggered release of cargo dye molecules from the adamantyl ester stoppered snap-top nanovalve system upon the addition of PLE. By contrast, no such release was observed for the amide-stoppered snap-top nanovalve. These results show that the snap-top design concept is a feasible one for the drug release application of MSN-based nanovalves.
 | ||
| Fig. 7 Release profile of enzyme-activated α-CD polypseudorotaxane-based snap-top nanovalves as a function of time. The emission intensity of RhB increases immediately after PLE is added for the ester adamantyl-stoppered MSNs (green trace) but not for the amide MSNs (blue trace). Inset shows the emission intensity before and after snap-top activation as a function of wavelength. Reprinted with permission from ref. 108. Copyright 2008 American Chemical Society. | ||
Another design also demonstrated the feasibility of this snap-top version of nanovalve motif, but instead using reductive chemistry for the nanovalve operation.102 In this example, MSNs were surface-functionalized with rotaxanes that incorporate disulfide bonds in their stalks which are encircled by α-CD rings. Only when reductive chemistry is performed, resulting in the snapping of the stalks of the surface-bound rotaxanes, the cargo molecules were released from the inside of the nanoparticles. On account of the modular nature of the assembly of these snap-top nanovalves, each component of the integrated system can be tailored to install targeting features and containment of small drug molecules and/or imaging agents. Due to the modularity and tunability of the integrated systems in addition to being relatively simple to assemble, the snap-top version of nanovalves promises to be useful for a range of non-biological as well as biological applications.
A key challenge for these integrated systems is whether they can be adapted for biological use through autonomously controlled nanovalve opening inside cells of interest. Very recently, Nel, Zink, Stoddart and Tamanoi’s109 four research groups reported together a novel MSN delivery system capable of drug delivery based on the function of β-CD nanovalves that are responsive to the endosomal acidification conditions in human differentiated myeloid (THP-1) and squamous carcinoma (KB-31) cell lines, and demonstrated the methods of optimizing the surface functionalization of the MSNs to provide a platform for the effective and rapid DOX release to the nuclei of KB-31 cells. In this work, the MSNs used are ∼100 nm spheres containing ordered two-dimensional hexagonal arrays of tubular pores with diameters of ∼2 nm, which is in the range needed to improve the targeting ability of a drug delivery system due to an enhanced permeability and retention (EPR) effect of cancerous tissue.110 These nanopores are large enough to contain common biological dyes and drugs, yet small enough to be gated by CDs. A CD-based nanovalve which is closed tightly at pH 7.4—the pH of blood—but self-open in acidifying endosomal compartments, is then constructed covalently to the nanopore openings as an effective gatekeeper. A series of aromatic amines were chosen as the stalk and β-CD as the cap of the nanovalve. As illustrated in Fig. 8, β-CD rings encircles the stalks through noncovalent bonding interactions under neutral pH conditions and effectively block the pores and traps the included cargo molecules. Lowering the pH results in the protonation of the aromatic amines, followed by β-CD cap de-threading and cargo release from the nanopores. Specifically, the N-methylbenzimidazole stalks with optimized pKa endow the nanovalves with the capability of binding the ring strongly at pH 7.4 and trapping drug molecules inside of MSNs, while deprotonation in acidifying endosomal compartments of pH < 6 leads to dissociation of the β-CD caps and release of the cargo molecules. The rate of release of the positively charged DOX molecules was increased by the interior surface modification of silica. MSNs loaded with Hoechst dye and the anticancer drug DOX were taken up into acidifying endosomal compartments in THP-1 and KB-31 cells. DOX release from within the LAMP-1 positive endosomal compartments causes apoptotic cell death in KB-31 cells. This design could improve the efficacy and safety of drug delivery for chemotherapeutic agents.
 | ||
| Fig. 8 Graphical representation of the pH responsive MSN nanovalve, showing the synthesis of the stalk, loading of the cargo, capping of the pore, and release of the cap and cargo under acidic conditions (a). Details of the protonation of the stalk and release of β-CD (b) and the TEM image of capped MSNs (c) were also shown. Reprinted with permission from ref. 109. Copyright 2010 American Chemical Society. | ||
Supramolecular nanovalves, relying on the hydrogen-bonding interaction between α-CD and the stalk which contains an anilino group this time, were also successfully constructed on a hollow mesoporous silica spherical framework.111 This type of MSN with a hollow core has proven to be an intriguing candidate for drug loading and storage because of the possibility of a higher storage capacity than that of conventional mesoporous particles. The successful gate-keeping control of the access to and from the interior with this simple supramolecular nanovalve based on α-CD/aniline supramolecular systems shows that hollow MSNs release as much as 2 times more cargo molecules upon activation by pH changes than do conventional MSNs, which could increase the delivery capabilities of drugs in different types of human cancer cells as required in some instances.
Biocompatible mechanized phosphonate-clothed MSNs have also been designed and fabricated in which the supramolecular machines covering the surfaces of the nanoparticles behaves like nano-pistons, releasing encapsulated guest molecules in a controlled fashion under acidic conditions.112 This nanovalve system consists of a monolayer of β-CD rings positioned selectively around the orifices of the nanopores of MSNs. A benzidine/RhB conjugate were used as the nano-pistons for movement in and out of the cylindrical cavities of the β-CD rings on the MSN surfaces. Fluorescence spectroscopy indicated that the nanovalve system could store small cargo molecules, i.e., 2,6-naphthalenedisulfonic acid disodium, within their nanopores at neutral pH, but release them by passing through CD cavities as soon as the pH was lowered to ∼5. Alternatively, the phosphonate-covered MSNs were functionalized with β-CD rings, but in this case, the seven linkers attaching the rings to the orifices surrounding the nanopores contained cleavable imine double bonds. The CD rings on MSN surfaces serve as gatekeepers for the storage of large cargo molecules, i.e., RhB, inside the MSNs under neutral conditions. Upon hydrolyzation of the imine bonds under acidic conditions, CDs could be cleaved off from the MSN surface when the pH was decreased to ∼6, releasing the relatively large cargo molecules. Since β-CD rings on the surfaces of the MSNs can form complexes with a variety of guest molecules in their hydrophobic cavities, molecular plugs can be designed and synthesized to respond to stimuli that trigger pH-, light-, or redox-operated drug delivery. These organic–inorganic hybrid nanoparticles can in principle be fabricated to display a dual-cargo release mechanism wherein the plugs and the β-CD rings retain small and large drug molecules, respectively, and release them at different conditions. Meanwhile, this system makes it possible to instruct MSNs to hunt down certain diseased cells prior to delivering a specific drug selectively to these cells owing to the readily functionalization of the plugs with targeting antenna. This research represents a significant step toward the development of pH-responsive nanovalve-based dual drug delivery vehicles that are potentially capable of being interfaced with biological systems.
Zink and Stoddart et al.113 functionalized the MSN surface with azobenzene derivatives, and their supramolecular recognition with β-CD can effectively block the MSN nanopores and then respond to a different external stimulus—light. The light-operated nanovalves are able to retain dye molecules and then release them upon exposure to light. The operation of this CD nanovalve is based on the association and light-activated dissociation of the β-CD and/or pyrene-β-CD rings with the azobenzene-containing stalks on the surfaces of the MCM-41 type of MSNs. Irradiating azobenzene with 351 nm light causes azobenzene to isomerize from the more stable trans to the less stable cis configuration. In this nanovalve system, β-CD will thread onto the stalks and bind to trans-azobenzene units, thus sealing the nanopores and stopping release of the cargo, e.g., RhB from the cargo-loaded MSNs. By contrast, upon irradiation with 351 nm light, the isomerization of trans-to-cisazobenzene units leads to the dissociation of β-CD rings from the stalks due to a lower binding affinity to cis configuration, thus opening the gates to the nanopores and releasing the cargo (Fig. 9). External control could help localize and optimize the process of drug delivery. The hydrophilic character of the mechanized MSNs and their ability to release the stored drug molecules in response to an external light stimulus make this system applicable to light-operated intracellular drug delivery systems.
