Second generation analogues of RKA182: synthetic tetraoxanes with outstanding in vitro and in vivoantimalarial activities†
Francesc
Marti
a,
James
Chadwick
a,
Richard K.
Amewu
a,
Hollie
Burrell-Saward
b,
Abhishek
Srivastava
c,
Stephen A.
Ward
c,
Raman
Sharma
a,
Neil
Berry
a and
Paul M.
O'Neill
*a
aDepartment of Chemistry, University of Liverpool, Liverpool, L69 7ZD, UK. E-mail: pmoneill@liverpool.ac.uk
bDepartment of Infectious and Tropical Disease, London School of Hygiene and Tropical Medicine, Keppel Street, London, WC1E 7HT, UK
cLiverpool School of Tropical Medicine, Pembroke Place, Liverpool, L3 5QA, UK
First published on 9th June 2011
Abstract
A series of polar dispiro-1,2,4,5-tetraoxanes have been designed and synthesized by parallel synthesis. From this series, endoperoxides with activity as low as 0.2 nM have been obtained and representatives of this group have excellent oral activities in the P. bergheiANKA mouse model of malaria.
Malaria is a parasitic disease that causes over 1 million deaths every year.1 The emergence of resistance to most available drugs,2 including the semi-synthetic artemisinin derivatives artemether and artesunate,3 has led to efforts to create new synthetic peroxides as potential antimalarial agents. Leading examples of synthetic endoperoxides include OZ277 (arterolane), a molecule in Phase III clinical trials in combination with piperaquine,4,5 and OZ439, a second generation derivative with improved pharmacokinetics and enhanced in vivoantimalarial activity.6 1,2,4,5-Tetraoxanes7–18 are another class of peroxides, which have proved to be an interesting pharmacophore that possesses excellent antimalarial activity against both chloroquine-resistant and chloroquine-sensitive strains of Plasmodium falciparum.9,18 Previously in our group, RKA182 (Fig. 1) was selected as a candidate for full preclinical development from a series of synthetic tetraoxane derivatives; this compound shows superior in vitro and in vivo activity to artemether and artesunate, has good oral bioavailability in rodent models and is more stable than arterolane in malaria infected human red blood cells.19
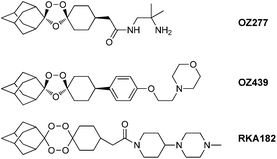 | ||
| Fig. 1 Structures of synthetic peroxides OZ277, OZ439 and RKA182. | ||
In this communication we describe the design and synthesis of a new series of tetraoxanes and present data on their in vitro and in vivoantimalarial activity. The new series was designed to eliminate any potential metabolic liability due to the amide linkage which is present in the lead candidate RKA182 (Fig. 2). Our hypothesis was that elimination of the amide linkage would be expected to provide compounds with increased metabolic stability and we provide results of a preliminary metabolic stability study comparing one of the compounds from the new series with candidate RKA182.
 | ||
| Fig. 2 Elimination of the amide linkage provides second generation analogues. | ||
We designed our synthesis to utilize a common intermediate for divergent parallel synthesis. The synthesis is depicted in Scheme 1 and begins with sodium borohydride reduction of 1,4-cyclohexanedione monoethylene ketal 1 to provide alcohol 2 which was immediately subjected to acidic conditions to produce the deprotected ketone 3.20Acetylation of 3 with acetyl chloride furnished ketone 4. Formation of a gem-dihydroperoxide followed by rhenium(VII) oxide-catalysed condensation with 2-adamantanone gave tetraoxane 5.21Deacetylation of 5 with lithium hydroxide gave alcohol 6 and oxidation of this alcohol with Jones reagent gave the key intermediate 7. With this intermediate in hand we prepared a small library of dispiro-1,2,4,5-tetraoxanes using a reductive amination approach. Reaction of 7 with various primary and secondary amines followed by reduction with sodium triacetoxyborohydride gave the analogues 8–24 depicted in Table 1.
