Alkynylation of N-(3-iodopyridin-2-yl)sulfonamide under Pd/C–Cu catalysis: a direct one pot synthesis of 7-azaindoles and their pharmacological evaluation as potential inhibitors of sirtuins†
Mohosin
Layek
ab,
Y.
Syam Kumar
a,
Aminul
Islam
a,
Ravikumar
Karavarapu
c,
Amrita
Sengupta
c,
Devyani
Halder
c,
K.
Mukkanti
b and
Manojit
Pal
*c
aDr Reddy's Laboratories Ltd, Bollaram Road, Miyapur, Hyderabad, 500049, India
bChemistry Division, Institute of Science and Technology, JNT University, Kukatpally, Hyderabad, 500072, India
cInstitute of Life Sciences, University of Hyderabad Campus, Gachibowli, Hyderabad, 500 046, India. E-mail: manojitpal@rediffmail.com; Fax: +91 40 6657 1581; Tel: +91 40 6657 1500
First published on 19th April 2011
Abstract
The Pd-mediated alkynylation of N-(3-iodopyridin-2-yl)sulfonamide was investigated in the presence of 2-aminoethanol as a base. The combination of Pd/C–Cu catalysts and 2-aminoethanol facilitated the reaction to proceed via a coupling-cyclization sequence in a single-pot. Unlike earlier Pd-mediated two-step process the present reaction proceeds via a tandem C–C and C–N bond forming reaction affording a direct synthesis of 2-substituted-7-azaindole derivatives. A variety of novel 2-substituted-7-azaindoles were prepared by using this one-pot method. The methodology was explored for a formal synthesis of a Variolin B analogue. When tested in vitro some of the compounds synthesized showed promising sirtuin inhibiting properties in yeast without showing significant cell toxicities. Docking studies using the active molecules were carried out to understand the nature of their interactions with Sir2 protein.
7-Azaindole (or 1H-pyrrolo[2,3-b]pyridine, Fig. 1), a member of azaindole family, is considered as a bioisostere of indole or purine moiety and found to be integral part of many bioactive molecules.1 Due to their natural occurrences and various physicochemical and pharmacological properties this class of compounds have attracted considerable interest.2,3 For example, Variolins isolated from Antartic sponge Kirkpatrickia varialosa were found to be active against P388 murine leukemia cells. A representative compound Variolin B (Fig. 1) was identified as the most active among them.4 Recently, an indole based compound (C, Fig. 1) i.e.6-chloro-2,3,4,9-tetrahydro-1H-carbazole-1-carboxamide or EX-527 (also known as SEN0014196) that was identified as a potent inhibitor of sirtuin5a is presently undergoing Phase 1a clinical trial and being developed for the treatment of Huntington's disease.5b This prompted us to examine a series of 2-substituted azaindole derivatives as potential inhibitors of sirtuins. We envisaged that due to the presence of an H-bond acceptor at “N–7” of the azaindole ring this class of compounds might show significant interactions with sirtuins. Moreover, replacing the indole ring by an azaindole moiety has resulted in mark improvement in pharmacokinetic properties in several cases earlier.5c Nevertheless, in spite of structural similarity with indoles the azaindole class has not been previously explored as probable inhibitors of sirtuins.