 | ||
| Fig. 9 Light-operated nanovalves based on azobenzene-modified stalks and β-CD or pyrene-β-CD. CD threads onto the trans-azobenzene stalks to seal the nanopores. Upon irradiation (351 nm), the isomerisation of trans-to-cisazobenzene units leads to the dissociation of CD rings from the stalks, thus opening the valves and releasing the cargo molecules. Reprinted with permission from ref. 112. Copyright 2010 American Chemical Society. | ||
It's worthy to note that recently Kim et al.114 reported a new special nanovalve system utilizing CD itself instead of CD-based molecular machines as gatekeepers, which shows enzyme-responsive release of guest molecules from MSNs in the presence of α-amylase or lipase. MSNs with a ∼60 nm diameter and an average pore diameter of 2.5 nm were employed. After loading with cargo dye molecules—calcein—these MSNs were blocked by click coupling mono-6-azido-β-CD onto the alkyne group-functionalized orifices of their nanopores. The results indicate that the guest molecules are kept in the nanopores without an enzymatic stimulus which can degrade the CD gatekeepers or cleave the stalk components. Upon treating the phosphate buffered saline solution of the nanocontainer systems with α-amylase, a significant increase in the fluorescence intensity of calcein was observed, which indicates the successful release of the calcein molecules from the pores due to degradation of CD gatekeepers by α-amylase (Fig. 10). In addition, since the stalk part of the gatekeeper in Si-MP-NBE-CD contains an ester bond which can be hydrolyzed by enzymes such as lipase and esterase, lipase treatment of the MSNs was investigated and showed an increase of the fluorescence intensity of calcein in Si-MP-NBE-CD through time-course fluorescence analysis, suggesting that the ester moiety was degraded by lipase to remove CD gatekeepers. More interestingly, a synergistic effect was observed by employing multiple stimuli for the guest release from Si-MP-NBE-CD which consists of the enzyme- and photo-responsive surface functionality (o-nitrobenzyl ester moiety works as a photocleavable linker). When both stimuli were applied to the MSN solution simultaneously, the fluorescence increase was accelerated, indicating that the cargo release was synergistically affected by the photo-stimulus of UV irradiation and enzymatic activity of α-amylase.
 | ||
| Fig. 10 Synthetic route to Si-MP-CD (a), FE-SEM image of Si-MP-CD (b), the structure of surface functional motifs on Si-MP-NBE-CD (c), and schematic illustration for enzyme-triggered guest release from the nanopores of CD-gated MSNs. Reprinted with permission from ref. 113. Copyright 2009 American Chemical Society. | ||
In collaboration with the Park group, the same research group115 also introduced CD gatekeepers linked via a disulfide unit to the surface of MSNs and demonstrated not only the entrapment of guest anticancer drugs in the pore reservoir but also the successful controlled release of the guest DOX in response to glutathione (GSH) which can remove the gatekeeper by cleavage of the disulfide stalk moiety. In addition, the GSH-induced release of DOX from the CD-capped MSNs was shown in A549 cancer cells, indicating the CD-capped MSN systems can be used as a versatile multifunctional nanocontainer for targeted controlled release in therapeutic systems.
3. Biocompatible nanovalves relying on polymers and dendrimer caps
In the previous section, we have discussed the operation of several major types of MSN nanovalves relying on the supramolecular interactions between rings and stalk components on MSN surfaces. In order to realize smart uptake and release of cargo molecules, e.g., drugs, designing nanovalves based on different type of materials besides those we mentioned above, i.e., inorganic and polymeric caps that can control the access of guest molecules to and from the nanopores of MSNs, are of great importance. Some notable gate-control “caps” such as Fe3O4,116 CdS117 and Au118–120nanoparticles have been well constructed on MSN surfaces by Lin and Amoró et al., and guest release from the nanovalves was achieved by the cleavage of the linkages between MSNs and nanoparticle caps by a variety of triggers, which has been reviewed in some recent articles.19,121–123 These functional MSNs were applied to the controlled release of drugs, genes, biocides, dyes, and proteins and for the detection of certain target cargos such as neurotransmitters, ATP, and fatty acids.19,121–125 In this section, capping the MSNs with polymer and dendrimers and activating them with different biocompatible external stimuli will be presented and evaluated.For the first time, a cross-linked polymeric network is used as a “gatekeeper” on the surface of MSNs to generate a tunable redox-responsive hybrid nanovalve, as reported by Feng et al.126 To avoid the uncontrolled release of guest molecules from polymer brush-coated MSN, poly(N-(acryloxy)succinimide) (PNAS)-coated MSNs were synthesized via surface-initiated reversible addition–fragmentation chain transfer (RAFT) polymerization and the polymer strands were cross-linked with cystamine, a disulfide-based bifunctional primary amine, via the reaction with N-oxysuccimide groups along the polymer chain to seal the MSN nanopore entrances. The uncapping can be easily achieved via the addition of dithiothreitol (DTT) to cleave the disulfide bonds of cystamine among the polymeric network around the pore opening, leading to the cargo escaping from the nanopores of the MSNs (Fig. 11). Since disulfide bonds can also be cleaved by using cell-produced antioxidants (e.g., dihydrolipoic acid or glutathione), this system is promising for biosensor and in vivo site-specific drug delivery.
 | ||
| Fig. 11 Schematic representation of redox-responsive nanovalves based on polymeric network-capped MSNs (top) and multi-responsive nanovalves based on supramolecular polymeric network-capped MSNs (bottom). In the first system, PNAS-coated MSNs were first loaded with dyes, then their valves closed with the cross-linking of the polymer chains by the addition of a disulfide-based cystamine. The polymeric network formed can then be reopened by cleaving the disulfide bond of cystamine in the presence of DTT, leading to the cargo release. In the second system, Poly-CD-MS filled with cargos were blocked by adding diazo-linker to cross-link the β-CD-bearing polymer chains. Release of cargo molecules—calcein—was achieved by the cleavage of the polymeric network using UV irradiation, competitive binding, or the addition of disulfide reducing agent—DTT. Reprinted with permission from ref. 126 & 127. Copyright 2008 & 2009 American Chemical Society. | ||
Most recently, they further modified PNAS-coated MSNs with β-CDvia a disulfide linkage. Through the addition of the diazo linker, a water-soluble ditopic guest molecule with an azobenzene group at each end, the polymer around the MSNs can be cross-linked to form a supramolecular polymeric network to block the transport of cargo molecules.127 This multi-responsive system integrated the advantages of polymeric network as capping agent with the versatile assembly/disassembly of CD-based supramolecular chemistry. The working principle is shown in Fig. 11. More importantly, the supramolecular polymeric network that blocks the nanopores of MSNs can then be opened by cleaving the linkage with multiple stimuli, i.e., (1) UV irradiation, (2) the addition of competitive host α-CD, which favors the formation of inclusion complexes with an azobenzene group more than β-CD does, and (3) also the addition of disulfide reducing agents such as DTT.
Hong et al.128 reported the construction of an intelligent, pH-responsive polymer shell on the exterior surface of MSNs by surface RAFT polymerization of acrylic acid. Trithiocarbonate functionality-coated MSNs were used as the RAFT agent: the MSN core can serve as a nanocontainer for guest molecules while the pH-sensitive poly(acrylic acid) nanoshell could be reversibly opened and closed by raising the pH and lowering the pH, acting as a nanovalve to regulate the smart loading and release of cargo molecules from the MSN nanocontainers.
In contrast, Feng et al.129 recently reported another type of pH-sensitive controlled release system employing a responsive polymer—poly(4-vinyl pyridine) (PVP). Instead of raising pH as shown in Hong's system,128 the guest release of this system was achieved by lowering the pH. PVP is commercially available and has been widely used in the construction of responsive colloid and surface systems because of its pH-triggered switching ability. In this system, the authors anchored PVP on MSN surface by simply grafting PVP with bromo-functionalized MSNs. At high pH, the deprotonation of the polymer produces a hydrophobic shrunken state and inhibits the release of entrapped cargos. The swollen state of the protonated PVP at low pH is permeable to molecule transport, resulting in the pH-responsive release of cargo molecules.
Liu et al.130 reported the fabrication of multifunctional fluorescent pH-sensing organic/inorganic hybrid MSNs with tunable redox-responsive guest release capability. Random copolymers composed of NAS, OEGMA and fluorescent pH-sensing monomer (NaphMA) were grafted onto the surfaces of MSNs via surface-initiated RAFT polymerization (Fig. 12). After loading with RhB and cross-linking the polymer brushes with cystamine, the obtained MSNs could respond to redox and release encapsulated cargo molecules via the addition of DTT.
 | ||
| Fig. 12 Schematic representation of opening and closing of the poly(acrylic acid) nanoshell as a nanovalve on MSN surfaces by pH trigger. The solubility and structure of poly(acrylic acid) are very sensitive to pH: it has good solubility at high pH values (such as pH ≥ 8.0), however it has poor solubility and even collapses entangled together to precipitate from solution at low pH values (such as pH ≤ 4.0). Reprinted with permission from ref. 130. Copyright 2010 American Chemical Society. | ||
Besides pH, redox, competitive binding, and photoirridiation, thermal control was also reported as an external stimulus for the operation of polymer-MSN hybrid nanovalves. Brock and Oupický et al.131 achieved temperature-dependent uptake and release of cargo molecules within the nanopores of MSNs by treatment of preformed thiol-functionalized MSNs with pyridyl disulfide-terminated poly(N-isopropylacrylamide) (PNIPAM). PNIPAM represents one of the most widely used temperature-responsive polymers with a lower critical solution temperature (LCST, the temperature at which the polymer undergoes a change from a hydrated to a dehydrated state) of ∼32 °C in water. Thus, at temperatures below the LCST, the polymer is in the coil (soluble) state, while above it, it is in the globule/collapsed (insoluble) state. The prepared PNIPAM-grafted MSNs show minimal leakage in the gate “closed” conformation (above LCST, <2% after 2 h), and a significant uptake and release of cargo molecules at room temperature (below LCST of the polymer). This is consistent with a mode of action in which fluorescein diffusion occurs readily when the polymer is in the random coil state but is significantly retarded when it is in the globule state.