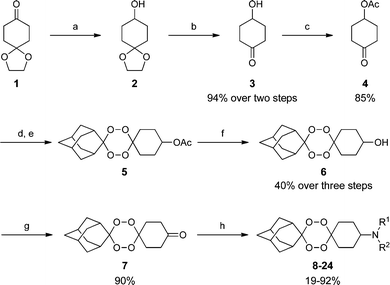 | ||
| Scheme 1 Reagents and conditions: (a) NaBH4, MeOH, 0 °C, 20 min; (b) 2 N aq. HCl, rt, 16 h; (c) AcCl, pyridine, CH2Cl2, 0 °C, 8 h; (d) H2O2, MeCN/HCO2H, 0 °C, 30 min; (e) 2-adamantanone, Re2O7 (2 mol%), CH2Cl2, 0 °C, 1 h; (f) LiOH, MeOH, rt, 2 h; (g) Jones reagent, acetone, rt, 3h; (h) amine, NaBH(OAc)3, CH2Cl2, 16 h. | ||
| Analogue | Amine side-chain (R1, R2) | IC50a/nM | |
|---|---|---|---|
| 3D7 | K1 | ||
| a IC50 values are the mean of at least two independent assays. ND = not determined. | |||
| 8 | R1, R2 = (CH2)4O | 0.4 | 0.4 |
| 9 | R1, R2 = (CH2)4 | 0.7 | 1.0 |
| 10 | R1 = H, R2 = CH(CH2)4CF2 | 0.4 | 0.9 |
| 11 | R1 = H, R2 = CH(CH2)4O | 0.7 | 0.4 |
| 12 | R1 = H, R2 = CH(CH2)4NCH3 | 0.4 | 1.1 |
| 13 | R1, R2 = N(CH2)4SO2 N-oxide | 3.7 | 2.5 |
| 14 | R1, R2 = (CH2)5 | 0.6 | 0.4 |
| 15 | R1, R2 = (CH2)4CF2 | 0.6 | 1.3 |
| 16 | R1, R2 = (CH2)4CHN(CH2)5 | 2.4 | 0.2 |
| 17 | R1 = H, R2 = CH(CH2)5 | 0.4 | 0.2 |
| 18 | R1, R2 = (CH2)4CHNH2 | 1.2 | 1.2 |
| 19 | R1 = H, R2 = CH(CH2)2 | 1.5 | ND |
| 20 | R1 = H, R2 = C(CH3)3 | 1.3 | ND |
| 21 | 2-Oxopiperazinyl | 3.5 | ND |
| 22 | R1, R2 = (CH2)4CHNHCOOC(CH3)3 | 1.0 | ND |
| 23 | 11 Hydrochloride salt | 1.0 | ND |
| 24 | 18 Ditosylate salt | 2.5 | ND |
| Chloroquine | 12.519 | 325 | |
| Artesunate | ND | 4.9 | |
| RKA182 | 0.819 | 1.119 | |
Table 1 shows the array of tetraoxanes synthesized (8–24) and their in vitroantimalarial activity against the 3D7 and K1 strains of Plasmodium falciparum. Against both these strains, all the compounds display excellent activities in the low nanomolar range and proved to be superior in potency to chloroquine and artesunate and similar to RKA182. Analogues 16 and 17 display an impressive 25-fold greater potency than artesunate against the chloroquine-resistant K1 strain of P. falciparum.
