Are pyridazines privileged structures?
Camille G.
Wermuth
Prestwick Chemical Inc., Boulevard Gonthier d'Andernach, 67400, ILLKIRCH, France. E-mail: camille.wermuth@prestwickchemical.fr
First published on 10th June 2011
Abstract
One can estimate that about 50% of all the drug molecules used in medicine contain a phenyl ring which can be substituted or not. The bioisosteric replacement of these phenyl rings by the corresponding pyridazine rings opens an access to several thousands of diaza analogues presenting more interaction possibilities, lower Log P values and improved crystalline salts. The use of pyridazine scaffolds in place of phenyl scaffolds entails additional interaction possibilities. Another interest of pyridazines is their capacity to act as original functional surrogates. Thus, aminopyridazines can be used as carboxamide, as well as amine surrogates. Finally, the many examples of pyridazines used either as a structural element or as a main scaffold, justify largely their status as privileged structures
Introduction
My interest in pyridazines goes back to my thesis work at the end of the fifties. At that time I examined the directed aldolization reaction between enolisable aldehydes (such as n-butanal) and pyruvic acid. As both partners possess a carbonyl function and a reactive α position, the objective was to investigate the reaction conditions of a selective attack either of the pyruvic acidcarbonyl or of the aldehydecarbonyl. Working with ethyl pyruvate and under mild conditions, we were able to prepare the substituted succinic semialdehyde as an ethyl ester (Fig. 1).1 Using potassium pyruvate and rather drastic conditions (KOH catalysis) we obtained the linear β-γ-ethylenic heptanoate, the strong basicity of the condensation milieu favouring polymerization of the aldehyde.2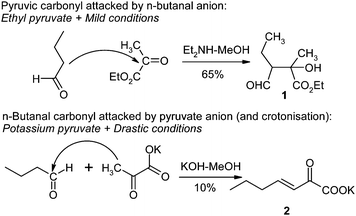 | ||
| Fig. 1 Directed aldolization between pyruvic acid and n-butanal. | ||
The treatment of the semisuccinic aldehydo-acid 3 with hydrazine yielded the hydroxy-pyridazinone4 which was easily dehydrated to the methyl-ethyl pyridazone5 (“MEP”) (Fig. 2).
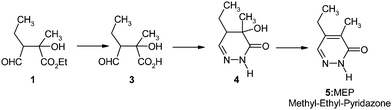 | ||
| Fig. 2 Preparation of the methyl-ethyl-pyridazone5 (“MEP”). | ||
As I had already an ongoing research program on GABA and γ-hydroxybutyrate with Henri Laborit, the inventor of chlorpromazine, I provided him with a sample of MEP with the hope that he would detect some interesting biological activity. A preliminary pharmacological study, based essentially on isolated organs and on EEG investigations,3 revealed some hypotensive activity. Henri Laborit decided then to proceed to some clinical assays. In humans no hypotensive activity was observed. However, three weeks after the injection of MEP one of the patients wrote a letter to Dr Laborit expressing her warmest thanks for the MEP treatment “which removed her shoulder ache”. Laborit therefore asked me if the MEP molecule did show any analogy with an already existing analgesic agent. Effectively this was the case insofar that the pyridazone ring of MEP could be compared to the typical pyrazolone functionality of antipyrine (Fig. 3).
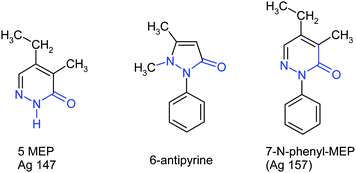 | ||
| Fig. 3 The pyridazone ring of compound 5 shows some similitude with the analgesicdrugantipyrine6. This analogy is even more pronounced with N-phenyl analogue 7. However, N-phenyl-MEP7 is quasi insoluble in water. | ||
This unexpected pharmacological activity of MEP was the starting-point of a long term research activity in pyridazine chemistry and medicinal chemistry. With my students and coworkers we examined more than one thousand pyridazine derivatives. Our main goal was to prepare compounds exhibiting enough biological activity to become drug candidates. The choice of pyridazines appeared to be timely because the chemistry of this heterocycle and its use in drug design were not very much explored.