 | ||
| Fig. 1 7-Azaindole (A), Variolin B (B) and inhibitor of sirtuin EX-527. | ||
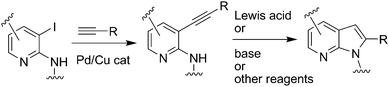
Despite their medicinal value, a straightforward synthesis of azaindoles is not common in the literature partly due to the electron-deficient nature of the key pyridine ring and the strong metal binding affinity of azaindoles. Moreover, the possibility of formation of N-isomers could complicate their synthesis. Nevertheless, one of the frequently used strategies for the synthesis of azaindole derivatives involves the construction of a pyrrole ring on a pyridine moiety and like indoles6–9 palladium catalyzed reactions have been explored as a key synthetic step.10–16 These include palladium catalyzed annulations of aryl halide with (i) terminal alkynes under Sonogashira conditions11–13 or (ii) internal alkynes under Larock conditions.15,16 Despite being quite versatile, the synthesis of 2-substituted-7-azaindoles employing terminal alkynes often involves a two step process (Fig. 2) i.e.Sonogashira coupling of 3-halo-2-aminopyridine with terminal alkynes followed by cyclization of the resulting 3-alkynyl-2-aminopyridine in the presence of (i) a base11a–ee.g.metal hydridei.e. KH in NMP or metal alkoxide i.e.KOtBu in NMP or DBU in MeOH or Et3N in DMF; (ii) a Lewis acid12a–de.g.AuCl3 in EtOH or CuI in DMF or InBr2 in toluene or (iii) iodine13 in acetonitrile. However most of these two-step methods suffer from either the requirement of longer reaction time (up to 72 h) or the use of harsh reaction conditions. Additionally, requirement of different catalysts or reagents for two individual steps made these approaches less attractive especially in scale-up synthesis. Some of these catalysts and reagents are either expensive or not recoverable/recyclable or their uses cause environmental problems. Thus, there was a need for the development of more general and effective method for the synthesis of 2-substituted-7-azaindoles. As part of our ongoing effort to build a compound library based on azaindoles17 we also required a simple and straightforward method to construct the 7-azaindole ring possessing various substituents at C-2 position. Herein, we report a highly efficient and practical method for the one pot synthesis of 7-azaindoles (3) from N-(3-iodopyridin-2-yl)sulfonamide (1, Z = Me or Ph) and terminal alkynes (2) using 10% Pd/C–PPh3–CuI as a catalyst system (Scheme 1). To the best of our knowledge, a one-pot synthesis of 2-substituted-7-azaindole under Pd/C–Cu catalysis has not been reported earlier.
 | ||
| Fig. 2 Previously reported two-step synthesis of 2-substituted-7-azaindoles.11–13 | ||
 | ||
| Scheme 1 Pd/C mediated synthesis of 7-azaindole. | ||
One of the key reactants i.e.sulfonamide (1) required for our azaindole synthesis was prepared from 2-amino-3-iodo-pyridine (4) following a modified literature procedure shown in Scheme 2.18 Initially, in order to establish the optimum reaction condition we chose to examine the coupling reaction of N-(3-iodopyridin-2-yl)benzenesulfonamide (1a) with phenylacetylene (2a) and the corresponding results are summarized in Table 1. Our present strategy to synthesize 7-azaindole was originally based on our earlier synthesis of 2-substituted indolesvia a Pd/C-catalyzed coupling-cyclization process in water.192-Aminoethanol was found to be an effective base in our previous study. Accordingly, the reaction of 1 with 2a was carried out using 10% Pd/C–PPh3–CuI as a catalyst system in water in the presence of 2-aminoethanol at 80 °C. While the starting compound (1) disappeared after 1 h (according to TLC) no desired product however was isolated from the reaction mixture (entry 1, Table 1). We then examined the use of few organic solventse.g.DMF (entry 2, Table 1), 1,4-dioxane (entry 3, Table 1) and acetonitrile (entry 4, Table 1). While the desired compound 3a was isolated