Edaravone containing isoindoline nitroxides for the potential treatment of cardiovascular ischaemia†
James R.
Walker
ab,
Kathryn E.
Fairfull-Smith
ab,
Kazunori
Anzai
c,
Shannen
Lau
d,
Paul J.
White
d,
Peter J.
Scammells
ad and
Steven E.
Bottle
*ab
aARC Centre of Excellence for Free Radical Chemistry and Biotechnology, Australia. E-mail: s.bottle@qut.edu.au; Fax: +61 7 3138 1804; Tel: +61 7 3138 1356
bChemistry Discipline, Faculty of Science and Technology, Queensland University of Technology, QLD 4001, Australia
cNihon Pharmaceutical University, 10281 Komura, Ina-machi, Kitaadachi-gun, Saitama, 362-0806, Japan
dMedicinal Chemistry and Drug Action, Monash Institute of Pharmaceutical Sciences, Monash University, 381 Royal Parade, Parkville, VIC 3052, Australia
First published on 17th March 2011
Abstract
A novel antioxidant for the potential treatment of ischaemia was designed by incorporating an isoindoline nitroxide into the framework of the free radical scavenger edaravone. 5-(3-Methyl-pyrazol-5-ol-1-yl)-1,1,3,3-tetramethylisoindolin-2-yloxyl 7 was prepared by N-arylation of 3-methyl-5-pyrazolone with 5-iodo-1,1,3,3-tetramethylisoindoline-2-yloxyl 8 in the presence of catalytic copper(I)iodide. Evaluation of 7, its methoxyamine derivative 10 and 5-carboxy-1,1,3,3-tetramethylisoindolin-2-yloxyl (CTMIO) against edaravone 1 in ischaemic rat atrial cardiomyocytes revealed significant decreases in cell death after prolonged ischaemia for each agent; however the protective effect of the novel antioxidant 7 (showing greater than 85% reduction in cell death at 100 μM) was significantly enhanced over that of edaravone 1 alone. Furthermore, the activity for 7 was found to be equal to or greater than the potent cardioprotective agent N6-cyclopentyladenosine (CPA). The methoxyamine adduct 10 and edaravone 1 showed no difference between the extent of reduction in cell death whilst CTMIO had only a modest protective effect.
Introduction
Ischaemia is a feature of both stroke and myocardial infarction and results in an inadequate supply of blood and oxygen to the brain or heart. The severity of brain or heart damage resulting from an ischaemic event depends on the degree and duration of ischaemia. It is therefore critical to shorten the ischaemic time in patients with cerebral or myocardial infarction in order to improve their immediate and long-term outcomes. Well established therapeutic strategies to limit infarct size involve early reperfusion with percutaneous coronary intervention (PCI) or thrombolytic therapy (administration of tissue plasminogen activator) and stabilization with antiplatelet agents.1,2 However, these strategies alone are not sufficient to prevent neuronal dysfunction or myocardial injury as reactive oxygen species (ROS) are generated both during ischaemia3 and when oxygen is reintroduced to ischaemic tissue (reperfusion injury).4,5Oxidative stress plays a crucial role in neurodegenerative, cerebrovascular and cardiovascular diseases. Under pathological conditions such as brain and heart injuries where energy generation is suppressed, the production of excessive reactive oxygen species (ROS) such as hydrogen peroxide (H2O2), hydroxyl radical (HO˙) and superoxide anion radical (O2˙−) can induce further damage to cell membranes, resulting in the development of cerebral edema, infarction6 and left ventricular dysfunction.7,8 Thus, the protection of cells from ROS attacks is considered to be an important therapeutic target during the acute phases of stroke and heart attack.