Pyridazine drugs—historical considerations
In the fifties, when we started to work on pyridazines, only two pyridazine-derived drugs were on the market, namely the vasodilator hydralazine and the antibacterial sulfamethoxypyridazine (Fig. 4). Apparently medicinal chemists feared possible toxicity problems arising from the presence of a hydrazinegroup in a drug molecule.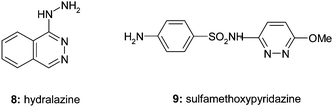 | ||
| Fig. 4 Historical pyridazinedrugs. | ||
Two review articles written by Heinisch and Frank analyse the pyridazine medicinal chemistry publications covering the period between 1975 and 1998.4,5 Clearly it appears that the results in terms of achieving marketable pyridazinedrug molecules were very modest and that none of the launched compounds reached the blockbuster status. A Google Scholar comparative examination of the “diazinedrug” consultations highlights the dominant position of the pyrimidines which have about ten times more entries than the pyridazines. As an example the entry “pyridazinedrugs” accounts for only 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 hits as compared to 141
100 hits as compared to 141![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 hits for the entry “pyrimidinedrugs”. A similar ratio is found with the entry “aminopyridazinedrugs” (21
000 hits for the entry “pyrimidinedrugs”. A similar ratio is found with the entry “aminopyridazinedrugs” (21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 hits) as compared with the “aminopyrimidinedrugs” (28
400 hits) as compared with the “aminopyrimidinedrugs” (28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 hits). A possible explanation may reside in the almost complete absence of pyridazines in living organisms whereas pyrimidines play an extremely important role in biology as constituents of nucleic acids, of ATP and various low molecular derivatives. Another point is the very modest place of the pyridazines in natural product chemistry as illustrated below.
600 hits). A possible explanation may reside in the almost complete absence of pyridazines in living organisms whereas pyrimidines play an extremely important role in biology as constituents of nucleic acids, of ATP and various low molecular derivatives. Another point is the very modest place of the pyridazines in natural product chemistry as illustrated below.
Natural pyridazines
A traditional source of lead structures results from natural compounds chemistry. Surprisingly only a very small number of natural compounds containing the typical pyridazine N–N bond is described in the literature. All of them have been isolated from Streptomyces culture broths and the knowledge of their biosynthesis is still limited. Pyridazomycin10 (Fig. 5), an antifungal and antibiotic isolated from Streptomyces violaceoniger sp. griseofucus, represents the first example of a naturally occurring fully unsaturated pyridazine.6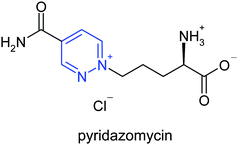 | ||
| Fig. 5 The structure of pyridazomycin10. | ||
The proposed pyridazomycinbiosynthesis7 involves the incorporation of the ornithine δ-amino function into a pyridazine scaffold which itself results from a succession of cyclization and several dehydrogenations of ornithine (Fig. 6). The biosynthesis of pyridazomycin is highly dependent on the availability of ornithine residues.
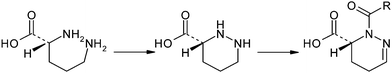 | ||
| Fig. 6 The pyridazine ring system of pyridazomycin results from a succession of cyclization and several dehydrogenation steps. | ||
Ornithine is not an amino acid coded for by DNA and, in that sense, is not involved in protein synthesis. However, in mammalian non-hepatic tissues, the main use of the urea cycle is in arginine biosynthesis, so, as an intermediate in metabolic processes, ornithine is quite important. It is believed not to be a part of genetic code because polypeptides containing unprotected ornithines undergo spontaneous lactamisation. The double necessity to start from ornithine and to create an N–N bond explains the small number of natural pyridazines. Beside pyridazomycin, azamerone 11 is the second natural product known to contain a pyridazine ring. The terpenoidphthalazinone azamerone was isolated from the marine sediment-derived bacterium Streptomyces sp. CNQ-766 (Fig. 7).8
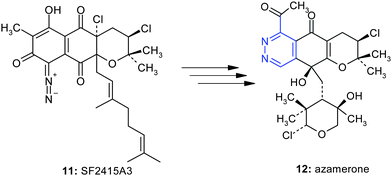 | ||
| Fig. 7 The key step of the biosynthesis of azamerone is the transformation of the diazoketone11 into the pyridazone12. | ||
It was first speculated that the pyridazine ring moiety in azamerone was also derived from the cyclization of amino acid residues, but biosynthetic investigations of azamerone7 suggested that azamerone may rather be derived from a naphthoquinone precursor (Fig. 7).