The in vivoantimalarial activity of selected compounds was studied in P. bergheiANKA infected mice using a 4-day Peters' suppressive test. The results are summarized in Table 2. All the compounds studied displayed potent activity by oral administration at a dose of 30 mg kg−1, most showing similar or better activity to artesunate. Following on from these promising results, four compounds (11, 15, 23, 24) were further assessed in a 4-day Peters' test to determine oral in vivoED50 and ED90 values against P. bergheiANKA (Table 3). These four compounds displayed high potency and 23 and 24 were more potent than artesunate in terms of ED90 in the mouse model of malaria.
| Analogue | ClogPa | % Suppression of parasitaemia at day 4 post-infection following 1 × 30 mg kg−1 oral dose |
|---|---|---|
| a ClogP calculated with Chem BioDraw Ultra. b Calculation on free base. | ||
| 9 | 3.48 | 99.8 |
| 12 | 2.46 | 99.2 |
| 15 | 3.42 | 99.8 |
| 16 | 3.84 | 99.7 |
| 19 | 2.98 | 99.7 |
| 20 | 3.64 | 98.7 |
| 21 | 2.59 | 98.8 |
| 24 (ditosylate of 18) | 2.05b | 99.9 |
| Artesunate | 99.6 |
| Analogue | ED50/mg kg−1 | ED90/mg kg−1 |
|---|---|---|
| 11 | 2.8 | 18 |
| 15 | 9.4 | 34 |
| 23 | 2.3 | 11 |
| 24 | 3.7 | 10 |
| Artesunate | 2.8 | 18 |
In order to gauge the metabolic stability of a representative of this series, the turnover of 18 and RKA182 were determined in human liver microsomes. These studies showed that 18 is more metabolically stable than RKA182 as can be seen in Table 4; following incubation for 1 hour with human liver microsomes there is 57% of 18 remaining whereas only 40% of RKA182 remains. Further studies comparing the metabolic stability of this series of compounds are ongoing.
| Time/minutes | % of parent compound remaining | |
|---|---|---|
| 18 | RKA182 | |
a
Drug incubations at 10 μg ml−1; n = 3.
b Microsomal protein content = 1 mg ml−1; NADPH concentration = 1 mg ml−1.
c Reaction was stopped by addition of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ice cold acetonitrile. 1 ice cold acetonitrile.
|
||
| 0 | 100.0 | 100.0 |
| 10 | 86.7 ± 2.5 | 62.9 ± 17.9 |
| 30 | 73.6 ± 8.0 | 40.6 ± 5.8 |
| 60 | 57.4 ± 4.6 | 39.5 ± 4.0 |
It has been well documented that antimalarially active endoperoxides can interact with heme22 and non-heme iron sources.23 With respect to the former mechanism, molecular dynamics (MD) simulations were performed in order to simulate the binding mode of 8 when complexed with the heme Fe-II system. Simulations were performed using the AMBER molecular dynamics package,24 as specified in the ESI†. This simulation allowed full flexibility of the ligand and heme moieties whilst sampling the possible equilibrium binding mode configurations of the complex.
Modelling of compound 8 reveals a single binding mode (Fig. 3) characterized by the morpholine group hydrogen bonding to the propionate group through the protonated nitrogen atom and peroxide O1 co-ordinated to the Fe(II) centre. Model studies performed with tetraoxane 8 and heme (produced by reduction of Fe(III)PPIX) confirmed that the formation of isolated adducts arises by preferential co-ordination of O1 with heme, as shown in Fig. 3A, leading to the intermediate depicted in Fig. 3B with eventual formation of heme adducts (only the δ-isomer is shown for simplicity).