The development of nanocarriers that can deliver large biomacromolecules selectively and effectively has also attracted much attention. Second generation of polyamidoamine (G2 PAMAM) dendrimers was successfully covalently linked to the surface of MSNs containing Texas Red in the pores to efficiently complex with plasmid DNA (pEGFP-C1) that codes for an enhanced green fluorescence protein (GFP), resulting in a gene transfection system (G2-MSN).124 The PAMAM-functionalized MCM-41 was then complexed with plasmid DNA, confirming the successful delivery of the plasmid to the cell nucleus (Fig. 13). The gene transfection efficancy, uptake mechanism and biocompatibility of the G2-MSN with neural glia (astrocytes), human cervical cancer (HeLa), and Chinese hamster ovarian (CHO) cells were investigated. This system has great potential to serve as a universal transmembrane carrier for intracellular drug delivery and many other biotechnological applications.
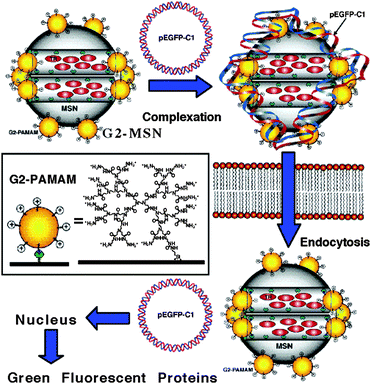 | ||
| Fig. 13 Schematic representation of controlled delivery of DNA plasmid pEGP-C1 in cellsvia a G2-PAMAM dendrimer-capped MSN based gene transfection system. PAMAM-grafted MSNs, loaded with Texas Red, were complexed with an enhanced GFP (Aequorea Victoria) plasmid DNA (pEGFP-C1). Once the MSNs were endocytosed into the cells, the pEGFP-C1 was delivered into the nucleus and GFPs produced. Reprinted with permission from ref. 124. Copyright 2004 American Chemical Society. | ||
4. Biocompatible nanovalves based on biomolecules
As shown above, supramolecular assemblies, polymers, dendrimers, inorganic nanoparticles have been used as the blocking caps in nanovalve delivery systems to control the opening and closing of pore entrances of MSNs. Different external stimuli, including enzymatic digestion of gatekeepers, temperature, competitive binding, light-irradiation, changes in pH, and changes in the redox potential, have been successfully applied as “triggers” to uncap the nanopores and release cargo molecules. Most recently, designing MSN-based systems based on nanovalves involving biomolecules, such as nucleic acids, proteins, antibodies, saccharides, amino acids, peptides, aptamers, lipid bilayers, and etc., to trigger cargo release from the pores of MSNs has become an important and emerging field. In this section, we will discuss some recent burgeoning developments of stimuli-responsive MSN systems involving biomolecules as nanovalves for controlled release of guest/drug molecules.4.1. Protein-capped MSN systems
Bein et al.132 described the use of a well-studied biotin–avidin system, which has been widely used in biorecognition, biosensing and biomedical applications, as a biomolecule-based, protease-responsive nanovalve system for MSNs and demonstrated the operation of this system for the controlled release of a model dye molecule—fluorescein. They used their developed strategy to integrate molecular functionality—protein-coupling sites—exclusively into the outer surface shell of MSNs in precisely controlled amounts, while leaving the inner pore system unfunctionalized. The resulting biotinylated MSNs (CMS-BIO) was then loaded with fluorescein and capped with avidin (CMS-AVI). The enzyme-responsive release properties were then investigated by fluorescence spectroscopy. The aqueous suspension of CMS-AVI was stored in a specially designed container, which could be closed by a holey lid lined with a dialysis membrane. This custom-made system fits on the opening of a fluorescence cuvette. MSNs are too large to diffuse through the dialysis membrane, but fluorescein can get into the free cuvette volume readily and be monitored by fluorescence spectroscopy at 37 °C. Upon the addition of the protease—trypsin—avidin was proteolytic digested, leading to the escape of cargo from MSNs. Interestingly, the release curve features a fairly slow release for the first hour after trypsin addition and fast release afterwards due to complete digestion of capping protein within 1h. A complementary thermo-responsive release experiment was carried out to compare the efficiency of the different valve-opening methods. In this case, the attached protein caps were opened by denaturation at high temperature to result in an immediate and simultaneous release of fluorescein molecules. The advantage of this system is the use of native biomolecules to avoid the creation of toxic and carcinogenic species.Antibody–antigen interaction is also employed as a powerful switchable method for the development of nanovalves on MSNs for controlled delivery applications. Martínez-Máñez, Maquieira, and Amorós et al.133 reported the design of a new controlled delivery system based on MSNs as the nanocarrier and a hapten–antibody complex as gatekeepers. The opening mechanism and delivery of entrapped cargo is related to a highly effective displacement reaction involving the presence in the solution of the antigen to which the antibody is selective. As a proof-of-the-concept, MCM-41 was loaded with cargo—[Ru(bipy)3]Cl2—and then surface modified with hapten 4-(4-aminobenzenesulfonylamino)benzoic acid. The nanopores were then capped with an antibody that shows good affinity and selectivity toward the anchored haptenvia suitable interaction through the two binding IgG regions of the former (solid S1–AB). As illustrated in Fig. 14, the presence in the solution of the corresponding target molecules (antigen, complementary to the antibody), to which the antibody binds selectively, induced the uncapping of the nanopores and release of the entrapped cargo molecules. The possibility of incorporating this antibody-dating protocol on diverse supports, the potential delivery of different cargos, and the opportunity of selecting antibodies or similar bioreceptors for a variety of possible antigens makes this approach highly appealing for a wide range of delivery applications in different fields.
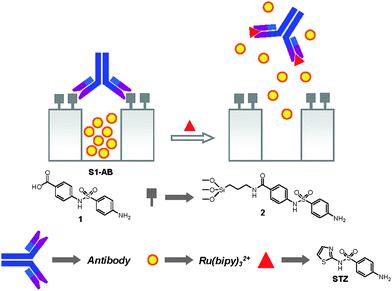 | ||
| Fig. 14 Schematic representation of the nanovalves consisting of antibody caps and chemical structures of hapten 1, hapten derivative 2, and the antigen sulfathiazole (STZ). Reprinted with permission from ref. 133. Copyright 2009 American Chemical Society. | ||
4.2. Nucleic acids-based molecular valve systems
Nucleic acids are attractive building blocks for nanotechnology and material science. The unique structural motif and self-recognition properties of double strand DNA, including temperature-dependent assembly, as well as the enzymatic recognition of specific encoded bases, can be applied as triggers for functional DNA manipulation.The Bein research group has recently centered on systems using biomolecules as components for the design of molecular valves. Besides the applicability of the biotin–avidin complex-based nanovalve,132 just discussed above, they recently designed a molecular nanovalve system134 that can be made programmable by encoding the desired behavior in a DNA sequence, part of the stimuli-responsive system. Biotin-labeled alkyne-modified DNA double strands of two different lengths were selectively attached to the pore orifices of azide-functionalized MSNs via a click chemistry approach. Subsequent pore closing was done through the addition of protein—avidin, and pore opening achieved by melting the DNA strands at the specific melting temperature of the oligonucleotides (Fig. 15). The release of model dye—fluorescein—was easily detected and quantified by fluorescence spectroscopy and UV/Vis spectroscopy. This nanodevice allows the temperature of guest release to be adjusted precisely for a desired application.
 | ||
| Fig. 15 Schematic representation of the programmable thermoresponsive DNA-based molecular nanovalve system. The avidin caps are opened by melting the DNA linkers at specifically encoded temperatures. Copyright 2010 Wiley. Used with permission from ref. 134. | ||
Ren and Quet al.135 described the design and construction of polyvalent nucleic acid/MSN “click” conjugates as dual stimuli-responsive vehicles for intracellular drug delivery. A self-complementary DNA duplex was attached to the pore openings of the MSNs by the highly efficient copper(I)-catalyzed azide-alkyne reaction and served as a cap for entrapping of guest molecules within the nanopores. RhB, anticancer drug camptothecin (CPT) and soluble drug floxuridine (FUDR) are successfully loaded into MSNs. An increase of the temperature to 50 °C resulted in the opening of the attached duplex caps by thermal denaturation, and led to the fast release of RhB. The addition of DNase I also could trigger the nanovalve system and the release of the encapsulated dyes owing to the enzymatic degradation of duplex DNA interconnects as a consequence of phosphodiester bond cleavage. Efficient intracellular controlled drug delivery in human liver cancer cells (HepG2) was observed when endogenous nuclease was used as an external stimulus. Importantly, novel nucleic acids such as aptamers and DNAzymes could also be incorporated into this system to construct multifunctional stimulus-responsive materials.