Edaravone (3-methyl-1-phenyl-2-pyrizolin-5-one) 1 is a free radical scavenger that has been approved for use in ischaemic stroke patients in Japan since 2001.9 The neuroprotective effects of edaravone 1 result from its ability to prevent impairment of the antioxidant defence system by reducing or restoring the amount of ROS increased by post-ischaemic reperfusion.10–12 By trapping hydroxyl radicals, edaravone 1 causes enhancement of prostacyclin production,13 radio-protection against ionizing radiation14,15 and the inhibition of both lipid peroxidation16 and the lipoxygenase pathway.13 The putative mechanism underlying the antioxidant action of edaravone 1 involves the transfer of electrons from the edaravone anion 2 to a peroxyl radical, yielding an edaravone radical 3 and a peroxyl anion (Scheme 1). Subsequently, the edaravone radical 3 forms a peroxyl radical of edaravone 4, which is transformed into a 4,5-dione 5 and then hydrolysed to afford 2-oxo-3-(phenylhydrazone)-butanoic acid 6.17
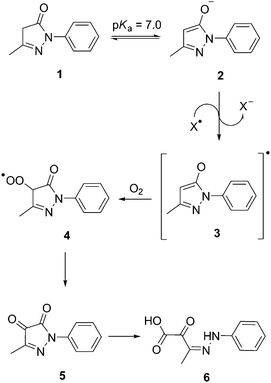 | ||
| Scheme 1 The proposed free radical scavenging mechanism of edaravone 1.17 | ||
The administration of edaravone 1 to rats after myocardial ischaemia-reperfusion also provided protection against the deterioration of cardiac function and suppressed the occurrence of lethal ventricular tachyarrhythmia.18 In addition, a reduction in myocardial infarction size following ischaemia-reperfusion has also been observed in rabbits upon treatment with edaravone 1.19,20 In patients with acute myocardial infarction, the administration of edaravone 1 before reperfusion was associated with smaller infarcts and better clinical outcomes.21,22
In addition to edaravone 1, other antioxidants have been attracting interest in recent years as increased levels of oxidative stress are implicated in many disease states, including Alzheimer's disease, Parkinson's disease, cardiovascular disease, cancer and aging. Nitroxides, a class of stable free radicals, are commonly used as potent antioxidants in biological systems.23,24 Their redox and radical trapping properties can lower levels of oxidative stress in cellular systems caused by ROS25–29 and they can also provide radio-protection towards ionising radiation.30–32 Nitroxides can also function as superoxide dismutase mimetics.33 A number of different ring class nitroxides have been used in mouse models of stroke and other ischaemic injuries (including cardioprotection from ischaemia-reperfusion)34,35 and have been shown to decrease the resultant infarct volume presumably via an antioxidant mode of action.36–38 A recent trend in medicinal chemistry research has been to combine tested and established pharmacophores within the one molecule.39 The work presented here reflects this approach and describes the synthesis and evaluation of a novel antioxidant, 5-(3-methyl-pyrazol-5-ol-1-yl)-1,1,3,3-tetramethylisoindolin-2-yloxyl 7, as a potential new treatment for cardiovascular ischaemia. This novel antioxidant was prepared by incorporating an isoindoline nitroxide into the edaravone framework.
Results and discussion
Synthesis
A number of routes were available for the synthesis of the target molecule, 5-(3-methyl-5-pyrazolone-1-yl)-1,1,3,3-tetramethylisoindolin-2-yloxyl 7. The parent edaravone compound 1 is typically prepared by the thermal condensation of phenyl hydrazine with ethyl acetoacetate.40 However, the corresponding 1,1,3,3-tetramethylisoindolin-2-yloxyl hydrazine analogue was envisioned to be unstable as hydrazines commonly reduce nitroxides to their corresponding hydroxylamines.41 Instead, a more concise synthesis which circumvented this unfavourable interaction was pursued. Transition metal catalysed N-arylation of amides using aryl halides is well known in the literature.42–47 In these C–N bond forming reactions, palladium and copper are the most commonly encountered catalysts. Associated ligands are generally phosphorus-based for palladium45 and chelating diamine ligands for copper48 but N–O mixed ligands are also used.46We have previously utilized halogenated nitroxides such as 5-bromo-1,1,3,3-tetramethylisoindoline-2-yloxyl and 5-iodo-1,1,3,3-tetramethylisoindoline-2-yloxyl 8 in palladium-catalysed Heck, Suzuki and Sonogashira cross-couplings.49–51 As an alternative route to the target molecule 7, we explored the use of halogenated nitroxides for C–N amidation reactions. Using the conditions of Filipski,46 the reaction of 3-methyl-5-pyrazolone with 5-bromo-1,1,3,3-tetramethylisoindoline-2-yloxyl in the presence of the ligand 8-hydroxyquinoline, potassium carbonate and catalytic copper(I) iodide in DMSO at 120 °C for 96 h gave unreacted starting materials. However, use of the more reaction iodo nitroxide 8 gave the desired product 7 in a low isolated yield (13%) after reacting at 130 °C for 3 h. As a marked colour change was observed in this reaction at around 85 °C, the reaction was repeated at 95 °C in an attempt to increase the yield of the desired product 7. After 8 h at this temperature, TLC analysis revealed complete consumption of starting materials, yet the isolated yield of 7 did not improve (Scheme 2). A large mass of a highly polar residue was, however, observed upon work-up. Subsequent variations to these reaction conditions including the use of the iodo amine, 5-iodo-1,1,3,3-tetramethylisoindoline, or the acetate protected iodo nitroxide, 2-acetoxy-5-iodo-1,1,3,3-tetramethylisoindoline, failed to improve the yield any further.