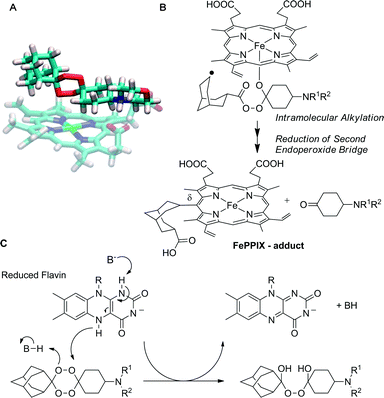 | ||
| Fig. 3 Potential mechanisms of bioactivation of adamantylidene fused tetraoxanes relevant to antimalarial mechanism of action: (A) snapshot of molecular dynamics simulation of heme (Fe(II)-PPIX) complexed with tetraoxane 8 and (B) structure of C-radical derived from homolytic cleavage and subsequent C–C β scission. The observed adducts produced are a mixture of three porphyrin regioisomers and in contrast to artemisinin and 1,2,4-trioxolanes an additional reduction step must occur to explain the formation of the isolated adduct. (C) Co-factor model (R = cofactor residue).25,26 Here, tetraoxanes operate through a non-iron activation pathway and function as co-factor oxidants; two equivalents of reduced flavin are required per tetraoxane molecule to produce carbonyl derived end-products (only a single reduction step is depicted). | ||
Whilst this modelling supports the proposed mechanism of bioactivation/decomposition by heme (with subsequent heme alkylation), the role of this pathway in the mechanism remains controversial and alternative non-heme pathways of bioactivation have recently been proposed.25,26 The tetraoxanes reported here are also likely to be capable of oxidising co-factors such as FADH2 in a manner similar to that proposed for sulfonamide based tetraoxane heterocycle and this model separates the tetraoxanes from 1,2,4-trioxanes and 1,2,4-trioxolanes by virtue of the additional oxidising capacity of the tetraoxane ring system. It remains to be seen whether this feature provides an advantage with respect to the spectrum of activity and potential resistance development.
Conclusions
In conclusion, we have developed a new series of tetraoxane analogues and screened them for their in vitro and in vivoantimalarial activity. All of the compounds synthesized show remarkable in vitro activity in the low nanomolar range (0.2–3.7 nM) and several demonstrate promising oral activity in the P. bergheiANKA mouse model of malaria. A preliminary study suggests that members of this series have improved metabolic stability compared with RKA182 and these data coupled with the excellent activity profiles, low ClogP and high aqueous solubilities (e.g.23, >40 mg ml−1) make this series worthy of further investigation.Experimental
Synthesis of 4-hydroxycyclohexanone (3)20
1,4-Cyclohexanedione monoethylene ketal (1) (15.0 g, 96 mmol) was dissolved in MeOH (300 ml) and cooled to 0 °C. Sodium borohydride (2.27 g, 60 mmol) was added over 20 minutes to form 2. After 20 minutes, the mixture was acidified with 2 M aq. HCl (pH 2–3) and left stirring for 16 hours. The mixture was then neutralized with sodium hydroxide. The solvent was evaporated and the crude mixture was extracted with EtOAc. The title compound was obtained without further purification as a colorless oil (10.3 g, 94%). 1H NMR (400 MHz, CDCl3) δ 4.34–4.15 (m, 1H), 2.77–2.53 (m, 2H), 2.48–2.25 (m, 2H), 2.19–1.91 (m, 4H); 13C NMR (100 MHz, CDCl3) δ 211.5, 66.7, 37.6, 34.1; MS (ES+) [M + H]+ 115.1 (100).Synthesis of 4-acetoxycyclohexanone (4)