Aptamers are single-stranded oligonucleotides that can specifically bind to their targets with high affinity and specificity. Compared with antibodies, aptamers, particularly DNA aptamers, are relatively easy to obtain, more stable to biodegradation, less vulnerable to denaturation, and flexible to modification, showing a promising future in the fields of bioscience and nanotechnology. Recently, Yang et al.136 reported the design of a novel bio-responsive controlled-release system based on MSNs gated with aptamer-modified gold nanoparticles (Fig. 16), which is stimuli-responsive to the aptamer–target interactions. In this work, MCM-41 tailed with amino group was selected as support, and further functionalized with adenosine-5′-carboxylic acid (adenosine–COOH)—a derivative of adenosine triphosphate (ATP) target—to give MSA. Gold nanoparticles were functionalized with ATP aptamer through Au–S bond to form AuNPs–aptamer. Because the binding reaction of ATP aptamer with adenosine resulted from the recognition of ATP aptamer to the adenine and ribose moieties, upon mixing AuNPs–aptamer with MSA, AuNPs would block the pores of MSA. The release of cargo molecules—fluorescein isothiocyanate (FITC) dye—from this AuNPs gated MSNs was triggered by the addition of ATP molecules which resulted in a competitive displacement reaction to the adenosine–aptamer interaction to uncap the pores of MSNs. As a control, the ATP analogues, i.e., cytosine 5′-triphosphate (CTP), guanosine 5′-triphosphate (GTP), and uridine 5′-triphosphate (UTP) did not induce a characteristic release similar to that for ATP. Interestingly, the complementary oligonucleotides (CDNA) and the random oligonucleotides (RDNA) to the ATP aptamer could also trigger the release of cargo molecules, and among them, CDNA shows higher delivery efficiency compared to ATP, owing to its high binding ability to ATP aptamer in comparision with that of ATP.
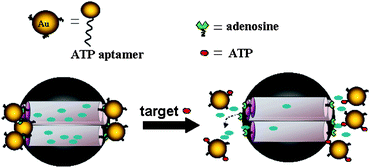 | ||
| Fig. 16 Schematic representation of a controlled-release system that responds to aptamer–target interaction. MSN was gated with AuNPs–aptamervia the binding of ATP aptamer to adenosine. Release of the trapped fluorescein was triggered by a displacement reaction in the presence of the target molecules (ATP). Reprinted with permission from ref. 136. Copyright 2011 American Chemical Society. | ||
Martínez-Máñez et al.137 also showed their design of new “bio-gates” able to respond selectively to “key” molecules. MSNs are first loaded with fluorescein, and then the external surface is functionalized with amino-propyl groups, which are partially charged at neutral pH in water and will interact with negatively charged oligonucleotides, resulting in the closing of the mesopores. Pore opening and cargo release were achieved by a highly effective displacement reaction in the presence of a target complementary strand contributing to the hybridization of the two oligonucleotides (Fig. 17).
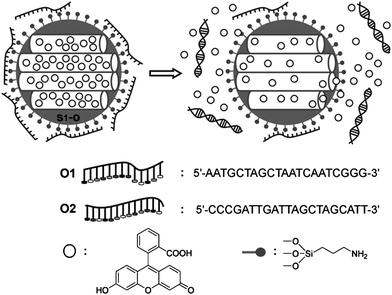 | ||
| Fig. 17 Schematic representation of a valve system based on MSNs functionalized with amino groups and capped with a single-stranded oligonucleotide (O1). Release of the trapped dye molecules is accomplished upon the addition of the complementary oligonucleotide (O2). The sequence of the oligonucleotides O1 and O2 is shown. Copyright 2010 Wiley. Used with permission from ref. 137. | ||
4.3. Saccharide-based molecular valve systems
Martínez-Máñez and Amorós et al.138,139 described the synthesis of saccharides-gated MSNs and its on-command guest delivery behavior in the presence of suitable enzymes. These nanovalve systems consists of nanoscopic MCM-41-based MSNs pore outlets functionalized with lactose and different “saccharide” derivatives (hydrolyzed starch, i.e., Glucidex 47, Glucidex 39, Glucidex 29) and loaded with dye molecules (Fig. 18). The anchoring of these saccharide derivatives inhibited cargo release due to the formation of a dense hydrogen-bonding interaction based saccharide network around the pore outlets. In the presence of pancreatin (containing amylases able to hydrolyze the 1→4 glycosidic bond between β-D-glucoses present in the starch) or β-D-galactosidase (able to hydrolyze the 1→4 glycosidic bond between β-D-galactose and β-D-glucose in lactose), the saccharide network gates were hydrolyzed, resulting in the uncapping of the pores of MSNs and the release of the entrapped cargo molecules in a controlled fashion. A clear control of the release rate was found depending on the used hydrolyzed starch derivative, which indicates the possibility of designing different delivery profiles via a simple selection of the hydrolyzation degree of the starch. It was also shown that the saccharide-gated MSNs can be efficiently taken up by both tumoral (HeLa) and nontumoral (LLC-PK1) cells, although a more efficient internalization in HeLa cells was observed. This cellular uptake is through the endocytosis targeting them to the autolysosomes, where the capping polysaccharides are hydrolyzed by lysosomal enzymes and the cargo is delivered. Chemotherapeutic agents such as DOX was loaded and delivered to cells, and a substantial reduction of cells was observed owing to the consequent release of the cytotoxic agent following the enzyme-dependent opening of the saccharide molecular gate. | ||
| Fig. 18 Schematic representation of nanovalve systems based on MSNs S1–S4 capped with carbohydrate derivatives and S5 functionalized with gluconamide. Addition of enzymes to S1–S4 uncapped the poresvia the selective hydrolysis of the saccharides mesoporous external surface, resulting in the cargo release. Reprinted with permission from ref. 139. Copyright 2010 American Chemical Society. | ||
4.4. Peptide sequences-based nanovalve system
Heise et al.140 describes the use of a simple tripeptide containing a 9-fluorenylmethyloxy-carbonyl (Fmoc) protecting group that regulates the pore closing on MSNs either by the π–π interactions between adjacent Fmoc moieties or simply due to the presence of bulky Fmoc groups that physically prevent the passage of cargo molecules from the particle core through steric hindrance. Successful enzymatic cleavage of the peptide sequence removes the Fmoc protecting group, enabling the peptide fragments remaining to become spatially independent of adjacent chains via electrostatic repulsion, therefore opening the gate to enable the passage of freely loaded guest molecules through the newly created spaces, offering the system highly selective responsiveness to release cargo molecules.Martínez-Máñez and Pérez-Payá et al.141 reported another example. MCM-41 support was first loaded with fluorescent dye—[Ru(bipy)3]Cl2—and then modified with azide group, which serves as a handle to attach the capping agents, i.e., a peptide sequence, in a suitable arrangement on MSN surface by “click” reaction to obtain the “zero release” material containing peptide sequences as gatekeepers. This valved nanodevice is specifically opened in the presence of targeted proteolytic enzymes (Fig. 19).
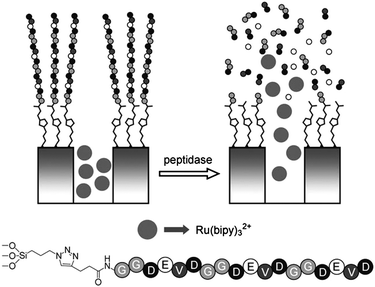 | ||
| Fig. 19 Schematic representation of nanovalve system based on MSNs capped with peptide sequence P1. The release of cargo molecules was achieved by enzymatic cleavage at the C-terminus amide bonds of the negatively charged amino acids contained in P1 (D and E). The sequence of the peptide is shown. Copyright 2011 Wiley. Used with permission from ref. 141. | ||
4.5. Lipid-bilayer-gated MSN delivery systems
An alternative design of capping systems involves the use of supported lipid bilayers to coat MSNs as gatekeepers, which mimic cellular envelopes and provide several natural advantages: (1) the lipid membrane enhances circulation time and accumulation in tumor cells; (2) lipid bilayers have the features of high biocompatibility, low toxicity and low immunogenicity which are very important for the use in cancer therapy; (3) nanoparticle-supported lipid membranes far exceed the structural stability of liposomes and offer narrow size distributions, both factors of key importance for successful drug delivery.142Bein et al.142 presented the efficient preparation of lipid bilayer coated MSNs (SLB@MSN), in which single colloidal MSNs are coated with an intact supported lipid bilayer using a solvent-exchange method. The effectiveness of the system for biomedical applications was demonstrated using colchicine, a drug binding to tubulin and inhibiting microtubule polymerization, as cargo molecule. Experiments indicate that colchicine can be effectively delivered into HuH7 liver cancer cells and released inside living cells to induce cell death after 120 min of inhibition of microtubule polymerization which shows an enhanced effect compared to the same dose of colchicine in solution. Herein, the uptake of colchicine-loaded SLB@MSN into the cells leads to cell death due to intracellular diffusive release of colchicine.