in these cases acetonitrile however, was identified as the best solvent in which the reaction was completed within 4 h affording 3a in 85% yield. The use of other bases e.g.triethylamine (entry 5, Table 1) and DIPA (entry 6, Table 1) in place of 2-aminoethanol decreased the product yield. Among the other Pd-catalysts examined (entries 7–9, Table 1), (PPh3)2PdCl2 was found to be effective (entry 9, Table 1) but afforded 3a in slightly lower yield. Nevertheless, we preferred Pd/C because it is cheaper, stable, and easy to handle and separable from the product. Moreover, the catalyst can be recycled.20
|
|
|||||
|---|---|---|---|---|---|
| Entry | Solvent | Catalysts | Base | Time (h) | % Yieldb |
| a All reactions were carried out by using 1 (0.832 mmol), 2 (1.247 mmol), 10% Pd/C or other Pd-catalyst (0.025 mmol), PPh3 (0.099 mmol), CuI (0.049 mmol) and a base (3.0 equiv) at 80 °C. b Isolated yields. c PPh3 was not used. | |||||
| 1. | H2O | 10%Pd/C-PPh3 | 2-Aminoethanol | 20 | 0 |
| 2. | DMF | 10%Pd/C-PPh3 | 2-Aminoethanol | 8 | 65 |
| 3. | 1,4-Dioxane | 10%Pd/C-PPh3 | 2-Aminoethanol | 20 | 50 |
| 4. | CH3CN | 10%Pd/C-PPh3 | 2-Aminoethanol | 4 | 85 |
| 5. | CH3CN | 10%Pd/C-PPh3 | Et3N | 20 | 40 |
| 6. | CH3CN | 10%Pd/C-PPh3 | (i-Pr)2NEt | 4 | 70 |
| 7. | CH3CN | Pd(PPh3)4 | 2-Aminoethanol | 5 | 40c |
| 8. | CH3CN | Pd(OAc)2 | 2-Aminoethanol | 6 | 45c |
| 9. | CH3CN | (PPh3)2PdCl2 | 2-Aminoethanol | 4 | 70c |
 | ||
| Scheme 2 Preparation of N-(3-iodopyridin-2-yl)substituted sulfonamide. | ||
Having prepared the 7-azaindole derivative 3a successfully we decided to explore the scope and generality of this one-pot coupling-cyclization process in the synthesis of other analogues especially varying the substituent at C-2. Accordingly, a variety of terminal alkynes were reacted with the sulfonamide 1 (Table 2) under the optimized conditions as presented earlier (Entry 4 of Table 1). As evident from Table 2, all the terminal alkynes participated well in this coupling-cyclization reaction affording the desired products in moderate to good yields. Various substituents such as alkyl or aryl groups present in the terminal alkyne were well tolerated. The alkyl side chain may contain a primary (entries 2, and 3, Table 2) or secondary alcohol (entry 4, Table 2). A chloro or cyano group on the alkyl side chain (entry 5 and 12, Table 2) was also tolerated. All the reactions were generally completed within 2–4 h irrespective of the nature of substituents present in the terminal alkynes (2a–k) except the alkyne 2i (entry 9, Table 2). The yields of products were found to be moderate when terminal alkynes containing amino acid residue e.g. (S)- phenylglycine methyl ester (2g) or (S)-leucine methyl ester (2h) were used (entry 7 and 8, Table 2). The moderate yield of product was also observed when hept-1-yne was used (entry 11, Table 2) possibly due to the slow evaporation of the reactant alkyne under the reaction conditions employed. Notably, arylalkynes that are known to undergo spontaneous dimerization under Pd–Cu catalysis were found to be effective under the present reaction conditions (entry 1 and 10, Table 2). All the compounds synthesized were well characterized by spectral and analytical data. Appearance of a singlet in the region 6.3–6.9 δ in the 1H NMR spectra and 104–110 ppm in 13C NMR spectra of all the compounds synthesized was due to the hydrogen at C-3 position which indicated the presence of azaindole ring.
| Entry | Iodide (1); Z = | Alkynes (2) R = | Products (3) | Time (h) | %Yieldb |
|---|---|---|---|---|---|
a All reactions were carried out by using 1 (1.0 equiv), 2 (1.5 equiv), 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio of 10% Pd/C: PPh3: CuI and 2-amino ethanol (3.0 equiv) in MeCN at 80 °C.
b Isolated yields. 2 ratio of 10% Pd/C: PPh3: CuI and 2-amino ethanol (3.0 equiv) in MeCN at 80 °C.
b Isolated yields.