 | ||
| Scheme 2 Reaction conditions (a) CuI, 8-hydroxyquinoline, K2CO3, DMSO, 95 °C, 8 h, 13%, (b) H2O2, FeSO4·7H2O, DMSO, 30 min, 69%. | ||
The use of the N-arylation conditions of Taillefer42 was also explored. Surprisingly, the reaction of 3-methyl-5-pyrazolone with iodo nitroxide 8 in the presence of Fe(acac)3, catalytic CuO and potassium carbonate in DMF at 90 °C for 48 h gave the corresponding secondary amine, 5-iodo-1,1,3,3-tetramethylisoindoline 9, in good yield (74%) (Scheme 3). This reduction did not occur in the absence of 3-methyl-5-pyrazolone and both metals together with 3-methyl-5-pyrazolone gave a far superior conversion of nitroxide 8 to secondary amine 9 than either metal on its own with 3-methyl-5-pyrazolone. Interestingly, this yield is significantly higher than the typical Fe/AcOH procedure for the reduction of nitroxides to amines (∼ 30–65%).24–26 Nonetheless, the only C–N amidation method providing access to the potential antioxidant 7 requires the use of 8-hydroxyquinoline, potassium carbonate and catalytic copper(I) iodide in DMSO.
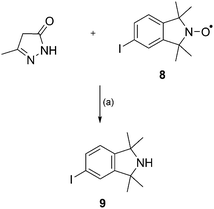 | ||
| Scheme 3 Reaction conditions (a) Fe(acac)3,CuO, K2CO3, DMF, 90 °C, 48 h, 74%. | ||
Synthesis of the methoxyamine analogue of 7, 5-(3-methyl-pyrazol-5-ol-1-yl)-2-methoxy-1,1,3,3-tetramethylisoindoline 10, was achieved in good yield (69%) from 7 using methyl radicals generated from DMSO, ferrous ions and hydrogen peroxide and provided further evidence for the formation of 7 (Scheme 2). Interestingly, 13C NMR analysis of methoxyamine 10 using DEPT revealed that the pyrazolone ring exists exclusively as the enol tautomer in CDCl3, whereas the parent edaravone 1 exists exclusively as the keto tautomer. As the enol tautomer is thought to be the active constituent of edaravone 1,52 this observation indicates that 7 has potential to be a more potent in vitro/in vivoantioxidant that the parent edaravone compound 1.
Cardioprotective effects
Cultured H9c2 embryonic rat atrial cardiomyocytes were exposed to conditions used previously to mimic in vivo ischaemia.34,35,53,54 This type of ischaemic model results in membrane dysfunction and allows entry of the fluorophore propidium iodide. Under simulated ischaemia conditions, 10–50% of all cells were stained positively for propidium iodide. The ischaemia model proceeded as per past experience,34,53,54 with reproducible levels of cell death occurring after 12 h of oxygen deprivation and metabolic stress. When cells were incubated in the simulated ischaemia conditions in the presence of edaravone 1, edaravone-TMIO 7, edaravone-TMIO-Me 10 and 5-carboxy-1,1,3,3-tetramethylisoindolin-2-yloxyl (CTMIO),55 significant decreases in H9c2 cell death after prolonged ischaemia were observed for each agent (Fig. 1). The maximal protective effect observed from all treatments was a reduction in cell death of 85.54 ± 2.13% produced by 100 μM edaravone-TMIO 7. The addition of the TMIO functional group significantly enhanced the protective effect of edaravone 1 (two-way ANOVA, p < 0.05, Fig. 1). The additional protection provided by the TMIO was not observed for the methoxyamine adduct edaravone-TMIO-Me 10; there was no significant difference between the extent of reduction in cell death produced by edaravone 1 to that produced by edaravone-TMIO-Me 10 (two-way ANOVA, p > 0.05). In fact, there was a trend towards the methoxyamine adduct 10 having lower activity than edaravone 1, possibly due to greater non-polar character in the side chain over the parent compound. The nitroxide (CTMIO) alone had only a modest protective effect which was not clearly concentration-dependent. | ||
| Fig. 1 Cardioprotection exerted by edaravone 1 and derivatives in a cell culture ischaemia model. Cells were incubated in hypoxic simulated ischaemia (SI) medium for 12 h. Propidium iodide exclusion was then used to determine the number of viable cells, and cell death calculated for each treatment, with the simulated ischaemia treatment normalised to 100%. * indicates a significant difference between edaravone 1 and edaravone-TMIO 7 (ANOVA; n = 3–4, p < 0.05). | ||
The adenosine A1receptor agonist N6-cyclopentyladenosine (CPA) produced a 75.4 ± 3.2% reduction in cell death when a supramaximal concentration (10 μM) was used. CPA was included to validate the assay and to provide context for any effects seen by the antioxidants tested, as we have previously shown CPA to be a potent protective agent in this model.34,53,54 The higher concentrations of edaravone-TMIO 7 produced reductions in cell death at least as high as that of CPA and possibly higher, although the trend towards a greater effect of 100 μM edaravone-TMIO 7 than CPA was not statistically significant. Thus, edaravone-TMIO 7 was found to be a potent protective agent in a model of ischaemic cell death, with an activity equal to or greater than the potent cardioprotective agent CPA.