Very recently, Brinker and Ashley et al.143 reported that the MSN-SLB (protocells, they called, that synergistically combine properties of liposomes and nanoporous particles) modified with a targeting peptide that binds to human hepatocellular carcinoma exhibits a 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000-fold greater affinity for human hepatocellular carcinoma than for hepatocytes, endothelial cells or immune cells. More significantly, the protocells can be loaded with combinations of therapeutic (drugs, small interfering RNA and toxins) and diagnostic (quantum dots) agents and modified to promote endosomal escape and nuclear accumulation of selected cargos (Fig. 20). The vast capacity of the high-surface-area MSNs and the enhanced targeting efficacy enabled by SLB facilitate a single protocell loaded with drugs to kill a drug-resistant human hepatocellular carcinoma cell, showing a 106-fold improvement over comparable liposomes. So far, no other nanoparticle-based drug-delivery nanocarrier has been reported to possess all of these attributes, making protocells the first example of a nanocarrier that simultaneously addresses the complex requirements of targeted, multi-component delivery for cancer therapy.
000-fold greater affinity for human hepatocellular carcinoma than for hepatocytes, endothelial cells or immune cells. More significantly, the protocells can be loaded with combinations of therapeutic (drugs, small interfering RNA and toxins) and diagnostic (quantum dots) agents and modified to promote endosomal escape and nuclear accumulation of selected cargos (Fig. 20). The vast capacity of the high-surface-area MSNs and the enhanced targeting efficacy enabled by SLB facilitate a single protocell loaded with drugs to kill a drug-resistant human hepatocellular carcinoma cell, showing a 106-fold improvement over comparable liposomes. So far, no other nanoparticle-based drug-delivery nanocarrier has been reported to possess all of these attributes, making protocells the first example of a nanocarrier that simultaneously addresses the complex requirements of targeted, multi-component delivery for cancer therapy.
 | ||
| Fig. 20 Schematic diagram depicting the successive steps of multivalent binding and internalization of targeted protocells, followed by endosomal escape and nuclear localization of protocell-encapsulated cargo. DOPC protocells (1) bind to HCC with high affinity owing to recruitment of SP94 targeting peptides (magenta) to the cell surface, (2) become internalized by receptor-mediated endocytosis and (3) release their cargo into the cytosol on endosome acidification and protonation of the H5WYG fusogenic peptide (blue). (4) Cargos modified with an NLS are transported through the nuclear pore complex and become concentrated in the nucleus. Reprinted by permission from Macmillan Publishers Ltd (ref. 143), copyright 2011. | ||
5. Conclusions
In summary, we have presented the recent development of supramolecule-, polymer- and biomolecule-based molecular and supramolecular nanovalves on the surface of MSNs for therapeutic drug delivery systems. The non-cytotoxicity of MSNs, along with their tunable rigid structures, ease of chemical synthesis and modification with organic compounds have made MSNs a superior platform on which to construct “smart” machinery for drug delivery. Scientists have made tremendous research progress towards the development of biocompatible molecular and supramolecular nanovalves to realize only the release of entrapped cargo molecules in response to a couple of external applied or other types of stimuli. Since the first construction and operation of nanovalves only in organic solvents, much effort has been thrown to the creation of robust nanovalves that operate in aqueous solution or biological relevant conditions to gain the biocompatibility for drug delivery, gene transfection and bioimaging applications. Although scientists have achieved great success towards operable biocompatible nanovalves based on MSNs, there still remain some challenges and questions that need further scientific attention and investigation, and there will be a long way to go for its final real application on human body. For example, studies need to be done to demonstrate (a) how to overcome the in vivo heterogeneity of biological systems (i.e., tumor microenvironments) when applying a specific nanovalve-based drug delivery system to different disease areas, (b) how one can prove the optimal operation of nanovalves that target a specific cancer or other diseases, just like the specificity of enzyme in biological world, (c) how one can make a general rule for the comparison or incorporation of nanovalve systems with different types of gates, such as molecular/supramolecular switches, polymeric valves, and biomolecular caps and screen for different cancer treatment, (d) what is the fate of these drug delivery systems in cells, animals and even human bodies, etc. However, only a few biological studies in cellular-based systems have been reported so far, with the primary achievements to date being largely platform design and construction of MSN-based nanovalves or gates due to the early developmental phase of this emerging field. To answer these questions and realize its final applications in curing cancer diseases, more biological studies, especially investigations in animal models and clinical trials, on selected biocompatible nanovalve systems based on MSNs have to be done to make them fit for therapeutic treatments and clinical use without or with only minimal undesired side-effects. Given that the focus is cancer therapy, we would predict that the incorporation of molecular/supramolecular nanovalve systems with biomolecular gates might prove optimal, but the recently developed systems or to-be-optimized systems with no matter what type of valves or gates hold promise in cancer therapy in human body in the near future. Furthermore, compared with mesoporous silicas in terms of applications under different external stimuli, we suggest that some newly developed mesoporous materials or metal-doped mesoporous materials, i.e., periodic mesoporous organosilicas (PMO),144–147 mesoporous carbon nanoparticles,148–151 and iron/silver/gold-doped MSNs,152 which possess better hydrothermal stability, biocompatibility, functionality and/or higher pore volumes will be more and more attractive to be used as the nanocontainer in a nanovalve system for drug delivery applications and deserve more attention.153 We believe that the proven safety of the nanovalve systems reviewed above will set the stage for clinical testing of some nanocarriers for cancer treatment, and eventually some of the biocompatible nanovalve systems will be on the market and deliver therapeutic agents to human bodies to cure the cancers that cause so many people to suffer.Acknowledgements
The author acknowledges the funding support from the State Key Lab of Supramolecular Structure & Materials and College of Chemistry at Jilin University. The author is grateful for the instruction given by Professor J Fraser Stoddart, Professor Jeffrey I. Zink, and Professor Fuyuhiko Tamanoi in our experimental efforts towards the development of biocompatible nanovalves, and for the contributions made by Sarah Angelos, Kaushik Patel, Niveen M. Khashab, Yaroslav Klichko, Hussam A. Khatib, Ali Trabolsi, Monty Liong, Eunshil Choi, Yuen Lau, Travis Pecorelli, etc. The author also thanks Prof. Sean Xiao-an Zhang and Prof. Minjie Li for their assistance during manuscript preparation. The author also wish to thank the journal editor, Dr Sylvie Garneau-Tsodikova, and the reviewers who raised valuable points that help improve the quality of the presentation of this review article.Notes and references
- J. M. Rosenholm, C. Sahlgren and M. Linden, Nanoscale, 2010, 2, 1870–1883 RSC.
- M. Vallet-Regi, A. Ramila, R. P. del Real and J. Perez-Pariente, Chem. Mater., 2001, 13, 308–311 CrossRef CAS.
- C. Barbe, J. Bartlett, L. G. Kong, K. Finnie, H. Q. Lin, M. Larkin, S. Calleja, A. Bush and G. Calleja, Adv. Mater., 2004, 16, 1959–1966 CrossRef CAS.
- D. Avnir, T. Coradin, O. Lev and J. Livage, J. Mater. Chem., 2006, 16, 1013–1030 RSC.
- Q. J. He and J. L. Shi, J. Mater. Chem., 2011, 21, 5845–5855 RSC.
- Z. M. Tao, B. Toms, J. Goodisman and T. Asefa, ACS Nano, 2010, 4, 789–794 CrossRef CAS.
- C. H. Lei, P. Liu, B. W. Chen, Y. M. Mao, H. Engelmann, Y. Shin, J. Jaffar, I. Hellstrom, J. Liu and K. E. Hellstrom, J. Am. Chem. Soc., 2010, 132, 6906–6907 CrossRef CAS.
- Q. J. He, Z. W. Zhang, Y. Gao, J. L. Shi and Y. P. Li, Small, 2009, 5, 2722–2729 CrossRef CAS.
- A. B. Descalzo, R. Martinez-Manez, F. Sancenon, K. Hoffmann and K. Rurack, Angew. Chem., Int. Ed., 2006, 45, 5924–5948 CrossRef CAS.
- R. Klajn, J. F. Stoddart and B. A. Grzybowski, Chem. Soc. Rev., 2010, 39, 2203–2237 RSC.
- P. DeMuth, M. Hurley, C. W. Wu, S. Galanie, M. R. Zachariah and P. DeShong, Microporous Mesoporous Mater., 2011, 141, 128–134 CrossRef CAS.
- J. M. Rosenholm, A. Meinander, E. Peuhu, R. Niemi, J. E. Eriksson, C. Sahlgren and M. Linden, ACS Nano, 2009, 3, 197–206 CrossRef CAS.