|
|||||
| 1 | Ph 1a | –Ph 2a |
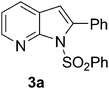
|
4.0 | 85 |
| 2 | 1a | –CH2OH 2b |

|
4.0 | 85 |
| 3 | 1a | -(CH2)2OH 2c |

|
3.0 | 70 |
| 4 | 1a | –CH(OH)CH32d |

|
3.0 | 80 |
| 5 | 1a | –(CH2)3Cl 2e |

|
3.0 | 75 |
| 6 | 1a | –CH2OC6H4NO2-o2f |

|
3.0 | 50 |
| 7 | 1a |
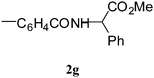
|

|
4.0 | 45 |
| 8 | 1a |

|

|
4.0 | 50 |
| 9 | 1a | –C(CH3)32i |

|
12.0 | 70 |
| 10 | Me 1b | –Ph 2j |

|
2.0 | 75 |
| 11 | 1a | –(CH2)4CH32k |

|
2.0 | 60 |
| 12 | 1a | –(CH2)3CN 2l |

|
3.0 | 72 |
We have shown that Pd/C–Cu catalysis in the presence of 2-aminoethanol afforded 2-substituted-7-azaindoles in a single-pot preparation of which required two distinct steps according to the previously reported methods. A plausible mechanism for the Pd/C–Cu mediated alkynylation of N-(3-iodopyridin-2-yl)sulfonamide 1via coupling-cyclization sequence is shown in Scheme 3. The alkynylation proceeds via generation of an active Pd(0) species in situ that undergoes oxidative addition with 1 to give the organo-Pd(II) species E-1. The active Pd(0) species is generated from the minor portion of the bound palladium (Pd/C) via a Pd leaching process into the solution.20 The leached Pd then becomes an active species by interacting with phosphine ligands. Thus, a dissolved Pd(0)–PPh3 complex is the active species that actually catalyzes the C–C bond forming reaction in solution. The catalytic cycle therefore works in solution rather than on the surface and at the end of the reaction re-precipitation of Pd occurs on the surface of the charcoal. Once generated, the organo-Pd(II) species E-1 then facilitates the stepwise formation of C–C bond via (i) trans organometallation with copper acetylide generated in situ from CuI and terminal alkyne followed by (ii) reductive elimination of Pd(0) to afford the internal alkyne E-2. The alkyne E-2 thus formed subsequently undergoes Cu-mediated21a ring closure in an intramolecular fashion to give the desired product (3). In general, the shorter reaction time (2–4 h except entry 9, Table 1) required for the preparation of 7-azaindoles in compared to the indole derivatives19 (3–24 h) is perhaps due to the higher reactivity of 1 aided by the π-electron deficiency of the pyridine ring. Moreover, the hydrogen bonding within the acid–base ion pair of 1 and 2-aminoethanol perhaps increased the reactivity of 1 towards Pd-mediated alkynation process.21b This also helps in polarizing the Cu-coordinated triple bond of E-2 thereby facilitating the intramolecular attack by the sulfonamide anion leading to the azaindole 3. Nevertheless, the overall reaction mechanism involves (a) generation of actual catalytic species, (b) the catalytic cycle for C–C bond formation followed by (c) intramolecular cyclization for C–N bond formation.
 | ||
| Scheme 3 Proposed mechanism for the one-pot synthesis of 2-substituted-7-azaindoles (3) under the catalysis of Pd/C–CuI–PPh3 | ||
Having prepared a variety of 2-subtituted-7-azaindoles (3), we envisaged that the core moiety of variolin (7) might be readily synthesized from the corresponding protected 2-amino-3-iodo pyridine (1a) using Pd/C–Cu catalyzed coupling-cyclization process as a key synthetic step (Scheme 4). Accordingly, the alcohol derivative (3b) prepared as above (entry 2, Table 2) was oxidized to the aldehyde (6) in the presence of MnO2 in chloroform. The aldehyde (6) could be converted to the target compound (7) following a known multistep method.22
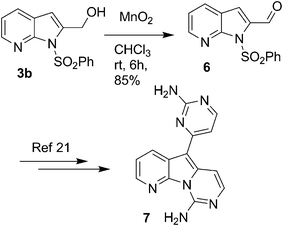 | ||
| Scheme 4 Syntheis of a variolin B analogue. | ||
We have a long standing interest in bioactive molecules.23 As part of our ongoing program24 on identification of novel modulators of sirtuins we tested some of the compounds synthesized for their sirtuin modulating properties in vitro. The sirtuins (class III NAD-dependent deacetylases) are being considered as important targets for cancer therapeutics as they are shown to be up-regulated in various types of cancer.25 Inhibition of sirtuins allows re-expression of silenced tumor suppressor genes, leading to reduced growth of cancer cells. Thus efforts have been devoted for identification of small molecules as inhibitors of sirtuins.26 In our effort to identify inhibitors of sirtuins we have used a yeast cell based reporter silencing assay as a model system for primary screening. Compounds were therefore tested at the concentration of 50 μM initially for their ability to inhibit yeast sirtuin family NAD-dependent histone deacetylase (HDAC) Sir 2 protein. Splitomicin, a known inhibitor of sirtuin, was used as a reference compound in this assay. Various 2-alkyl/aryl substituted azaindoles (3) were tested for their ability to inhibit Sir2 protein by estimating inhibition of growth of yeast strain containing Ura3 gene at telomeric locus, in presence of 5-fluoroorotic acid (5-FOA) as described in experimental procedure.27 Data for three compounds i.e.3b, 3i and 3j are presented in Fig. 3. A compound having the sirtuin inhibitory effect would inhibit the Sir2 protein, and thus the URA3 gene would be de-repressed resulting the death of the yeast cell in presence of 5-FOA. A parallel screen was done in absence of 5-FOA to check the cytotoxicity of the compounds. Among all the compounds tested 3i and 3j showed significant inhibition i.e. 42% and 40% respectively in the presence of 5-FOA. In a dose response study compound 3j showed dose dependent inhibition across all the doses tested. None of these compounds showed significant toxic effect as can be seen from yeast growth in the absence of 5-FOA. The other compounds that showed significant growth inhibitory activity at the concentration of 50 μM are 3a (37%) and 3d (30%). The compounds that showed moderate to low activity include 3c (15%), 3e (12%), 3k (10%) and 3l (10%). Notably all these compounds contain a linear side chain at C-2. Thus the presence of a bulky group at C-2 of the azaindole ring seems to be crucial for displaying sirtuin inhibitory properties of this class of compounds. Nevertheless, to understand the nature of interactions between these compounds and the Sir2 protein docking simulation studies were carried out using 3i and 3j.