Antrimycin 9 and cirratiomycins are dihydropyridazines. Antrimycin13, Fig. 8 (R = CH3), was isolated from Streptomyces xanthocidicusMG-125-CF1, and shows activity in vitro against Mycobacterium smegmatis and Mycobacterium tuberculosis. Cirratiomycin A 14, Fig. 8 (R = iso-Bu), was isolated from Streptomyces cirratus248-Sq2, and is active in vitro against a narrow range of Lactobacilli and some strains of Streptococcus.10
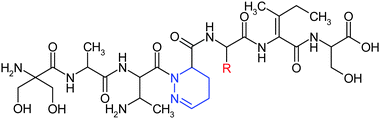 | ||
| Fig. 8 Structures of the partially saturated dihydropyridazines: antrimycin (R = CH3) and cirratiomycin A (R = iso-Bu). | ||
Also a dihydropyridazinedrug candidate is compound L 365209 15, a structurally unique, potent, and selective oxytocinantagonist derived from Streptomyces silvensis (Fig. 9).11
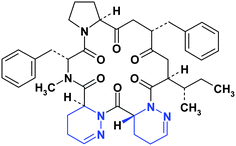 | ||
| Fig. 9 Structure of the dihydropyridazine L 365209 15. | ||
Physicochemical properties of pyridazines
The dipole moments in dioxane solution at 35 °C have been determined for the three unsubstituted diazines (Fig. 10).12Pyridazine presents the most elevated dipolar moment of 3.9 Debye units due to a concentration on one side of the molecule of the electronegative nitrogen lone pairs. This value has to be compared with those of the isomeric diazines: pyrimidine (2.42 D) and pyrazine (0.6 D).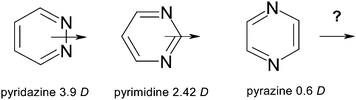 | ||
| Fig. 10 Dipole moments of the diazines. | ||
Consequences
A first consequence concerns the interactions with the receptorprotein. The two nitrogen atoms of pyridazine moieties are capable of forming H-bond interactions whereas the carbocycles are not able to act in this way (Fig. 11). Another consequence of the isosteric replacement of a phenyl ring by a pyridazine is the change of the ADME profile and of the bioactivity and toxicity (see below).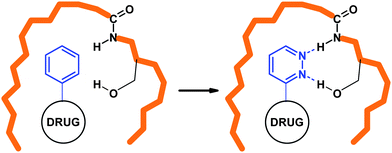 | ||
| Fig. 11 The two nitrogens of the pyridazines are capable to give interactions where the carbocycles do not give. | ||
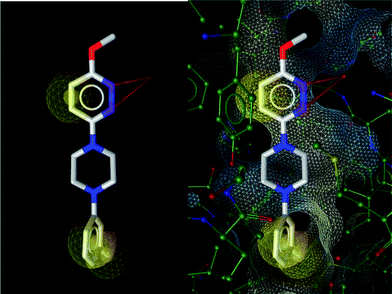
The binding of the antirhinoviral agent R 61837 to human rhinovirus 14 has been examined by X-ray crystallographic methods.13 It appears clearly that the two pyridazinenitrogens of the compound R 61837 establish two acceptor hydrogen bonds with the receptorprotein (Fig. 12).
A pharmacophore model was derived from pdb structure 1R09 using LigandScout: red arrows represent H-bonds, yellow spheres hydrophobic areas of the ligand interacting with the protein.