- J. M. Rosenholm, E. Peuhu, J. E. Eriksson, C. Sahlgren and M. Linden, Nano Lett., 2009, 9, 3308–3311 CrossRef CAS.
- M. Liong, S. Angelos, E. Choi, K. Patel, J. F. Stoddart and J. I. Zink, J. Mater. Chem., 2009, 19, 6251–6257 RSC.
- J. L. Vivero-Escoto, I. I. Slowing, B. G. Trewyn and V. S. Y. Lin, Small, 2010, 6, 1952–1967 CrossRef CAS.
- Y. Klichko, M. Liong, E. Choi, S. Angelos, A. E. Nel, J. F. Stoddart, F. Tamanoi and J. I. Zink, J. Am. Ceram. Soc., 2009, 92, S2–S10 CrossRef CAS.
- I. I. Slowing, J. L. Vivero-Escoto, B. G. Trewyn and V. S. Y. Lin, J. Mater. Chem., 2010, 20, 7924–7937 RSC.
- Y. B. Zheng, B. Kiraly and T. J. Huang, Nanomedicine, 2010, 5, 1309–1312 CrossRef CAS.
- B. G. Trewyn, I. I. Slowing, S. Giri, H. T. Chen and V. S. Y. Lin, Acc. Chem. Res., 2007, 40, 846–853 CrossRef CAS.
- S. Angelos, E. Johansson, J. F. Stoddart and J. I. Zink, Adv. Funct. Mater., 2007, 17, 2261–2271 CrossRef CAS.
- J. M. Rosenholm, E. Peuhu, L. T. Bate-Eya, J. E. Eriksson, C. Sahlgren and M. Linden, Small, 2010, 6, 1234–1241 CAS.
- A. M. Chen, M. Zhang, D. G. Wei, D. Stueber, O. Taratula, T. Minko and H. X. He, Small, 2009, 5, 2673–2677 CrossRef CAS.
- C. P. Tsai, C. Y. Chen, Y. Hung, F. H. Chang and C. Y. Mou, J. Mater. Chem., 2009, 19, 5737–5743 RSC.
- P. A. Gale, Chem. Soc. Rev., 2007, 36, 141–142 RSC.
- C. Caltagirone and P. A. Gale, Chem. Soc. Rev., 2009, 38, 520–563 RSC.
- J. H. Hartley, T. D. James and C. J. Ward, J. Chem. Soc., Perkin Trans. 1, 2000, 3155–3184 RSC.
- Y. W. Yang, Y. Chen and Y. Liu, Inorg. Chem., 2006, 45, 3014–3022 CrossRef CAS.
- Y. Liu, Y. W. Yang, L. Li and Y. Chen, Org. Biomol. Chem., 2004, 2, 1542–1548 CAS.
- Y. Liu, Z. Fan, H. Y. Zhang, Y. W. Yang, F. Ding, S. X. Liu, X. Wu, T. Wada and Y. Inoue, J. Org. Chem., 2003, 68, 8345–8352 CrossRef CAS.
- Y. Liu, Y. W. Yang, R. Cao, S. H. Song, H. Y. Zhang and L. H. Wang, J. Phys. Chem. B, 2003, 107, 14130–14139 CrossRef CAS.
- Y. Liu, Y. W. Yang, Y. Song, H. Y. Zhang, F. Ding, T. Wada and Y. Inoue, ChemBioChem, 2004, 5, 868–871 CrossRef CAS.
- Y. Liu, Y. W. Yang, H. Y. Zhang, B. W. Hu, F. Ding and C. J. Li, Chem. Biodiversity, 2004, 1, 481–488 CAS.
- Y. Liu, Y. W. Yang, E. C. Yang and X. D. Guan, J. Org. Chem., 2004, 69, 6590–6602 CrossRef CAS.
- V. Balzani, A. Credi, F. M. Raymo and J. F. Stoddart, Angew. Chem., Int. Ed., 2000, 39, 3348–3391 CrossRef CAS.
- T. B. Gasa, C. Valente and J. F. Stoddart, Chem. Soc. Rev., 2011, 40, 57–78 RSC.
- J. F. Stoddart, Chem. Soc. Rev., 2009, 38, 1802–1820 RSC.
- F. M. Raymo and J. F. Stoddart, Chem. Rev., 1999, 99, 1643–1663 CrossRef CAS.
- S. J. Rowan, S. J. Cantrill, G. R. L. Cousins, J. K. M. Sanders and J. F. Stoddart, Angew. Chem., Int. Ed., 2002, 41, 898–952 CrossRef.
- B. K. Juluri, A. S. Kumar, Y. Liu, T. Ye, Y. W. Yang, A. H. Flood, L. Fang, J. F. Stoddart, P. S. Weiss and T. J. Huang, ACS Nano, 2009, 3, 291–300 CrossRef CAS.
- Y. B. Zheng, Y. W. Yang, L. Jensen, L. Fang, B. K. Juluri, A. H. Flood, P. S. Weiss, J. F. Stoddart and T. J. Huang, Nano Lett., 2009, 9, 819–825 CrossRef CAS.
- L. Fang, M. Hmadeh, J. S. Wu, M. A. Olson, J. M. Spruell, A. Trabolsi, Y. W. Yang, M. Elhabiri, A. M. Albrecht-Gary and J. F. Stoddart, J. Am. Chem. Soc., 2009, 131, 7126–7134 CrossRef CAS.
- Y. W. Yang, H. Y. Zhang and Y. Liu, Supramol. Chem., 2008, 20, 731–736 CrossRef CAS.
- D. B. Li, W. F. Paxton, R. H. Baughman, T. J. Huang, J. F. Stoddart and P. S. Weiss, MRS Bull., 2009, 34, 671–681 CrossRef CAS.
- T. J. Huang and B. K. Juluri, Nanomedicine, 2008, 3, 107–124 CrossRef CAS.
- T. J. Huang, MRS Bull., 2008, 33, 226–231 CrossRef CAS.
- T. J. Huang, B. Brough, C. M. Ho, Y. Liu, A. H. Flood, P. A. Bonvallet, H. R. Tseng, J. F. Stoddart, M. Baller and S. Magonov, Appl. Phys. Lett., 2004, 85, 5391–5393 CrossRef CAS.
- T. J. Huang, H. R. Tseng, L. Sha, W. X. Lu, B. Brough, A. H. Flood, B. D. Yu, P. C. Celestre, J. P. Chang, J. F. Stoddart and C. M. Ho, Nano Lett., 2004, 4, 2065–2071 CrossRef CAS.
- P. R. Ashton, D. Philip, N. Spencer and J. F. Stoddart, J. Chem. Soc., Chem. Commun., 1991, 1677–1679 RSC.
- J. O. Jeppesen, J. Perkins, J. Becher and J. F. Stoddart, Angew. Chem., 2001, 113, 1256–1261 CrossRef.
- Y. Liu, A. H. Flood, P. A. Bonvallett, S. A. Vignon, B. H. Northrop, H. R. Tseng, J. O. Jeppesen, T. J. Huang, B. Brough, M. Baller, S. Magonov, S. D. Solares, W. A. Goddard, C. M. Ho and J. F. Stoddart, J. Am. Chem. Soc., 2005, 127, 9745–9759 CrossRef CAS.
- T. D. Nguyen, K. C. F. Leung, M. Liong, C. D. Pentecost, J. F. Stoddart and J. I. Zink, Org. Lett., 2006, 8, 3363–3366 CrossRef CAS.
- K. C. F. Leung, T. D. Nguyen, J. F. Stoddart and J. I. Zink, Chem. Mater., 2006, 18, 5919–5928 CrossRef CAS.
- N. K. Mal, M. Fujiwara and Y. Tanaka, Nature, 2003, 421, 350–353 CrossRef CAS.
- N. G. Liu, D. R. Dunphy, P. Atanassov, S. D. Bunge, Z. Chen, G. P. Lopez, T. J. Boyle and C. J. Brinker, Nano Lett., 2004, 4, 551–554 CrossRef CAS.
- T. D. Nguyen, K. C. F. Leung, M. Liong, Y. Liu, J. F. Stoddart and J. I. Zink, Adv. Funct. Mater., 2007, 17, 2101–2110 CrossRef CAS.
- R. Hernandez, H. R. Tseng, J. W. Wong, J. F. Stoddart and J. I. Zink, J. Am. Chem. Soc., 2004, 126, 3370–3371 CrossRef CAS.
- T. D. Nguyen, H. R. Tseng, P. C. Celestre, A. H. Flood, Y. Liu, J. F. Stoddart and J. I. Zink, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 10029–10034 CrossRef CAS.
- T. D. Nguyen, Y. Liu, S. Saha, K. C. F. Leung, J. F. Stoddart and J. I. Zink, J. Am. Chem. Soc., 2007, 129, 626–634 CrossRef CAS.