A solution of acetyl chloride (11 ml, 155.8 mmol) in anhydrous CH2Cl2 (20 ml) was added to a solution of 3 (8.89 g, 77.9 mmol) in anhydrous CH2Cl2 (40 ml) and pyridine (25 ml, 311.5 mmol) at 0 °C. After stirring for 8 hours, 2 M aq. HCl was added until the reaction mixture was acidic (pH 2–3) and the mixture was stirred for 20 minutes. The mixture was then extracted with CH2Cl2 and the organic extracts were washed with saturated aq. NaHCO3. The combined organic extracts were dried over MgSO4, filtered and concentrated in vacuo. Purification of the product viaflash chromatography on silica gel (CH2Cl2 100%) gave 4 (10.2 g, 85%) as a colourless oil. 1H NMR (400 MHz, CDCl3) δ 5.24–5.12 (m, 1H), 2.61–2.49 (m, 2H), 2.43–2.32 (m, 2H), 2.21–2.02 (m, 7H); 13C NMR (100 MHz, CDCl3) δ 210.2, 170.8, 69.0, 37.7, 30.8, 21.7; MS (ES+) [M + H]+ 157.1 (100).Synthesis of dispiro[cyclohexane-1,3′-[1,2,4,5]tetroxane-6′,2′′-tricyclo[3.3.1.13,7]decan]-4-yl acetate (5)
To a stirred solution of 4 (2.79 g, 17.9 mmol) in formic acid/acetonitrile (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 40 ml) at 0 °C was added 30% aqueous hydrogen peroxide (10 ml, 85 mmol) and the mixture was allowed to stir at room temperature for 30 minutes. The mixture was then poured into ice-cold water and extracted with CH2Cl2. The combined organic extracts were dried over Na2SO4, filtered and concentrated in vacuo. The residue was dissolved in CH2Cl2 (130 ml) and 2-adamantanone (4.0 g, 26.8 mmol) was added followed by Re2O7 (2 mol%, 173 mg, 0.36 mmol). The mixture was allowed to stir for 1 h at room temperature. The reaction mixture was filtered through a pad of silica and concentrated. Isolation of analytically pure 5 by flash chromatography on silica gel was not possible since it co-elutes with 2-adamantanone. Therefore the product was carried forward to the next step. MS (ES+) [M + Na]+ 361.0 (100).
1, 40 ml) at 0 °C was added 30% aqueous hydrogen peroxide (10 ml, 85 mmol) and the mixture was allowed to stir at room temperature for 30 minutes. The mixture was then poured into ice-cold water and extracted with CH2Cl2. The combined organic extracts were dried over Na2SO4, filtered and concentrated in vacuo. The residue was dissolved in CH2Cl2 (130 ml) and 2-adamantanone (4.0 g, 26.8 mmol) was added followed by Re2O7 (2 mol%, 173 mg, 0.36 mmol). The mixture was allowed to stir for 1 h at room temperature. The reaction mixture was filtered through a pad of silica and concentrated. Isolation of analytically pure 5 by flash chromatography on silica gel was not possible since it co-elutes with 2-adamantanone. Therefore the product was carried forward to the next step. MS (ES+) [M + Na]+ 361.0 (100).
Synthesis of dispiro[cyclohexane-1,3′-[1,2,4,5]tetroxane-6′,2′′-tricyclo[3.3.1.13,7]decan]-4-ol (6)
To a solution of 5 (10.4 g, 30.7 mmol) (impure with 2-adamantanone) in MeOH (600 ml) was added lithium hydroxide monohydrate (1.93 g, 45.1 mmol) and the reaction mixture was stirred at room temperature for 2 hours. The crude reaction mixture was concentrated in vacuo and saturated aq. NH4Cl (150 ml) was added followed by extraction with CH2Cl2. The combined organic extracts were dried over MgSO4, filtered and concentrated in vacuo. Purification of the product by flash chromatography on silica gel (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 n-Hex/EtOAc) gave 6 (5.65 g, 40% over three steps) as a white solid. 1H NMR (400 MHz, CDCl3) δ 4.00–3.82 (m, 1H), 3.17 (br s, 1H), 2.67 (br s, 1H), 2.14 (br s, 1H), 2.10–1.92 (m, 5H), 1.90–1.80 (m, 6H), 1.78–1.68 (m, 4H), 1.67–1.64 (m, 4H); 13C NMR (100 MHz, CDCl3) δ 110.9, 107.7, 68.2, 37.3, 34.7, 33.5, 30.6, 30.0, 28.6, 27.4, 26.1; MS (ES+) [M + Na]+ 319.1 (100); HRMS calculated for C16H24O5 319.1521, found 319.1516.