By comparison, edaravone 1 is used clinically for stroke patients twice a day with the dose of 30 mg for each treatment taking 30 min by intravenous infusion. An effective dose of 3 mg kg−1via intravenous infusion was reported against ischaemic brain injury models of rats.56,57 Peak plasma concentrations of edaravone 1 from clinical trials of this agent have been reported to be around 20 μM after a moderate bolus intravenous dose (1.5 mg kg−1).58 Thus, the protective effects of edaravone analogues seen in the present study occurred at concentrations that were clinically relevant.
Experimental
General chemistry methods
Air-sensitive reactions were carried out under an atmosphere of ultra-high purity argon. 5-Bromo-1,1,3,3-tetramethylisoindoline-2-yloxyl,595-iodo-1,1,3,3-tetramethylisoindoline-2-yloxyl 860 and 5-carboxy-1,1,3,3-tetramethylisoindolin-2-yloxyl (CTMIO)55 were synthesised using established literature procedures. All other reagents were purchased from commercial suppliers and used without further purification. 1H and 13C NMR spectra were recorded on a Bruker Avance 400 spectrometer and referenced to the relevant solvent peak. Low and high resolution mass spectra were recorded at the Australian National University (ANU) using either a Micromass autospec double focusing magnetic sector mass spectrometer (EI + spectra) or a Bruker Apex 3 fourier transform ion cyclotron resonance mass spectrometer with a 4.7 T magnet (ESI + spectra). Formulations were calculated in the elemental analysis programs of Mass Lynx 4.0 or Micromass Opus 3.6. Fourier transform infrared (FTIR) spectra were recorded on a Nicolet 870 Nexus Fourier Transform Infrared Spectrometer equipped with a DTGS TEC detector and an ATR objective. Melting points were measured on a GallenKamp Variable Temperature Apparatus by the capillary method and are uncorrected. Analytical HPLC was carried out on an Agilent Technologies HP 1100 Series HPLC system using an Agilent Prep-C18 scalar column (4.6 × 150 mm, 10 μm) with a flow rate of 1 mL min−1.Synthesis of 5-(3-methyl-pyrazol-5-ol-1-yl)-1,1,3,3-tetramethylisoindolin-2-yloxyl 7
5-Iodo-1,1,3,3-tetramethylisoindolin-2-yloxyl 8 (300 mg, 0.95 mmol), 3-methyl-5-pyrazolone (124 mg, 1.27 mmol, 1.3 equiv.), 8-hydroxyquinoline (23 mg, 0.16 mmol, 17 mol%), CuI (30 mg, 0.16 mmol, 17 mol%) and K2CO3 (160 mg, 1.16 mmol, 1.2 equiv.) were combined in a Schlenk tube. The tube was evacuated and an atmosphere of argon was introduced. DMSO (6 cm3) was added and the reaction mixture submitted to three freeze-evacuate-thaw cycles after which time a positive pressure of argon was introduced. The reaction was heated at 95 °C for 8 h. The resulting solution was diluted with EtOAc and run through a short silica column. The reaction residue was repeatedly extracted with EtOAc (3 × 10 cm3) and these organics were also run through the short silica column to remove insoluble impurities. Thorough elution with EtOAc ensured the desired compound eluted from the column. The combined column fractions were washed with H2O, dried (Na2SO4) and the solvent removed under reduced pressure to yield a dark brown residue. Silica gel chromatography (85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15, EtOAc:Hex) yielded 7 (36 mg, 0.126 mmol, 13.3%) as a pale yellow powder. mp 185 °C (decomp.). MS (EI): m/z (%) = 286 (50) [M+]. HRMS (EI): m/z: calcd. for C16H20N3O2 [M+]: 286.1556; found 286.1558. IR: 1430 cm−1 (N–O˙). The purity of 7 was confirmed to be >96% using Analytical HPLC with a mobile phase of 60% MeOH/40% H2O.