Coordination with metals
Thanks to the presence in the diazines of two nitrogen lone pairs they are able to coordinate with elements such as platinum to yield dinuclear platinum(II) complexes (Fig. 13). The cytotoxicity of these compounds is comparable to that of cisplatin but they present the advantage of circumventing cross-resistance to cisplatin.14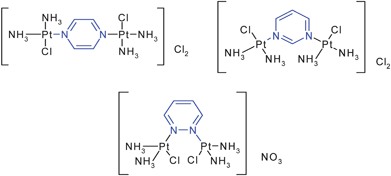 | ||
| Fig. 13 New isomeric azine-bridged dinuclear platinum(II) complexes circumvent cross-resistance to cisplatin. | ||
Increase in aqueous solubility
An interesting use of pyridazines consists of increasing the water-solubility of an otherwise too lipophilic drug candidate. In replacing for example the phenyl ring of diazepam by the isosteric pyridazine ring an approximately two-unit decrease in the log P is produced (Fig. 14).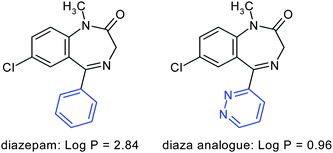 | ||
| Fig. 14 Replacement of the phenyl ring of diazepam by the isosteric pyridazine ring produces an approximately two-unit decrease in the log P (calculated values). | ||
Similarly the bioisosteric change in compound 16 of a pyridine ring into a pyridazine ring15 yielded improved water solubility (6 μg ml−1 instead of less than 0.5 μg ml−1) (Fig. 15).
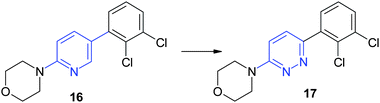 | ||
| Fig. 15 Replacement of the pyridine by a pyridazine improves the solubility in water. | ||
Formation of crystalline, water-soluble salts
Many drug molecules contain basic functions and, as free bases, are not suited for industrial production. The free bases are often oily or have a low melting point. They are not very stable and become colored through oxidation or degradation. Finally, often they are not water-soluble (Fig. 16).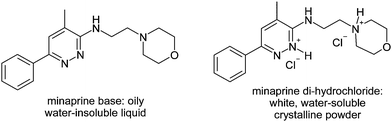 | ||
| Fig. 16 The presence of a pyridazine ring in a bioactive molecule allows the preparation of solid, water-soluble salts. | ||
However, when a pyridazine ring is present in a bioactive molecule, salt formation becomes possible, yielding colorless, water-soluble crystalline salts. Thus, for the compound 17, the hydrochloride solubility increases from 6 μg ml−1 to 120 mg ml−1.15
Bioisosteric considerations
In classical diazine bioisosteryphenyl or pyridyl rings are replaced by the corresponding pyridazine rings. Examples are given below with ridogrel analogues and with cannabinoid CB2agonists.In the series of diazine analogues of ridogrel18, replacement of the 3-pyridyl group by a 2-pyrazinyl, 4-pyridazinyl or 5-pyrimidinyl moiety led to compounds that inhibit thromboxane A2 synthetase and may serve as bioisosteric substitute of a 3-pyridyl group in antiplatelet agents (Fig. 17).16
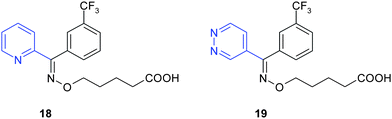 | ||
| Fig. 17 Replacement of the 3-pyridyl ring of ridogrel by 4-pyridazinyl ring yields highly potent anti-platelet agents. | ||
Starting from a pyridinelead active as selective cannabinoid CB2agonist, Gleave et al.15 identified pyridazine analogues with high potency in an in vivo model of inflammatory pain (Fig. 18).
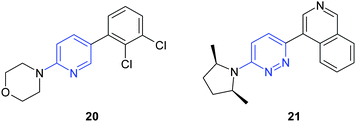 | ||
| Fig. 18 An essential step in optimization of the pyridine lead20 was the replacement of the pyridine ring by a pyridazine. Additional modifications led to compound 21. | ||
Functional equivalents
Beside incorporating them as a part of scaffolds, pyridazines can be used as functional equivalents. Fig. 19 for example shows how aminopyridazines can work as carboxamide surrogates. Thus, during their studies of sulpiride-derived selective dopaminergic antagonists Rognan et al.17 observed that the inclusion of an aminopyridazine ring at the place of the carboxamide functionality of sulpiride yielded potent and original bioisosteres with a similar activity profile (Fig. 19).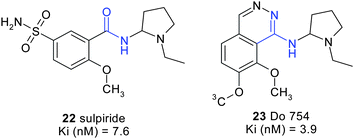 | ||
| Fig. 19 Bioisosteric replacement of a carboxamide by an aminopyridazine. | ||
Attachment of a butyric acid chain to the endo-amidinonitrogen of a 3-aminopyridazine yields “pyridazinyl-GABA's”, highly potent and selective antagonists of the GABA-A receptor (Fig. 20) and useful research tools for neuropharmacology.18,19 One of these compounds, Gabazine, is commercially available.