 | ||
| Fig. 3 Inhibition of Sir2 protein mediated transcriptional silencing at the telomeric locus in yeast by 3b, 3i and 3j. (A). The growth inhibition of yeast in presence of 3b, 3i and 3j which is due to inhibition of HDAC activity of Sir2 protein. (B) Representative % growth inhibitory activity of the compound 3b, 3i and 3j. | ||
The budding yeast contain five silent information regulator-2 (Sir2) homologuese.g. Sir2-Hist1-4. The yeast Sir2 protein deacetylates histones H3 and H4 and requires the cofactor NAD+ for catalytic activity. The X-ray studies28–31 have shown that a conserved 270 amino acid catalytic domain with variable N- and C- termini is present in all Sir2 structures. The catalytic domain consists of a small Zn-binding domain and a large Rossmann-fold. Based on the interactions with various parts of the NAD+ cofactor (e.g.adenine, ribose and nicotinamide) the interface between large and the small subdomain is subdivided into A (adenine), B (ribose), and C (nicotinamide) pocket. While a similar interaction has been observed with adenine and ribose in all sirtuin X-ray structures the interaction with nicotinamide is less clearly understood. The observed productive and non-productive conformations of nicotinamide in the crystal structure indicate the high flexibility of this part of cofactor. Studies have shown that the acetylated peptide binds in a gap between the two domains. The aliphatic chain of the acetyllysine residue inserts into a conserved hydrophobic pocket (where NAD+ binds nearby) and makes extensive van der Waals interactions. Based on the docking studies we carried out for yeast Sir2 using compounds 3i and 3j (Fig. 4 and 5) it was observed that these compounds interact with the nicotinamide or C subpocket. The X-ray structure of yeast Sir2 was used for an automated ligand docking using Schrodinger molecular modeling software. Both the molecules were found to interact with the Asn484 and Cys233 amino acid residues and bind with the similar active site of Sir2. For example, the “N–7” of the azaindole moiety of both the molecules formed H-bonding with the Asn484 residue. Similarly, the SO2 moiety of the N-sulfonyl group formed hydrogen bond with the “–SH” group of the Cys233 residue (the other commonly found amino acids in the binding region include Lys 501, Hie501, Ser473, Glu504, Ala503). Overall, the binding energy of 3i (−4.81 Kcal/mol) and 3j (−6.65 Kcal/mol) indicates that both the molecules interact significantly with yeast Sir2.