- S. Saha, K. C. F. Leung, T. D. Nguyen, J. F. Stoddart and J. I. Zink, Adv. Funct. Mater., 2007, 17, 685–693 CrossRef CAS.
- K. K. Coti, M. E. Belowich, M. Liong, M. W. Ambrogio, Y. A. Lau, H. A. Khatib, J. I. Zink, N. M. Khashab and J. F. Stoddart, Nanoscale, 2009, 1, 16–39 RSC.
- M. M. Boyle, R. A. Smaldone, A. C. Whalley, M. W. Ambrogio, Y. Y. Botros and J. F. Stoddart, Chem. Sci., 2011, 2, 204–210 RSC.
- S. Angelos, E. Johansson, J. F. Stoddart and J. I. Zink, Adv. Funct. Mater., 2007, 17, 2261–2271 CrossRef CAS.
- J. Lagona, P. Mukhopadhyay, S. Chakrabarti and L. Isaacs, Angew. Chem., Int. Ed., 2005, 44, 4844–4870 CrossRef CAS.
- K. Kim, N. Selvapalam, Y. H. Ko, K. M. Park, D. Kim and J. Kim, Chem. Soc. Rev., 2007, 36, 267–279 RSC.
- J. W. Lee, S. Samal, N. Selvapalam, H. J. Kim and K. Kim, Acc. Chem. Res., 2003, 36, 621–630 CrossRef CAS.
- K. Kim, Chem. Soc. Rev., 2002, 31, 96–107 RSC.
- A. Harada, Acc. Chem. Res., 2001, 34, 456–464 CrossRef CAS.
- Y. Chen and Y. Liu, Chem. Soc. Rev., 2010, 39, 495–505 RSC.
- J. Li and X. J. Loh, Adv. Drug Delivery Rev., 2008, 60, 1000–1017 CrossRef CAS.
- G. Wenz, B. H. Han and A. Muller, Chem. Rev., 2006, 106, 782–817 CrossRef CAS.
- E. Engeldinger, D. Armspach and D. Matt, Chem. Rev., 2003, 103, 4147–4173 CrossRef CAS.
- V. J. Stella, V. M. Rao, E. A. Zannou and V. Zia, Adv. Drug Delivery Rev., 1999, 36, 3–16 CrossRef CAS.
- F. Hirayama and K. Uekama, Adv. Drug Delivery Rev., 1999, 36, 125–141 CrossRef CAS.
- Y. Liu and Y. Chen, Acc. Chem. Res., 2006, 39, 681–691 CrossRef CAS.
- Y. Liu, S. H. Song, Y. Chen, Y. L. Zhao and Y. W. Yang, Chem. Commun., 2005, 1702–1704 RSC.
- Y. Liu, Y. W. Yang and Y. Chen, Chem. Commun., 2005, 4208–4210 RSC.
- Y. Liu, Y. W. Yang, Y. Chen and H. X. Zou, Macromolecules, 2005, 38, 5838–5840 CrossRef CAS.
- Y. Liu, Y. W. Yang, Y. Chen and F. Ding, Bioorg. Med. Chem., 2005, 13, 963–971 CrossRef CAS.
- H. Gao, Y. W. Yang, Y. G. Fan and J. B. Ma, J. Controlled Release, 2006, 112, 301–311 CrossRef CAS.
- S. M. Liu, C. Ruspic, P. Mukhopadhyay, S. Chakrabarti, P. Y. Zavalij and L. Isaacs, J. Am. Chem. Soc., 2005, 127, 15959–15967 CrossRef CAS.
- A. Day, A. P. Arnold, R. J. Blanch and B. Snushall, J. Org. Chem., 2001, 66, 8094–8100 CrossRef CAS.
- R. Behrend, E. Meyer and F. Rusche, Justus Liebigs Ann. Chem., 1905, 339, 1–37 CrossRef.
- C. Marquez, R. R. Hudgins and W. M. Nau, J. Am. Chem. Soc., 2004, 126, 5806–5816 CrossRef CAS.
- D. Tuncel and J. H. G. Steinke, Chem. Commun., 2001, 253–254 RSC.
- D. Tuncel, O. Ozsar, H. B. Tiftik and B. Salih, Chem. Commun., 2007, 1369–1371 RSC.
- D. Tuncel and J. H. G. Steinke, Chem. Commun., 2002, 496–497 RSC.
- D. Tuncel and J. H. G. Steinke, Chem. Commun., 1999, 1509–1510 RSC.
- W. L. Mock, T. A. Irra, J. P. Wepsiec and T. L. Manimaran, J. Org. Chem., 1983, 48, 3619–3620 CrossRef CAS.
- W. L. Mock, T. A. Irra, J. P. Wepsiec and M. Adhya, J. Org. Chem., 1989, 54, 5302–5308 CrossRef CAS.
- T. C. Krasia and J. H. G. Steinke, Chem. Commun., 2002, 22–23 RSC.
- R. Huisgen, G. Szeimies and L. Möbius, Chem. Ber., 1967, 100, 2494–2507 CrossRef CAS.
- R. Huisgen, Pure Appl. Chem., 1989, 61, 613–628 CrossRef CAS.
- H. C. Kolb, M. G. Finn and K. B. Sharpless, Angew. Chem., Int. Ed., 2001, 40, 2004–2021 CrossRef CAS.
- V. V. Rostovtsev, L. G. Green, V. V. Fokin and K. B. Sharpless, Angew. Chem., Int. Ed., 2002, 41, 2596–2599 CrossRef CAS.
- C. W. Tornoe, C. Christensen and M. Meldal, J. Org. Chem., 2002, 67, 3057–3064 CrossRef CAS.
- W. R. Dichtel, O. S. Miljanic, J. M. Spruell, J. R. Heath and J. F. Stoddart, J. Am. Chem. Soc., 2006, 128, 10388–10390 CrossRef CAS.
- O.Š. Miljanić, W. R. Dichtel, I. Aprahamian, R. D. Rohde, H. D. Agnew, J. R. Heath and J. Fraser Stoddart, QSAR Comb. Sci., 2007, 26, 1165–1174 Search PubMed.
- S. Angelos, Y. W. Yang, K. Patel, J. F. Stoddart and J. I. Zink, Angew. Chem., Int. Ed., 2008, 47, 2222–2226 CrossRef CAS.
- S. Angelos, N. M. Khashab, Y. W. Yang, A. Trabolsi, H. A. Khatib, J. F. Stoddart and J. I. Zink, J. Am. Chem. Soc., 2009, 131, 12912–12914 CrossRef CAS.
- S. Angelos, Y. W. Yang, N. M. Khashab, J. F. Stoddart and J. I. Zink, J. Am. Chem. Soc., 2009, 131, 11344–11346 CrossRef CAS.
- N. M. Khashab, M. E. Belowich, A. Trabolsi, D. C. Friedman, C. Valente, Y. N. Lau, H. A. Khatib, J. I. Zink and J. F. Stoddart, Chem. Commun., 2009, 5371–5373 RSC.
- M. W. Ambrogio, T. A. Pecorelli, K. Patel, N. M. Khashab, A. Trabolsi, H. A. Khatib, Y. Y. Botros, J. I. Zink and J. F. Stoddarrt, Org. Lett., 2010, 12, 3304–3307 CrossRef CAS.
- C. R. Thomas, D. P. Ferris, J. H. Lee, E. Choi, M. H. Cho, E. S. Kim, J. F. Stoddart, J. S. Shin, J. Cheon and J. I. Zink, J. Am. Chem. Soc., 2010, 132, 10623–10625 CrossRef CAS.
- N. M. Khashab, A. Trabolsi, Y. A. Lau, M. W. Ambrogio, D. C. Friedman, H. A. Khatib, J. I. Zink and J. F. Stoddart, Eur. J. Org. Chem., 2009, 1669–1673 CrossRef CAS.
- J. S. Liu and X. Z. Du, J. Mater. Chem., 2010, 20, 3642–3649 RSC.
- J. S. Liu, X. Z. Du and X. F. Zhang, Chem.–Eur. J., 2011, 17, 810–815 CrossRef CAS.
- C. Park, K. Oh, S. C. Lee and C. Kim, Angew. Chem., Int. Ed., 2007, 46, 1455–1457 CrossRef CAS.
- K. Patel, S. Angelos, W. R. Dichtel, A. Coskun, Y. W. Yang, J. I. Zink and J. F. Stoddart, J. Am. Chem. Soc., 2008, 130, 2382–2383 CrossRef CAS.
- H. Meng, M. Xue, T. Xia, Y.-L. Zhao, F. Tamanoi, J. F. Stoddart, J. I. Zink and A. E. Nel, J. Am. Chem. Soc., 2010, 132, 12690–12697 CrossRef CAS.
- K. Greish, J. Drug Targeting, 2007, 15, 457–464 CrossRef CAS.