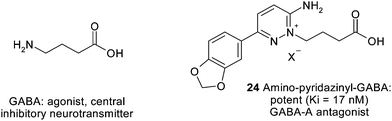 | ||
| Fig. 20 Bioisosteric replacement of the GABAamino function by an aminopyridazine. | ||
Choice between different heterocycles
When an initial hit or lead contains a heterocycle the question arises of its bioisosteric replacement. Usually a great number of variations are envisaged as well for structure–activity exploration as for extension of the patent protection. The strategy is then to identify rapidly and to synthesize the closest heterocyclic bioisosteres first. A thumb rule useful to identify the closest analogues of a given heterocycle consists of the comparison of the boiling points of the corresponding parent heterocycles.As an example, when the initial hit contains a pyridazine ring several bioisosteres can be considered a priori (Fig. 21): pyridine, pyrimidine, pyrazine, 1,2,4-triazines, 3,4-oxadiazoles, 3,4-thiadiazoles, 2,4-oxadiazoles, 2,4-thiadiazoles and many others. We made the assumption that the boiling points of these heterocycles reflect their dipole moments and that the closest heterocycles in terms of boiling points are also the closest analogues in terms of pharmacological activity.
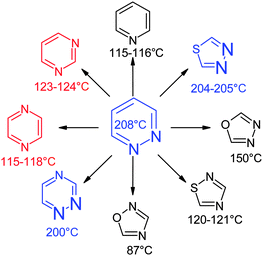 | ||
| Fig. 21 Boiling points of pyridazine bioisosteres. | ||
In reserpine-induced ptosis and in 5-HT potentiation the three most potent compounds (in blue) are the pyridazine, the thiadiazole or the 1,2,4-diazine ring, all three characterized by boiling points close to 200 °C (Table 1). The less potent pyrimidine and pyrazine derivatives (in red) boil respectively at 123–124 and 115–118 °C. However, in the turning behavior test (values in black) no discrimination is found.
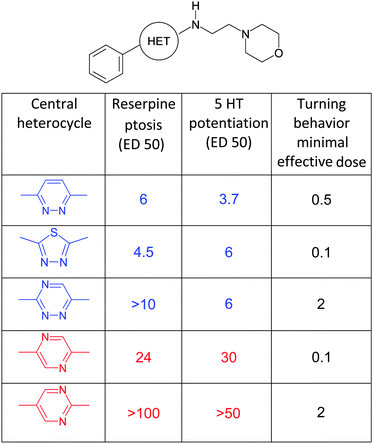
|
Recent trends
During the last decade several hundred articles dealing with bioactive pyridazines belonging to almost all therapeutic classes were published. The analysis of these publications reveals a great interest for sophisticated structures, particularly structures in which the pyridazines take place in fused bi- or tricyclic scaffolds. The following four examples are illustrative of this trend (Fig. 22).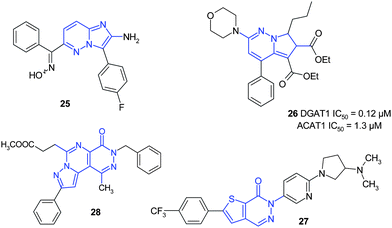 | ||
| Fig. 22 Pyridazines as parts of fused bi- or tricyclic scaffolds. | ||
Picornaviruses cause numerous human diseases, including poliomyelitis, acute hepatitis, myocarditis and common cold. Compound 25 and congenate imidazo[1,2-b]pyridazines were found to be active against human picornaviruses.20
Pyrrolopyridazines 26 are novel acyl CoA:diacylglycerol acyltransferase (DGAT1) inhibitors.21 As DGAT1 catalyzes the esterification of 1,2-diacylglycerol with fatty acyl CoA to form triglycerides, their inhibition may be useful in lowering corporeal concentration and absorption of triglycerides.