1 n-Hex/EtOAc) gave 6 (5.65 g, 40% over three steps) as a white solid. 1H NMR (400 MHz, CDCl3) δ 4.00–3.82 (m, 1H), 3.17 (br s, 1H), 2.67 (br s, 1H), 2.14 (br s, 1H), 2.10–1.92 (m, 5H), 1.90–1.80 (m, 6H), 1.78–1.68 (m, 4H), 1.67–1.64 (m, 4H); 13C NMR (100 MHz, CDCl3) δ 110.9, 107.7, 68.2, 37.3, 34.7, 33.5, 30.6, 30.0, 28.6, 27.4, 26.1; MS (ES+) [M + Na]+ 319.1 (100); HRMS calculated for C16H24O5 319.1521, found 319.1516.
Synthesis of 4H-dispiro[cyclohexane-1,3′-[1,2,4,5]tetroxane-6′,2′′-tricyclo[3.3.1.13,7]decan]-4-one (7)
To a solution of 6 (1.5 g, 5.06 mmol) in acetone (8 ml) was added Jones reagent (2 ml) and the reaction mixture was allowed to stir at room temperature for 3 hours. Water was then added followed by saturated aq. NaHCO3 and the mixture was extracted with EtOAc. The combined organic extracts were washed with saturated aq. NaCl, dried over MgSO4, filtered and concentrated in vacuo. Compound 7 (1.30 g, 90%) was obtained as a white solid with no further purification required. 1H NMR (400 MHz, CDCl3) δ 3.20 (br s, 1H), 2.72 (br s, 2H), 2.48 (br s, 4H), 2.22–1.92 (m, 7H), 1.87 (br s, 3H), 1.85–1.69 (m, 5H); 13C NMR (100 MHz, CDCl3) δ 209.6, 111.3, 107.0, 37.224, 36.8, 36.0, 34.7, 33.5, 30.7, 30.5, 28.2, 27.4. MS (ES+) [M + Na]+ 317.1 (100); HRMS calculated for C16H22O5 317.1365, found 317.1353.General procedure for the reductive amination reaction
To a solution of compound 7 (1 eq., 0.05 M) in anhydrous CH2Cl2 was added the respective amine (1.5 eq.) and the mixture was allowed to stir at room temperature for 30 minutes followed by the addition of sodium triacetoxyborohydride (1.5 eq.). After stirring at room temperature for 16 hours, saturated aq. NaHCO3 was added and the aqueous phase was extracted with CH2Cl2. The combined organic extracts were washed with saturated aq. NaCl, dried over MgSO4, filtered and concentrated in vacuo. The product was purified by flash chromatography on silica gel followed by recrystallisation if necessary.Representative example: synthesis of 4-(dispiro[cyclohexane-1,3′-[1,2,4,5]tetroxane-6′,2′′-tricyclo[3.3.1.13,7]decan]-4-yl) morpholine (8)
8 was isolated as a pale yellow solid (52% yield) according to the general procedure utilising morpholine as the amine and following purification by flash chromatography on silica gel (100% EtOAc). 1H NMR (400 MHz, CDCl3) δ 3.83–3.66 (m, 4H), 3.16 (s, 1H), 3.05 (s, 1H), 2.66–2.50 (m, 4H), 2.36 (t, J = 9.8 Hz, 1H), 1.97 (br s, 4H), 1.85 (br s, 3H), 1.80–1.65 (m, 8H), 1.62 (s, 5H); 13C NMR (100 MHz, CDCl3) δ 110.8, 107.9, 67.8, 62.5, 50.1, 37.3, 34.7, 33.5, 30.6, 28.2, 27.4, 24.2, 23.7. MS (ES+) [M + H]+ 366.1 (100); HRMS calculated for C20H32NO5 366.2280, found 366.2295; Elemental analysis C: 65.69, H: 8.76, N: 3.75 (required values C: 65.73, H: 8.55, N: 3.83%).Full experimental details for the synthesis and characterisation of 9–24, along with the in vitro and in vivoantimalarial screening and the metabolic stability studies can be found in the ESI†.
Acknowledgements
The research leading to these results has received funding from AntiMal, an FP6-funded integrated project under contract number LSHP-CT-2005-0188.Notes and references
- World Malaria Report 2009, World Health Organization, Geneva, 2009 Search PubMed.