 | ||
| Fig. 4 Docking studies showing H-bond interactions (marked by yellow dashed line) of amino acid residues of yeast Sir 2 with 3i. | ||
 | ||
| Fig. 5 Docking studies showing H-bond interactions (marked by yellow dashed line) of amino acid residues of yeast Sir 2 with 3j. | ||
In conclusion, Pd-mediated alkynylation of N-(3-iodopyridin-2-yl)sulfonamide was investigated and the use of a combination of Pd/C–Cu catalysts and 2-aminoethanol facilitated the coupling-cyclization sequence to proceed in a single-pot. This resulted in the development of an direct and straightforward method for the synthesis of 2-substituted-7-azaindole derivatives via a tandem C–C and C–N bond formation between N-(3-iodopyridin-2-yl)sulfonamide and a terminal alkyne. A number of compounds were prepared for the identification of novel inhibitors of sirtuins by using this methodology. To the best of our knowledge this is the first example of Pd/C-mediated synthesis of 2-substituted-7-azaindole in a single pot. The methodology was found to be general as it worked with a variety of terminal alkynes and well tolerated with a range of functional groups. The methodology is amenable for the synthesis of the core moiety of variolin and other 7-azaindole based complex heterocycles of potential pharmacological interest. The methodology therefore has potential to become a practical alternative to the previously reported methods. All the 2-substituted-7-azaindoles synthesized were screened for their sirtuins inhibitory properties in vitro and docking studies were carried out using the most active compounds to understand the nature of interactions. Due to the medicinal value of 7-azaindoles the methodology presented here would find wide applications.
Mr. M. L. thanks Dr D. Kalita and Dr V. Dahanukar for his encouragement. The authors thank the analytical group of DRL for spectral data.
Notes and references
- For a review, see: F. Popowycz, S. Routier, B. Joseph and J.-Y. Meérour, Tetrahedron, 2007, 63, 1031 Search PubMed.
- (a) P. Bamborough, M. D. Barker, S. A. Campos, R. P. C. Cousins, P. Faulder, H. Hobbs, D. S. Holmes, M. J. Johnston, J. Liddle, J. J. Payne, J. M. Pritchard and C. Whitworth, World Patent Application WO 2008034860 A1, March 27, 2008; (b) J. Liddle, P. Bamborough, M. D. Barker, S. Campos, R. P. C. Cousins, G. J. Cutler, H. Hobbs, D. S. Holmes, C. Ioannou, G. W. Mellor, M. A. Morse, J. J. Payne, J. M. Pritchard, K. J. Smith, D. T. Tape, C. Whitworth and R. A. Williamson, Bioorg. Med. Chem. Lett., 2009, 19, 2504 CrossRef CAS.
- (a) N. Hofgen, U. Egerland, T. Kronbach, D. Marx, S. Szelenyi, H. Kuss and E. Polymeropoulos, World Patent Application WO 20080207689, August 28, 2008; (b) A. Kodimuthali, S. S. L. Jabaris and M. Pal, J. Med. Chem., 2008, 51, 5471 CrossRef CAS.
- (a) N. B. Perry, L. Ettouatti, M. Litaudon, J. W. Blunt, M. H. G. Munro, S. Parkin and H. Hope, Tetrahedron, 1994, 50, 3987 CrossRef CAS; (b) G. Trimurtulu, D. J. Faulkner, N. B. Perry, L. Ettouatti, M. Litaudon, J. W. Blunt, M. H. G. Munro and G. B. Jameson, Tetrahedron, 1994, 50, 3993 CrossRef CAS.
- (a) A. J. Tervo, S. Kyrylenko, P. Niskanen, A. Salminen, J. Leppanen, T. H. Nyronen, T. Jarvinen and A. Poso, J. Med. Chem., 2004, 47, 6292 CrossRef CAS; (b) 11 Jan 2010 (http://www.medicalnewstoday.com/articles/175585.php); (c) See for example: T. Wang, Z. Yin, Z. Zhang, J. A. Bender, Z. Yang, G. Johnson, Z. Yang, L. M. Zadjura, C. J. D'Arienzo, P. D. DiGiugno, C. Gesenberg, G. A. Yamanaka, Y. F. Gong, H. T. Ho, H. Fang, N. Zhou, B. V. McAuliffe, B. J. Eggers, L. Fan, B. Nowicka-Sans, I. B. Dicker, Q. Gao, R. J. Colonno, P. F. Lin, N. A. Meanwell and J. F. Kadow, J. Med. Chem., 2009, 52, 7778 Search PubMed.
- G. R. Humphrey and J. T. Kuethe, Chem. Rev., 2006, 106, 2875 CrossRef CAS.
- K. Krüger, A. Tillack and M. Beller, Adv. Synth. Catal., 2008, 350, 2153 CrossRef.
- L. Ackermann, Synlett, 2007, 507 CrossRef CAS.
- L. Joucla and L. Djakovitch, Adv. Synth. Catal., 2009, 351, 673 CrossRef CAS.
- J. J. Song, J. T. Reeves, F. Gallou, Z. Tan, N. K. Yeea and C. H. Senanayake, Chem. Soc. Rev., 2007, 36, 1120 RSC.