- L. Du, S. J. Liao, H. A. Khatib, J. F. Stoddart and J. I. Zink, J. Am. Chem. Soc., 2009, 131, 15136–15142 CrossRef CAS.
- Y. L. Zhao, Z. X. Li, S. Kabehie, Y. Y. Botros, J. F. Stoddart and J. I. Zink, J. Am. Chem. Soc., 2010, 132, 13016–13025 CrossRef CAS.
- D. P. Ferris, Y. L. Zhao, N. M. Khashab, H. A. Khatib, J. F. Stoddart and J. I. Zink, J. Am. Chem. Soc., 2009, 131, 1686–1688 CrossRef CAS.
- C. Park, H. Kim, S. Kim and C. Kim, J. Am. Chem. Soc., 2009, 131, 16614–16615 CrossRef CAS.
- H. Kim, S. Kim, C. Park, H. Lee, H. J. Park and C. Kim, Adv. Mater., 2010, 22, 4280–4283 CrossRef CAS.
- S. Giri, B. G. Trewyn, M. P. Stellmaker and V. S. Y. Lin, Angew. Chem., Int. Ed., 2005, 44, 5038–5044 CrossRef CAS.
- C. Y. Lai, B. G. Trewyn, D. M. Jeftinija, K. Jeftinija, S. Xu, S. Jeftinija and V. S. Y. Lin, J. Am. Chem. Soc., 2003, 125, 4451–4459 CrossRef CAS.
- F. Torney, B. G. Trewyn, V. S. Y. Lin and K. Wang, Nat. Nanotechnol., 2007, 2, 295–300 CrossRef CAS.
- J. L. Vivero-Escoto, I. I. Slowing, C. W. Wu and V. S. Y. Lin, J. Am. Chem. Soc., 2009, 131, 3462–3463 CrossRef CAS.
- E. Aznar, M. D. Marcos, R. Martinez-Manez, F. Sancenon, J. Soto, P. Amoros and C. Guillem, J. Am. Chem. Soc., 2009, 131, 6833–6843 CrossRef CAS.
- I. I. Slowing, B. G. Trewyn, S. Giri and V. S. Y. Lin, Adv. Funct. Mater., 2007, 17, 1225–1236 CrossRef CAS.
- I. I. Slowing, B. G. Trewyn and V. S. Y. Lin, J. Am. Chem. Soc., 2007, 129, 8845–8849 CrossRef CAS.
- B. G. Trewyn, S. Giri, I. I. Slowing and V. S. Y. Lin, Chem. Commun., 2007, 3236–3245 RSC.
- D. R. Radu, C. Y. Lai, K. Jeftinija, E. W. Rowe, S. Jeftinija and V. S. Y. Lin, J. Am. Chem. Soc., 2004, 126, 13216–13217 CrossRef CAS.
- C. Coll, E. Aznar, R. Martinez-Manez, M. D. Marcos, F. Sancenon, J. Soto, P. Amoros, J. Cano and E. Ruiz, Chem.–Eur. J., 2010, 16, 10048–10061 CrossRef CAS.
- R. Liu, X. Zhao, T. Wu and P. Y. Feng, J. Am. Chem. Soc., 2008, 130, 14418–14419 CrossRef CAS.
- R. Liu, Y. Zhang and P. Y. Feng, J. Am. Chem. Soc., 2009, 131, 15128–15129 CrossRef CAS.
- C. Y. Hong, X. Li and C. Y. Pan, J. Mater. Chem., 2009, 19, 5155–5160 RSC.
- R. Liu, P. H. Liao, J. K. Liu and P. Y. Feng, Langmuir, 2011, 27, 3095–3099 CrossRef CAS.
- X. J. Wan, D. Wang and S. Y. Liu, Langmuir, 2010, 26, 15574–15579 CrossRef CAS.
- Y. Z. You, K. K. Kalebaila, S. L. Brock and D. Oupicky, Chem. Mater., 2008, 20, 3354–3359 CrossRef CAS.
- A. Schlossbauer, J. Kecht and T. Bein, Angew. Chem., Int. Ed., 2009, 48, 3092–3095 CrossRef CAS.
- E. Climent, A. Bernardos, R. Martinez-Manez, A. Maquieira, M. D. Marcos, N. Pastor-Navarro, R. Puchades, F. Sancenon, J. Soto and P. Amoros, J. Am. Chem. Soc., 2009, 131, 14075–14080 CrossRef CAS.
- A. Schlossbauer, S. Warncke, P. M. E. Gramlich, J. Kecht, A. Manetto, T. Carell and T. Bein, Angew. Chem., Int. Ed., 2010, 49, 4734–4737 CAS.
- C. E. Chen, J. Geng, F. Pu, X. J. Yang, J. S. Ren and X. G. Qu, Angew. Chem., Int. Ed., 2011, 50, 882–886 CrossRef CAS.
- C. L. Zhu, C. H. Lu, X. Y. Song, H. H. Yang and X. R. Wang, J. Am. Chem. Soc., 2011, 133, 1278–1281 CrossRef CAS.
- E. Climent, R. Martinez-Manez, F. Sancenon, M. D. Marcos, J. Soto, A. Maquieira and P. Amoros, Angew. Chem., Int. Ed., 2010, 49, 7281–7283 CrossRef CAS.
- A. Bernardos, E. Aznar, M. D. Marcos, R. Martinez-Manez, F. Sancenon, J. Soto, J. M. Barat and P. Amoros, Angew. Chem., Int. Ed., 2009, 48, 5884–5887 CrossRef CAS.
- A. Bernardos, L. Mondragon, E. Aznar, M. D. Marcos, R. Martinez-Manez, F. Sancenon, J. Soto, J. M. Barat, E. Perez-Paya, C. Guillem and P. Amoros, ACS Nano, 2010, 4, 6353–6368 CrossRef CAS.
- P. D. Thornton and A. Heise, J. Am. Chem. Soc., 2010, 132, 2024–2028 CrossRef CAS.
- C. Coll, L. Mondragon, R. Martinez-Manez, F. Sancenon, M. D. Marcos, J. Soto, P. Amoros and E. Perez-Paya, Angew. Chem., Int. Ed., 2011, 50, 2138–2140 CrossRef CAS.
- V. Cauda, H. Engelke, A. Sauer, D. Arcizet, C. Brauchle, J. Radler and T. Bein, Nano Lett., 2010, 10, 2484–2492 CrossRef CAS.
- C. E. Ashley, E. C. Carnes, G. K. Phillips, D. Padilla, P. N. Durfee, P. A. Brown, T. N. Hanna, J. W. Liu, B. Phillips, M. B. Carter, N. J. Carroll, X. M. Jiang, D. R. Dunphy, C. L. Willman, D. N. Petsev, D. G. Evans, A. N. Parikh, B. Chackerian, W. Wharton, D. S. Peabody and C. J. Brinker, Nat. Mater., 2011, 10, 389–397 CrossRef CAS.
- W. Guo, J. Wang, S. J. Lee, F. Dong, S. S. Park and C. S. Ha, Chem.–Eur. J., 2010, 16, 8641–8646 CrossRef CAS.
- S. Hudson, J. Cooney, B. K. Hodnett and E. Magner, Chem. Mater., 2007, 19, 2049–2055 CrossRef CAS.
- S. Inagaki, S. Guan, Y. Fukushima, T. Ohsuna and O. Terasaki, J. Am. Chem. Soc., 1999, 121, 9611–9614 CrossRef CAS.
- W. Cai, I. R. Gentle, G. Q. Lu, J.-J. Zhu and A. Yu, Anal. Chem., 2008, 80, 5401–5406 CrossRef CAS.
- J. L. Gu, S. S. Su, Y. S. Li, Q. J. He and J. L. Shi, Chem. Commun., 2011, 47, 2101–2103 RSC.
- Y. Fang, D. Gu, Y. Zou, Z. X. Wu, F. Y. Li, R. C. Che, Y. H. Deng, B. Tu and D. Y. Zhao, Angew. Chem., Int. Ed., 2010, 49, 7987–7991 CrossRef CAS.
- T. W. Kim, P. W. Chung, I. I. Slowing, M. Tsunoda, E. S. Yeung and V. S. Y. Lin, Nano Lett., 2008, 8, 3724–3727 CrossRef CAS.
- B. Hu, K. Wang, L. H. Wu, S. H. Yu, M. Antonietti and M. M. Titirici, Adv. Mater., 2010, 22, 813–828 CrossRef CAS.
- M. Liong, J. Lu, M. Kovochich, T. Xia, S. G. Ruehm, A. E. Nel, F. Tamanoi and J. I. Zink, ACS Nano, 2008, 2, 889–896 CrossRef CAS.
- M. W. Ambrogio, C. R. Thomas, Y.-L. Zhao, J. I. Zink and J. F. Stoddart, Acc. Chem. Res., 2011 DOI:10.1021/ar200018x.
| This journal is © The Royal Society of Chemistry 2011 |