Thienopyridazinones, as melanin-concentrating hormone 1 antagonists, are potent in vivo anorectic agents.22 In obese rats compound 27 demonstrated a dose-dependent reduction in feeding and body weight with doses between 1 and 10 mg kg−1.
Pyrazolopyrimidopyridazinones 28 exhibit potent and selective phosphodiesterase 5 (PDE5) inhibitory activity.23 Compound 28 displays high potency (IC50 = 8.3 nM) with a high selectivity versusPDE6 (240-fold).
Pyridazines as privileged structures
The term “privileged structure” originates from Evans and coworkers who stated “what is clear is that certain privileged structures are capable of providing useful ligands for more than one receptor.”24 A typical example of a privileged structure is provided by the benzodiazepine scaffold which has led to cholecystokinin (CCK) antagonists, to ocytocinantagonists, to C5aantagonists, to GABA A agonists, to potassium channel blockers, to γ-secretase inhibitors, to PDE (IV) inhibitors and to Src kinase inhibitors.25 Other privileged structures were since identified: diphenylmethanes, spiropiperidines, biphenyltetrazoles, phenylethylamines, benzazepines, 2,2-dimethylbenzopyrans.26 The present review focuses on a new privileged structure candidate, namely the pyridazine scaffold. Starting from the antidepressantminaprine containing the pyridazine moiety, we rapidly became interested in the search for backup compounds, and our studies eventually revealed to us the vast potentiality of the pyridazines to accede to various bioactive molecules (Fig. 23).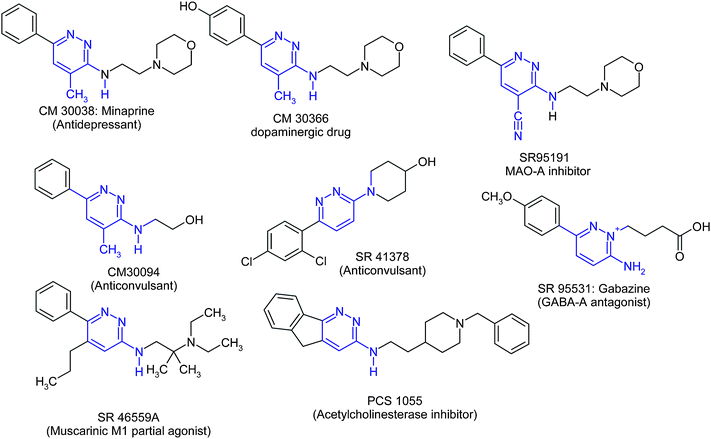 | ||
| Fig. 23 A great variety of activity profiles derived from pyridazines scaffold. | ||
Minaprine exhibits dopaminergic properties in vivo and the hydroxyl-ethylmetabolite CM 30094 combines sedative and anticonvulsant properties. Potent anticonvulsant properties were also found for compound SR 41378. Compound SR 95531, a combination of a 6-aryl-pyridazine and GABA, turned out to be a potent and selective GABA-A receptor antagonist commercialized under the name Gabazine as a research tool for the neuropharmacologists. Exchanging the 4-methylgroup of minaprine to a 4-cyanogroup yielded SR 95191 which in addition to the antidepressant properties is a potent monoamineoxidase A inhibitor. Further investigations on minaprine analogues led to SR 46559A, a muscarinic M1 partial agonist, and to PCS 1055, a nanomolar acetylcholinesterase inhibitor.
Considering the various advantages of pyridazines in drug design: modulation of the physico-chemical properties, save ADME and toxicity profile, easy and diverse synthetic methods of access, reasonable unexplored medicinal chemistry routes, would be arguments enough to work with pyridazines.
But, even more, given their affinity for a great number of receptorproteinspyridazines can without doubt be considered as privileged structures. Therefore it becomes fully justified to include pyridazines in any drug candidate research program. For the same reason I recommend to make massive use of pyridazine libraries in screening programs.
Acknowledgements
The author wishes to express his warmest thanks to Thierry Langer, Bruno Giethlen, Christophe Morice and Jean-Marie Contreras for helpful discussions, suggestions and corrections.References
- C. G. Wermuth, Etude de la condensation de l'acide pyruvique avec les aldéhydes aliphatiques non substitutes en α. I.- Produits de condensation dont la chaîne carbonée est ramifiée: semialdéhydes méthyl-alcoyl maliques et semialdéhydes méthyl-alcoyl maléiques, Bull. Soc. Chim. Fr., 1965, 2242–2249 Search PubMed.