- K. Hayton and X. Z. Su, Curr. Drug Targets: Infect. Disord., 2004, 4, 1–10 Search PubMed.
- A. M. Dondorp, F. Nosten, P. Yi, D. Das, A. P. Phyo, J. Tarning, K. M. Lwin, F. Ariey, W. Hanpithakong, S. J. Lee, P. Ringwald, K. Silamut, M. Imwong, K. Chotivanich, P. Lim, T. Herdman, S. S. An, S. Yeung, P. Singhasivanon, N. P. J. Day, N. Lindegardh, D. Socheat and N. J. White, N. Engl. J. Med., 2009, 361, 455–467 Search PubMed.
- J. L. Vennerstrom, S. Arbe-Barnes, R. Brun, S. A. Charman, F. C. K. Chiu, J. Chollet, Y. X. Dong, A. Dorn, D. Hunziker, H. Matile, K. McIntosh, M. Padmanilayam, J. S. Tomas, C. Scheurer, B. Scorneaux, Y. Q. Tang, H. Urwyler, S. Wittlin and W. N. Charman, Nature, 2004, 430, 900–904 CrossRef CAS.
- Y. X. Dong, S. Wittlin, K. Sriraghavan, J. Chollet, S. A. Charman, W. N. Charman, C. Scheurer, H. Urwyler, J. S. Tomas, C. Snyder, D. J. Creek, J. Morizzi, M. Koltun, H. Matile, X. F. Wang, M. Padmanilayam, Y. Tang, A. Dorn, R. Brun and J. L. Vennerstrom, J. Med. Chem., 2010, 53, 481–491 CrossRef CAS.
- S. A. Charman, S. Arbe-Barnes, I. C. Bathurst, R. Brun, M. Campbell, W. N. Charman, F. C. K. Chiu, J. Chollet, J. C. Craft, D. J. Creek, Y. Dong, H. Matile, M. Maurer, J. Morizzi, T. Nguyen, P. Papastogiannidis, C. Scheurer, D. M. Shackleford, K. Sriraghavan, L. Stingelin, Y. Tang, H. Urwyler, X. Wang, K. L. White, S. Wittlin, L. Zhou and J. L. Vennerstrom, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 4400–4405 Search PubMed.
- K. J. McCullough and M. Nojima, Curr. Org. Chem., 2001, 5, 601–636 CAS.
- Y. Dong, Mini-Rev. Med. Chem., 2002, 2, 113–123 CrossRef CAS.
- J. L. Vennerstrom, H. N. Fu, W. Y. Ellis, A. L. Ager, J. K. Wood, S. L. Andersen, L. Gerena and W. K. Milhous, J. Med. Chem., 1992, 35, 3023–3027 CrossRef CAS.
- Y. X. Dong and J. L. Vennerstrom, J. Org. Chem., 1998, 63, 8582–8585 CrossRef CAS.
- Y. X. Dong, H. Matile, J. Chollet, R. Kaminsky, J. K. Wood and J. L. Vennerstrom, J. Med. Chem., 1999, 42, 1477–1480 CrossRef CAS.
- K. Tsuchiya, Y. Hamada, A. Masuyama, M. Nojima, K. J. McCullough, H. S. Kim, Y. Shibata and Y. Wataya, Tetrahedron Lett., 1999, 40, 4077–4080 CrossRef CAS.
- Y. Nonami, T. Tokuyasu, A. Masuyama, M. Nojima, K. J. McCullough, H. S. Kim and Y. Wataya, Tetrahedron Lett., 2000, 41, 4681–4684 CrossRef CAS.
- J. L. Vennerstrom, Y. X. Dong, S. L. Andersen, A. L. Ager, H. N. Fu, R. E. Miller, D. L. Wesche, D. E. Kyle, L. Gerena, S. M. Walters, J. K. Wood, G. Edwards, A. D. Holme, W. G. McLean and W. K. Milhous, J. Med. Chem., 2000, 43, 2753–2758 Search PubMed.