15, EtOAc:Hex) yielded 7 (36 mg, 0.126 mmol, 13.3%) as a pale yellow powder. mp 185 °C (decomp.). MS (EI): m/z (%) = 286 (50) [M+]. HRMS (EI): m/z: calcd. for C16H20N3O2 [M+]: 286.1556; found 286.1558. IR: 1430 cm−1 (N–O˙). The purity of 7 was confirmed to be >96% using Analytical HPLC with a mobile phase of 60% MeOH/40% H2O.
Synthesis of 5-(3-methyl-pyrazol-5-ol-1-yl)-2-methoxy-1,1,3,3-tetramethylisoindoline 10
A solution of 5-(3-methyl-pyrazol-5-ol-1-yl)-1,1,3,3-tetramethylisoindolin-2-yloxyl 7 (44 mg, 0.154 mmol) and FeSO4·7H2O (87 mg, 0.313 mmol, 2 equiv.) in DMSO (∼1 cm3) was prepared. Hydrogen peroxide (30%, 35 μL, 0.37 mmol, 2.4 equiv.) was then added dropwise with stirring. After stirring at room temperature for 30 min, the reaction was quenched with H2O and extracted with EtOAc (3 × 30 cm3). The combined organics were dried (Na2SO4) and the solvent removed under reduced pressure. Silica gel column chromatography (70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 EtOAc:Hex) yielded 10 (32 mg, 69%) as a brown solid. mp 198–203 °C (decomp.). 1H NMR (400 MHz, CDCl3): δ = 7.25 (dd, 1H, J1 = 7.2 Hz, J2 = 2.2 Hz, 6-H), 7.17 (d, 1H, J1 = 7.2 Hz, 7-H) 7.10 (d, 1H, J2 = 2.2 Hz, 4-H), 5.60 (s, 1H, 4′–H), 3.81 (s, 3H, O–CH3), 2.27 (s, 3H, 3′–CH3), 1.48 (s, 12H, 2 × C(CH3)2). 13C NMR (100 MHz, CDCl3): δ = 162.7 (C5′), 146.3 (C3′), 144.1 (C3a), 140.7 (C7a), 138.0(C5), 123.6 (C6), 122.2 (C7), 118.1 (C4), 93.2 (C4′), 67.1 (C1/C3), 65.5 (O–CH3), 12.7 (N = C–CH3). MS (EI): m/z (%) = 301 (25) [M+], 286 (100). HRMS (EI): m/z: calcd. for C17H23N3O2 [M+]: 301.1790; found 301.1785. The purity of 10 was confirmed to be >96% using Analytical HPLC with a mobile phase of 60% MeOH/40% H2O.
30 EtOAc:Hex) yielded 10 (32 mg, 69%) as a brown solid. mp 198–203 °C (decomp.). 1H NMR (400 MHz, CDCl3): δ = 7.25 (dd, 1H, J1 = 7.2 Hz, J2 = 2.2 Hz, 6-H), 7.17 (d, 1H, J1 = 7.2 Hz, 7-H) 7.10 (d, 1H, J2 = 2.2 Hz, 4-H), 5.60 (s, 1H, 4′–H), 3.81 (s, 3H, O–CH3), 2.27 (s, 3H, 3′–CH3), 1.48 (s, 12H, 2 × C(CH3)2). 13C NMR (100 MHz, CDCl3): δ = 162.7 (C5′), 146.3 (C3′), 144.1 (C3a), 140.7 (C7a), 138.0(C5), 123.6 (C6), 122.2 (C7), 118.1 (C4), 93.2 (C4′), 67.1 (C1/C3), 65.5 (O–CH3), 12.7 (N = C–CH3). MS (EI): m/z (%) = 301 (25) [M+], 286 (100). HRMS (EI): m/z: calcd. for C17H23N3O2 [M+]: 301.1790; found 301.1785. The purity of 10 was confirmed to be >96% using Analytical HPLC with a mobile phase of 60% MeOH/40% H2O.