- C. G. Wermuth, Etude de la condensation de l'acide pyruvique avec les aldéhydes aliphatiques non substitutes en α. II.- Produits de condensation dont la chaîne carbonée est linéaire: acides α-cétoniques β γ-éthyléniques et α-céto γ-butyrolactones, Bull. Soc. Chim. Fr., 1966, 1435–1444 CAS.
- H. Laborit, C. G. Wermuth, F. Leterrier and C. Baron, La méthyl-4 éthyl-5 pyridazone (MEP) (Ag 147), Agressologie, 1965, 6, 401–410 Search PubMed.
- G. Heinisch and H. Frank, Pharmacologically Active Pyridazines. Part 1, in Progress in Medicinal Chemistry, ed. G. P. Ellis and G. B. West, Elsevier, Amsterdam, 1990, vol. 27, pp. 1–49 Search PubMed.
- G. Heinisch and H. Kopelenk-Frank, Pharmacologically Active Pyridazines. Part 2, in Progress in Medicinal Chemistry, ed. G. P. Ellis and D. K. Luscombe, Elsevier, Amsterdam, 1992, vol. 29, pp. 141–183 Search PubMed.
- R. Grote, Y. Chen and A. Zeeck, Metabolic products of microorganisms 243. Pyridazomycin, a new antifungal antibiotic produced by Streptomyces violaceoniger, J. Antibiot., 1988, 41, 595–601 CAS.
- H. Bockholt, J. M. Beale and J. Rohr, Biosynthesestudien an pyridazomycin, Angew. Chem., Int. Ed. Engl., 1994, 33, 1648–1651 CrossRef.
- J. M. Winter, A. L. Jansma, T. M. Handel and B. S. Moore, Formation of the pyridazine natural product azamerone by biosynthetic rearrangement of an aryl diazoketone, Angew. Chem., Int. Ed., 2009, 48, 767–770 CrossRef.
- Y. Nakamura, A. Ito and C. Shin, Dehydrooligopeptides. XVI. convenient syntheses of two kinds of antrimycins Av and Dv containing dehydrovaline residues, Bull. Chem. Soc. Jpn., 1994, 67, 2151–2161 CAS.
- P. Hughes and J. Clardy, Total synthesis of 3(S)-carboxy-4(S)-hydroxy-2,3,4,5-tetrahydropyridazine, an unusual amino acid constituent of luzopeptin A, J. Org. Chem., 1989, 54, 3260–3264 CrossRef CAS.
- D. J. Pettibone, B. V. Clineschmidt, P. S. Anderson, R. M. Freidinger, G. F. Lundell, L. R. Koupal, C. D. Schwartz, J. M. Williamson, M. A. Goetz, O. D. Hensens, J. M. Liesch and J. P. Springer, A structurally unique, potent, and selective oxytocin antagonist derived from Streptomyces silvensis, Endocrinology, 1989, 125, 117–122 Search PubMed.
- W. Schneider, The dipole moments of diazines, J. Am. Chem. Soc., 1948, 70, 627–630 Search PubMed.
- M. S. Chapman, I. Minor, M. G. Rossmann, G. D. Diana and K. Andries, Human rhinovirus 14 complexed with antiviral compound R 61837, J. Mol. Biol., 1991, 217, 455–463 Search PubMed.
- S. Komeda, G. V. Kalayda, M. Lutz, A. L. Spek, Y. Yamanaka, T. Sato, M. Chikuma and J. Reedijk, New isomeric azine-bridged dinuclear platinum(II) complexes circumvent cross-resistance to cisplatin, J. Med. Chem., 2003, 46, 1210–1219 CrossRef CAS.
- R. J. Gleave, P. J. Beswick, A. J. Brown, G. M. P. Giblin, P. Goldsmith, C. P. Haslam, W. L. Mitchell, N. H. Nicholson, L. W. Page, S. Patel, S. Roomans, B. P. Slingsby and M. E. Swarbrick, Synthesis and evaluation of 3-amino-6-aryl-pyridazines as selective CB2 agonists for the treatment of inflammatory pain, Bioorg. Med. Chem. Lett., 2010, 20, 465–468 Search PubMed.