- H. S. Kim, Y. Nagai, K. Ono, K. Begum, Y. Wataya, Y. Hamada, K. Tsuchiya, A. Masuyama, M. Nojima and K. J. McCullough, J. Med. Chem., 2001, 44, 2357–2361 CrossRef CAS.
- D. Opsenica, G. Pocsfalvi, W. K. Milhous and B. A. Solaja, J. Serb. Chem. Soc., 2002, 67, 465–471 Search PubMed.
- J. Iskra, D. Bonnet-Delpon and J. P. Begue, Tetrahedron Lett., 2003, 44, 6309–6312 CrossRef CAS.
- R. Amewu, A. V. Stachulski, S. A. Ward, N. G. Berry, P. G. Bray, J. Davies, G. Labat, L. Vivas and P. M. O'Neill, Org. Biomol. Chem., 2006, 4, 4431–4436 RSC.
- P. M. O'Neill, R. K. Amewu, G. L. Nixon, F. B. ElGarah, M. Mungthin, J. Chadwick, A. E. Shone, L. Vivas, H. Lander, V. Barton, S. Muangnoicharoen, P. G. Bray, J. Davies, B. K. Park, S. Wittlin, R. Brun, M. Preschel, K. S. Zhang and S. A. Ward, Angew. Chem., Int. Ed., 2010, 49, 5693–5697 CrossRef CAS.
- M. M. Kayser and C. M. Clouthier, J. Org. Chem., 2006, 71, 8424–8430 CrossRef CAS.
- P. Ghorai and P. H. Dussault, Org. Lett., 2009, 11, 213–216 Search PubMed.
- A. Robert, J. Cazelles and B. Meunier, Angew. Chem., Int. Ed., 2001, 40, 1954–1957 CrossRef CAS.
- P. A. Stocks, P. G. Bray, V. E. Barton, M. Al-Helal, M. Jones, N. C. Araujo, P. Gibbons, S. A. Ward, R. H. Hughes, G. A. Biagini, J. Davies, R. Amewu, A. E. Mercer, G. Ellis and P. M. O'Neill, Angew. Chem., Int. Ed., 2007, 46, 6278–6283 CrossRef CAS.
- D. A. Case, T. A. Darden, T. E. Cheatham, III, C. L. Simmerling, J. Wang, R. E. Duke, R. Luo, R. C. Walker, W. Zhang, K. M. Merz, B. Roberts, B. Wang, S. Hayik, A. Roitberg, G. Seabra, I. Kolossváry, K. F. Wong, F. Paesani, J. Vanicek, J. Liu, X. Wu, S. R. Brozell, T. Steinbrecher, H. Gohlke, Q. Cai, X. Ye, J. Wang, M.-J. Hsieh, G. Cui, D. R. Roe, D. H. Mathews, M. G. Seetin, C. Sagui, V. Babin, T. Luchko, S. Gusarov, A. Kovalenko and P. A. Kollman, AMBER 11, University of California, San Francisco, 2010 Search PubMed.
- R. K. Haynes, W. C. Chan, H. N. Wong, K. Y. Li, W. K. Wu, K. M. Fan, H. H. Y. Sung, I. D. Williams, D. Prosperi, S. Melato, P. Coghi and D. Monti, ChemMedChem, 2010, 5, 1282–1299 Search PubMed.
- R. K. Haynes, K. W. Cheu, M. M. K. Tang, M. J. Chen, Z. F. Guo, Z. H. Guo, P. Coghi and D. Monti, ChemMedChem, 2011, 6, 279–291 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Full experimental details for the synthesis, antimalarial screening, in vitro metabolic stability studies and the molecular dynamics simulations. See DOI: 10.1039/c1md00102g |
| This journal is © The Royal Society of Chemistry 2011 |