Method for simulated ischaemia
Conclusions
The protection of cells from ROS insult is an important therapeutic target during the acute phases of ischaemia. A novel antioxidant was designed for this purpose and combines edaravone 1, the free radical scavenger approved for use in ischaemic patients in Japan, with a nitroxide moiety. The synthesis of the target molecule was achieved by N-arylation of 3-methyl-5-pyrazolone with 5-iodo-1,1,3,3-tetramethylisoindoline-2-yloxyl 8 in the presence of catalytic copper(I)iodide in DMSO in a low yield (13%). The use of 5-iodo-1,1,3,3-tetramethylisoindoline, or the acetate protected nitroxide, 2-acetoxy-5-iodo-1,1,3,3-tetramethylisoindoline, in place of 8 under the same reaction conditions failed to improve the yield of obtained product. Employing alternative reaction conditions involving Fe(acac)3, catalytic CuO and potassium carbonate in DMF surprisingly reduced the nitroxide to its secondary amine in good yield (74%). The methoxyamine derivative 10 was prepared from 7 in good yield (69%) by reaction with methyl radicals formed using Fenton chemistry. Assessment of 7, its methoxyamine adduct 10 and 5-carboxy-1,1,3,3-tetramethylisoindolin-2-yloxyl (CTMIO) against edaravone 1 in ischaemic rat atrial cardiomyocytes showed a significant decrease in cell death after prolonged ischaemia for all agents. The maximal protective effect was produced by treatment with nitroxide 7 at 100 μM with an observed cell death of 85.54 ± 2.13%. The concentration-dependent reduction in cell death by 7 after 12 h simulated ischaemia was significantly greater than that of edaravone 1 alone. Furthermore, the activity for 7 was found to be equal to or greater than the potent cardioprotective agent N6-cyclopentyladenosine (CPA). The methoxyamine derivative 10 and edaravone 1 showed no difference between the extent of reduction in cell death whilst CTMIO had only a modest protective effect which was not clearly-concentration dependent. Thus, the novel antioxidant 7 described herein shows promise for the treatment of cardiovascular ischaemia. Evaluation of this compound 7 as a potential treatment for ischaemic stroke is in progress and will be reported in due course.Acknowledgements
We thank the Australian Research Council Centre of Excellence for Free Radical Chemistry and Biotechnology (CEO 0561607) for financial support.Notes and references
- W. Hacke, T. Brott, L. Caplan, D. Meier, C. Fieschi, R. Von Kummer, G. Donnan, W. Heiss, N. Wahlgren, M. Spranger, G. Boysen and J. R. Marler, Neurology, 1999, 53, S3 CAS.
- W. Hacke, M. Kaste, E. Bluhmki, M. Brozman, A. Davalos, D. Guidetti, V. Larrue, K. R. Lees, Z. Medeghri, T. Machnig, D. Schneider, R. von Kummer, N. Wahlgren and D. Toni, N. Engl. J. Med., 2008, 359, 1317 CrossRef CAS.
- B. R. S. Broughton, D. C. Reutens and C. G. Sobey, Stroke, 2009, 40, e331 CrossRef.
- B. J. Lee, Y. Egi, K. van Leyen, E. H. Lo and K. Arai, Brain Res., 2010, 1307, 22 CrossRef CAS.
- M. T. Dirksen, G. J. Laarman, M. L. Simoons and D. J. G. M. Duncker, Cardiovasc. Res., 2007, 74, 343 CrossRef CAS.
- I. Klatzo, J. Neuropathol. Exp. Neurol., 1967, 26, 1 CrossRef CAS.
- J. T. Flaherty, Am. J. Med., 1991, 91, 3C79S.
- H. Tsutsui, Circ. J., 2004, 68, 1095 CrossRef CAS.
- Edaravone Acute Infarction Study Group, Cerebrovascular Diseases, 2003, 15, 222 Search PubMed.
- M. Tosaka, Y. Hashiba, N. Saito, H. Imai, T. Shimizu and T. Sasaki, Acta Neurochirurgica, 2002, 144, 1305 Search PubMed.
- T. Yamaguchi, K. Oishi, M. Uchida and H. Echizen, Biol. Pharm. Bull., 2003, 26, 1706 CrossRef CAS.
- H. Kawai, H. Nakai, M. Suga, S. Yuki, T. Watanabe and K. I. Saito, J. Pharmacol. Exp. Ther., 1997, 281, 921 CAS.
- H. Watanabe, I. Morita, H. Nishi and S. Murota, Prostaglandins, Leukotrienes Essent. Fatty Acids, 1988, 33, 81 CrossRef.
- K. Anzai, M. Furuse, A. Yoshida, A. Matsuyama, T. Moritake, K. Tsuboi and N. Ikota, J. Radiat. Res., 2004, 45, 319 Search PubMed.
- N. Sasano, A. Enomoto, Y. Hosoi, Y. Katsumura, Y. Matsumoto, K. Shiraishi, K. Miyagawa, H. Igaki and K. Nakagawa, J. Radiat. Res., 2007, 48, 495 Search PubMed.
- T. Watanabe, S. Yuki, M. Egawa and H. Nishi, J. Pharmacol. Exp. Ther., 1994, 268, 1597 CAS.