- G. Heinisch, W. Holzer, F. Kuntz, T. Langer, P. Lukavski, P. Pechlaner and H. Weissenberger, On the bioisosteric potential of diazines: diazine analogues of the combined thromboxane A2 receptor antagonist and synthetase inhibitor ridogrel, J. Med. Chem., 1996, 39, 4058–4064 CrossRef CAS.
- D. Rognan, P. Sokokoff, A. Mann, M.-P. Martres, J.-C. Schwartz, J. Costentin and C. G. Wermuth, Optically active benzamides as predictive tools for mapping the dopamine D2 receptor, Eur. J. Pharmacol., 1990, 189, 59–70 Search PubMed.
- C. G. Wermuth and K. Bizière, Pyridazinyl-GABA derivatives: a new class of synthetic GABA-A antagonists, Trends Pharmacol. Sci., 1986, 7, 421–424 CrossRef CAS.
- C. G. Wermuth, J.-J. Bourguignon, G. Schlewer, J.-P. Gies, A. Schoenfelder, A. Melikian, M.-J. Bouchet, D. Chantreux, J.-C. Molimard, M. J.-P. C. Heaulme and K. Bizière, Synthesis and structure–activity relationships of a series of aminopyridazine derivatives of gamma-aminobutyric acid acting as selective GABA-A antagonists, J. Med. Chem., 1987, 30, 239–249 CrossRef CAS.
- C. Hamdouchi, C. Sanchez-Martinez, J. Gruber, M. del Prado, J. Lopez, A. Rubio and B. A. Heinz, Imidazo[1,2-b]pyridazines, novel nucleus with potent and broad spectrum activity against human picornaviruses: design, synthesis and biological evaluation, J. Med. Chem., 2003, 46, 4333–4341 Search PubMed.
- B. M. Fox, K. Iio, K. Li, R. Choi, I. Takashi, S. Jackson, S. Sagawa, B. Shan, M. Tanaka, A. Yoshida and F. Kayser, Discovery of pyrrolopyridazines as novel DGAT 1 inhibitors, Bioorg. Med. Chem. Lett., 2010, 20, 6030–6033 Search PubMed.
- B. Dyck, S. Markison, L. Zhao, J. Tamiya, J. Grey, M. W. Rowbottom, M. Zhang, T. Vickers, K. Sorensen, C. Norton, J. Wen, C. E. Heise, J. Saunders, P. Conlon, A. Madan, D. Schwarz and V. S. Goodfellow, A thienopyridazinone-based melanin-concentrating hormone receptor 1 antagonist with potent in vivo anorectic properties, J. Med. Chem., 2006, 49, 3753–3756 CrossRef CAS.
- M. P. Giovannoni, C. Vergelli, C. Biancalani, N. Cesari, A. Graziano, P. Biagini, J. Gracia, A. Gavalda and V. Dal Paz, Novel pyrazolopyrimidopyridazinones with potent and selective phosphodiesterase 5 (PDE5) inhibitory activity as potential agents for treatment of erectile dysfunction, J. Med. Chem., 2006, 49, 5363–5371 Search PubMed.
- B. E. Evans, K. E. Rittle, M. G. Bock, R. M. DiPardo, R. M. Freidinger, W. L. Whitter, G. F. Lundell, D. F. Veber, P. S. Anderson, R. S. L. Chang, V. J. Lotti, D. J. Cerino, T. B. P. J. K. Chen, K. A. Kunkel, J. P. Springer and J. Hirshfield, Methods for Drug Discovery: Development of Potent, Selective, Orally Effective Cholecystokinin Antagonists, J. Med. Chem., 1988, 31, 2235–2246 Search PubMed.
- K. Russell and W. F. Michne, The Value of Chemical Genetics in Drug Discovery, in Chemogenomics in Drug Discovery, ed. H. Kubinyi and G. Müller, VCH, 2004, pp. 69–96 Search PubMed.
- A. Kolinsky, Lead-Likeness and Drug-Likeness, in The Practice of Medicinal Chemistry, ed. C. G. Wermuth, Elsevier, San Diego, 2008, pp. 244–254 Search PubMed.
| This journal is © The Royal Society of Chemistry 2011 |