- (a) C. Koradin, W. Dohle, A. L. Rodriguez, B. Schmid and P. Knochel, Tetrahedron, 2003, 59, 1571 CrossRef CAS; (b) A. L. Rodriguez, C. Koradin, W. Dohle and P. Knochel, Angew. Chem., Int. Ed., 2000, 39, 2488 CrossRef CAS; (c) C. Harcken, Y. Ward, D. Thomson and D. Riether, Synlett, 2005, 3121 CAS; (d) L.-P. Sun, X.-H. Huang and J.-X. Wang, J. Chinese Chem. Soc., 2007, 54, 569 Search PubMed; (e) K. C. Majumdar and S. Mondal, Tetrahedron Lett., 2007, 48, 6951 CrossRef CAS.
- (a) K. C. Majumdar, S. Samanta and B. Chattopadhyay, Tetrahedron Lett., 2008, 49, 7213 CrossRef CAS; (b) V. Kumar, J. A. Dority, E. R. Bacon, B. Singh and G. Y. Lesher, J. Org. Chem., 1992, 57, 6995 CrossRef CAS; (c) M. C. de Mattos, S. Alatorre-Santamaría, V. Gotor-Fernández and V. Gotor, Synthesis, 2007, 2149; (d) N. Sakai, K. Annaka, A. Fujita, A. Sato and T. Konakahara, J. Org. Chem., 2008, 73, 4160 CrossRef CAS.
- M. Amjad and D. W. Knight, Tetrahedron Lett., 2004, 45, 539 CrossRef CAS.
- L. Xu, I. R. Lewis, K. D. Steven and J. B. Summers, Tetrahedron Lett., 1998, 39, 5159 CrossRef CAS.
- S. S. Park, J.-K. Choi and E. K. Yum, Tetrahedron Lett., 1998, 39, 627 CrossRef CAS.
- F. Ujjainwalla and T. F. Walsh, Tetrahedron Lett., 2001, 42, 6441 CrossRef CAS.
- M. Layek, V. Gajare, D. Kalita, A. Islam, K. Mukkanti and M. Pal, Tetrahedron, 2009, 65, 4814 CrossRef CAS.
- (a) J. A. Turner, J. Org. Chem., 1983, 48, 3401 CrossRef CAS; (b) A. Yasuhara, M. Kameda and T. Sakamoto, Chem. pharm. Bull., 1999, 47, 809 CAS; (c) C. Hamdouchi, C. Sanchez and J. Ezquerra, Synthesis, 1998, 867 CrossRef CAS.
- (a) M. Pal, V. Subramanian, V. R. Batchu and I. Dager, Synlett, 2004, 1965 CrossRef CAS; (b) M. Layek, U. Lakshmi, D. Kalita, D. K. Barange, A. Islam, K. Mukkanti and M. Pal, Beilstein J. Org. Chem., 2009, 5(No 46) DOI:10.3762/bjoc.5.46.
- M. Pal, Synlett, 2009, 2896 CrossRef CAS.
-
(a) For Cu(I)-mediated similar cyclization process; see: N. T. Patil, H. Wu and Y. Yamamoto, J. Org. Chem., 2005, 70, 4531 Search PubMed;
(b) The reaction of H-bonded ion pair of 1 and 2-aminoethanol can be presented schematically as shown below
. - P. Molina, P. M. Fresneda and S. Delgado, J. Org. Chem., 2003, 68, 489 CrossRef CAS.
- (a) M. Pal, Drug Discovery Today, 2009, 14, 784 CrossRef CAS; (b) M. Pal, Curr. Med. Chem., 2009, 16, 3858 CrossRef CAS; (c) S. H. Havale and M. Pal, Bioorg. Med. Chem., 2009, 17, 1783 CrossRef CAS; (d) R. Gupta, S. S. Walunj, R. K. Tokala, K. V. L. Parsa, S. K. Singh and M. Pal, Curr. Drug Targets, 2009, 10, 71 Search PubMed; (e) A. Kodimuthali, S. S. L. Jabaris and M. Pal, J. Med. Chem., 2008, 18, 5471 CrossRef; (f) M. Pal, S. Angaru, A. Kodimuthali and N. Dhingra, Curr. Pharm. Des., 2009, 15, 1008 CrossRef CAS; (g) M. Pal and S. Pillarisetti, Curr. Med. Chem. –Cardiovascular and Hematological Agents, 2007, 5, 55 Search PubMed; (h) N. Mulakayala, U. Reddy CH., J. Iqbal and M. Pal, Tetrahedron, 2010, 66, 4919 CrossRef CAS.