- Y. Yamamoto, T. Kuwahara and K. Watanabe, Redox Rep., 1996, 2, 333 Search PubMed.
- H. Yagi, S. Horinaka and H. Matsuoka, J. Cardiovasc. Pharmacol., 2005, 46, 46 CrossRef CAS.
- A. Fukuda, S. Okubo, Y. Tanabe, Y. Hoshiba, H. Shiobara, K. Harafuji, Y. Kobori, M. Fujinawa, T. Okubo and A. Yamashina, J. Int. Med. Res., 2006, 34, 475 Search PubMed.
- H. Onogi, S. Minatoguchi, X.-H. Chen, N. Bao, H. Kobayashi, Y. Misao, S. Yasuda, T. Yamaki, R. Maruyama, Y. Uno, M. Arai, G. Takemura and H. Fujiwara, Clin. Exp. Pharmacol. Physiol., 2006, 33, 1035 CrossRef CAS.
- K. Tsujita, H. Shimomura, H. Kawano, J. Hokamaki, M. Fukuda, T. Yamashita, S. Hida, Y. Nakamura, Y. Nagayoshi, T. Sakamoto, M. Yoshimura, H. Arai and H. Ogawa, Am. J. Cardiol., 2004, 94, 481 CrossRef CAS.
- K. Tsujita, H. Shimomura, K. Kaikita, H. Kawano, J. Hokamaki, Y. Nagayoshi, T. Yamashita, M. Fukuda, Y. Nakamura, T. Sakamoto, M. Yoshimura and H. Ogawa, Circ. J., 2006, 70, 832 CrossRef CAS.
- M. C. Krishna and A. Samuni, Methods Enzymol., 1994, 234, 580 CAS.
- J. B. Mitchell, M. C. Krishna, A. Samuni, A. Russo, S. M. Hahn, in Reactive Oxygen Species in Biological Systems: An Interdisciplinary Approach, ed. D. L. Gilbert, C. A. Colton, Kluwer Academic Publishers, New York, 1999 Search PubMed.
- R. Schubert, L. Erker, C. Barlow, H. Yakushiji, D. Larson, A. Russo, J. B. Mitchell and A. Wynshaw-Boris, Hum. Mol. Genet., 2004, 13, 1793 CrossRef CAS.
- K. Hosokawa, P. Chen, M. F. Lavin and S. E. Bottle, Free Radical Biol. Med., 2004, 37, 946–952 CrossRef CAS.
- M. C. Krishna, W. DeGraff, O. H. Hankovszky, C. P. Sar, T. Kalai, J. Jeko, A. Russo, J. B. Mitchell and K. Hideg, J. Med. Chem., 1998, 41, 3477 CrossRef CAS.
- A. M. Samuni and Y. Barenholz, Free Radical Biol. Med., 2003, 34, 177 CrossRef CAS.
- A. M. Samuni, W. DeGraff, J. A. Cook, M. C. Krishna, A. Russo and J. B. Mitchell, Free Radical Biol. Med., 2004, 37, 1648 CrossRef CAS.
- S. M. Hahn, M. C. Krishna, A. M. DeLuca, D. Coffin, J. B. Mitchell, Free Radical Bio. Med., 28, p. 953 Search PubMed.
- S. M. Hahn, M. C. Krishna, A. Samuni, W. DeGraff, D. O. Cuscela, P. Johnstone and J. B. Mitchell, Cancer Res., 1994, 54, 2006s CAS.
- S. M. Hahn, L. Wilson, M. C. Krishna, J. Liebmann, W. DeGraff, J. Gamson, A. Samuni, D. Venzon and J. B. Mitchell, Free Radical Biol. Med., 2000, 28, 1257 CrossRef CAS.
- A. Samuni, C. M. Krishna, J. B. Mitchell, C. R. Collins and A. Russo, Free Radical Res., 1990, 9, 241 CrossRef CAS.
- A. Gregg, S. E. Bottle, S. M. Devine, H. Figler, J. Linden, P. White, C. W. Pouton, V. Urmaliya and P. J. Scammells, Bioorg. Med. Chem. Lett., 2007, 19, 5437 CrossRef.
- S. M. Devine, A. Gregg, H. Figler, K. McIntosh, V. Urmaliya, J. Linden, C. W. Pouton, P. J. White, S. E. Bottle and P. J. Scammells, Bioorg. Med. Chem., 2010, 18, 3078 CrossRef CAS.
- D. Arieli, G. Nahmany, N. Casap, D. Ad-El and Y. Samuni, Free Radical Res., 2008, 42, 114 CrossRef CAS.
- A. Hoffman, S. Goldstein, A. Samuni, J. B. Borman and H. Schwalb, Biochem. Pharmacol., 2003, 66, 1279 CrossRef CAS.
- N. Kato, K. Yanaka, K. Hyodo, K. Homma, S. Nagase and T. Nose, Brain Res., 2003, 979, 188 CrossRef CAS.
- L. M. Ruilope, A. Dukat, M. Boehm, Y. Lacourciere, J. Gong and M. P. Lefkowitz, Lancet, 1255, 375 Search PubMed.
- Q. Tao, S. Wang and Z. Hao, Zhongguo Yiyao Gongye Zazhi, 2004, 35, 643 Search PubMed.
- D. Gigmes, A. Gaudel-Siri, S. R. A. Marque, D. Bertin, P. Tordo, P. Astolfi, L. Greci and C. Rizzoli, Helv. Chim. Acta, 2006, 89, 2312 CrossRef CAS.
- M. Taillefer, N. Xia and A. Ouali, Angew. Chem., Int. Ed., 2007, 46, 934 CrossRef CAS.
- E. R. Strieter, D. G. Blackmond and S. L. Buchwald, J. Am. Chem. Soc., 2005, 127, 4120 CrossRef CAS.
- J. Yin and S. L. Buchwald, Org. Lett., 2000, 2, 1101 CrossRef CAS.
- W. C. Shakespeare, Tetrahedron Lett., 1999, 40, 2035 CrossRef CAS.
- K. J. Filipski, J. T. Kohrt, A. Casimiro-Garcia, C. A. Van Huis, D. A. Dudley, W. L. Cody, C. F. Bigge, S. Desiraju, S. Sun, S. N. Maiti, M. R. Jaber and J. J. Edmunds, Tetrahedron Lett., 2006, 47, 7677 CrossRef CAS.
- J. E. Golden, S. D. Sanders, K. M. Muller and R. W. Buerli, Tetrahedron Lett., 2008, 49, 794 CrossRef CAS.
- A. Klapars, X. Huang and S. L. Buchwald, J. Am. Chem. Soc., 2002, 124, 7421 CrossRef CAS.
- D. J. Keddie, T. E. Johnson, D. P. Arnold and S. E. Bottle, Org. Biomol. Chem., 2005, 3, 2593 RSC.
- D. J. Keddie, K. E. Fairfull-Smith and S. E. Bottle, Org. Biomol. Chem., 2008, 6, 3135 RSC.
- K. E. Fairfull-Smith and S. E. Bottle, Eur. J. Org. Chem., 2008, 5391 CrossRef CAS.
- K. Watanabe, Y. Morinaka, K. Iseki, T. Watanabe, S. Yuki and H. Nishi, Redox Rep., 2003, 8, 151 Search PubMed.
- V. B. Urmaliya, J. E. Church, I. M. Coupar, R. B. Rose'Meyer, C. W. Pouton and P. J. White, J. Cardiovasc. Pharmacol., 2009, 53, 424 CrossRef CAS.
- V. B. Urmaliya, C. W. Pouton, S. M. Devine, J. M. Haynes, L. Warfe, P. J. Scammells and P. J. White, J. Cardiovasc. Pharmacol., 2010, 56, 282 CrossRef CAS.
- S. E. Bottle, D. G. Gillies, D. L. Hughes, A. S. Micallef, A. I. Smirnov and L. H. Sutcliffe, J. Chem. Soc., Perkin Trans. 2, 2000, 1285 RSC.
- K. Abe, S. Yuki and K. Kogure, Stroke, 1988, 19, 480 CAS.
- H. Nishi, T. Watanabe, H. Sakurai, S. Yuki and A. Ishibashi, Stroke, 1989, 20, 1236 CAS.
- H. Shibata, S. Arai, M. Izawa, M. Murasaki, Y. Takamatsu, O. Izawa, C. Takahashi and M. Tanaka, Jpn J. Clin. Pharm. Ther., 1998, 29, 863 Search PubMed.
- A. S. Micallef, R. C. Bott, S. E. Bottle, G. Smith, J. M. White, K. Matsuda and H. Iwamura, J. Chem. Soc., Perkin Trans. 2, 1999, 65 RSC.
- K. E. Fairfull-Smith, J. P. Blinco, D. J. Keddie, G. A. George and S. E. Bottle, Macromolecules, 2008, 41, 1577 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Spectral characterisation data and analytical HPLC chromatograms for compounds 7 and 10 are included in the supporting information. See DOI: 10.1039/c1md00041a/ |
| This journal is © The Royal Society of Chemistry 2011 |