- (a) M. Layek, A. Reddy M., A. V. D. Rao, M. Alvala, M. K. Arunasree, A. Islam, K. Mukkanti, J. Iqbal and M. Pal, Org. Biomol. Chem., 2011, 9, 1004 RSC; (b) R. M. Rao, U. Reddy, C. H. Alinakhi, N. Mulakayala, M. Alvala, M. K. Arunasree, R. R. Poondra, J. Iqbal and M. Pal, Org. Biomol. Chem., 2011 10.1039/c0ob01161d , in press.
- (a) S. Michan and D. Sinclair, Biochem. J., 2007, 404, 1–13 CrossRef CAS; (b) A. A. Sauve, C. Wolberger, V. L. Schramm and J. D. Boeke, Annu. Rev. Biochem., 2006, 75, 435 CrossRef CAS; (c) L. R. Saunders and E. Verdin, Oncogene, 2007, 26, 5489 CrossRef CAS; (d) M. F. Fraga, R. Agrelo and M. Esteller, Ann. N. Y. Acad. Sci., 2007, 1100, 60 CrossRef CAS; (e) W. Zhao, J. P. Kruse, Y. Tang, S. Y. Jung, J. Qin and W. Gu, Nature, 2008, 451, 587 CrossRef CAS.
- (a) A. Bedalov, T. Gatbonton, W. P. Irvine, D. E. Gottschling and J. A. Simon, Proc. Natl. Acad. Sci. U. S. A., 2001, 98, 15113 CrossRef CAS; (b) A. J. Tervo, T. Suuronen, S. Kyrylenko, E. Kuusisto, P. H. Kiviranta, A. Salminen, J. Leppanen and A. Poso, J. Med. Chem., 2006, 49, 7239 CrossRef CAS; (c) J. Trapp, R. Meier, D. Hongwiset, M. U. Kassack, W. Sippl and M. Jung, ChemMedChem, 2007, 2, 1419 CrossRef CAS; (d) C. Gey, S. Kyrylenko, L. Hennig, L. H. Nguyen, A. Buttner, H. D. Pham and A. Giannis, Angew. Chem., Int. Ed., 2007, 46, 5219 CrossRef CAS; (e) Y. Cen, Biochim. Biophys. Acta, Proteins Proteomics, 2010, 1804, 1635 CrossRef CAS; (f) M. Gutiérrez, E. H. Andrianasolo, W. Kyo Shin, D. E. Goeger, A. Yokochi, J. Schemies, M. Jung, D. France, S. Cornell-Kennon, E. Lee and W. H. Gerwick, J. Org. Chem., 2009, 74, 5267 CrossRef CAS; (g) W. K. Oh, K. B. Cho, T. T. Hien, T. H. Kim, H. S. Kim, T. T. Dao, H. K. Han, S. M. Kwon, S. G. Ahn, J. H. Yoon, T. H. Kim, Y. G. Kim and K. W. Kang, Mol. Pharmacol., 2010, 78, 855 CrossRef CAS; (h) D. Rotili, V. Carafa, D. Tarantino, G. Botta, A. Nebbioso, L. Altucci and A. Mai, Bioorg. Med. Chem., 2011 DOI:10.1016/j.bmc.2011.01.025 , in press.
- C. M. Grozinger, E. D. Chao, H. E. Blackwell, D. Moazed and S. L Schreiber, J. Biol. Chem., 2001, 276, 38837 CrossRef CAS.
- J. Min, J. Landry, R. Sternglanz and R.-M. Xu, Cell, 2001, 105, 269 CrossRef CAS.
- J. L. Avalos, K. M. Bever and C. Wolberger, Mol. Cell, 2005, 17, 855 CrossRef CAS.
- M. S. Finnin, J. R. Donigian and N. P. Pavletich, Nat. Struct. Biol., 2001, 8, 621 CrossRef CAS.
- R. Marmorstein, Biochem. Soc. Trans., 2004, 32, 904 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c1md00029b |
| This journal is © The Royal Society of Chemistry 2011 |


