Discovery and characterization of novel potent PARP-1 inhibitors endowed with neuroprotective properties: From TIQ-A to HYDAMTIQ†
Roberto
Pellicciari
*a,
Emidio
Camaioni
a,
Adam M.
Gilbert
b,
Antonio
Macchiarulo
a,
Jack A.
Bikker
b,
Falgun
Shah‡
b,
Joel
Bard
e,
Gabriele
Costantino
d,
Antimo
Gioiello
a,
Graeme M.
Robertson
a,
Paola
Sabbatini
a,
Francesco
Venturoni
a,
Paride
Liscio
a,
Andrea
Carotti
a,
Daniele
Bellocchi
a,
Andrea
Cozzi
c,
Andrew
Wood§
b,
Cathleen
Gonzales
b,
Margaret M.
Zaleska
b,
John W.
Ellingboe¶
b and
Flavio
Moroni
c
aDipartimento di Chimica e Tecnologia del Farmaco, Università di Perugia, Via del Liceo 1, 06123, Perugia, Italy. E-mail: rp@unipg.it; Fax: +39 075 5855124; Tel: +39 075 5855120
bPfizer Global Research and Development, Neuroscience Research Unit, 558 Eastern Point Road, Groton, CT 06340, USA
cDipartimento di Farmacologia Preclinica e Clinica, Università di Firenze, V.le Pieraccini 6, 53100, Firenze, Italy
dDipartimento Farmaceutico, Università di Parma, V.le G.P. Usberti 27/A, 43124, Parma, Italy
ePfizer Global Research and Development, Global Biotherapeutic Technologies, 200 Cambridgepark Drive, Cambridge, MA USA
First published on 7th April 2011
Abstract
Activation of poly(ADP-ribose) polymerase (PARP) is an important factor in controlling cell survival or death. As a consequence, therapeutic interventions with PARP-1 inhibitors are sought in different pathological conditions such as cancer, cardiovascular and inflammatory diseases, as well as brain ischemia. In the first part of this work, as a continuation of our efforts in the field, we report the design, synthesis and biological appraisal of novel potent PARP-1 inhibitors. A crystallization experiment is carried out to ascertain the mode of binding to PARP-1 of the most potent compound, namely 2-((dimethylamino)methyl)-9-hydroxythieno[2,3-c]isoquinolin-5(4H)-one (HYDAMTIQ), whilst molecular modeling studies are performed to infer the role of water molecules in ligand binding. In the second part of the work, we discuss the results of HYDAMTIQ in models of brain ischemia as well as its preliminary physicochemical and pharmacokinetic characterization. Collectively, the data obtained qualify HYDAMTIQ as a novel lead candidate for advancement to clinical settings of brain ischemia.
Introduction
Poly(ADP-ribose) polymerase-1 (PARP-1) is an abundant, chromatin bound, nuclear enzyme able to promote poly(ADP-ribosyl)ation of a significant number of nuclear proteins and to facilitate DNA repair.1,2 It is strongly activated by DNA damage and its activity plays key roles in modulating gene expression and in controlling cell survival or death pathways.3–6PARP-1 is considered an interesting target for innovative therapeutic agents and has been particularly studied in cancer chemotherapy. Indeed, PARP-1 inhibitors or deletion of the parp-1gene may facilitate the action of DNA damaging chemotherapeutic agents.7 Furthermore, cells with defects in other DNA repairing enzymes such as BRCA1 or BRCA2 are particularly dependent on PARP-1 activity8–11 and promising clinical results have been obtained using PARP-1 inhibitors in patients with BRCA mutations.12,13
Administration of PARP-1 inhibitors or deletion of the parp-1gene in mice also reduces excitotoxic and post-ischemic brain damage in stroke models14,15 possibly because an excessive activation of this enzyme in neurons may reduce NAD levels leading to severe ATP shortage, energy failure and cell necrosis. It has been proposed that the ischemia-induced glutamate release results in NMDA receptor stimulation, a massive influx of Ca2+ into the neurons, activation of nitric oxide synthase (nNOS) and a number of other enzymes with production of free radical species able to cause DNA strand breaks and pathological activation of PARP.16–18
PARP-1 seems therefore implicated in multiple aspects of DNA damage response and PARP-1 inhibitors are considered interesting agents for different therapeutic applications.19
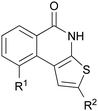
In this framework, we previously reported the design and synthesis of a thieno[2,3-c]isoquinolin-5(4H)-one derivative (TIQ-A, 1, Chart 1) as a potential anti-ischemic agent.20
![Structures of thieno[2,3-c]isoquinolin-5(4H)-one derivatives.](/image/article/2011/MD/c1md00021g/c1md00021g-c1.gif) | ||
| Chart 1 Structures of thieno[2,3-c]isoquinolin-5(4H)-one derivatives. | ||
This compound was endowed with good potency in inhibiting the catalytic activity of PARP-1. In vitro (IC50 = 0.9 μM) and in vivo studies demonstrated that it reduced PARP activity in the brain because it was able to penetrate the blood brain barrier. In rats with transient middle cerebral artery occlusion (tMCAO), TIQ-A (10 mg kg−1i.p.) significantly reduced stroke volumes.20,21
Interestingly, TIQ-A (1, Chart 1) prevented inflammatory cytokine secretion and eosinophil recruitment in a murine model of allergic airway inflammation, thus suggesting that PARP-1 inhibition could be a novel therapeutic strategy for asthma treatment.22,23
More recently, TIQ-A (1) was reported to induce regression of atherosclerotic plaques in animal models of atherosclerosis.24
Despite these outstanding results, however, the poor water solubility of TIQ-A (1) (<100 μg ml−1) still represents a major drawback to advance the compound to clinical settings. Thus, on the basis of these considerations, we focused our attention on the optimization of TIQ-A (1) both in terms of pharmacokinetic and anti-ischemic properties. In this paper, the design, synthesis as well as early physicochemical and biological appraisals of DAMTIQ (2) and HYDAMTIQ (3) are reported as follow-up compounds of TIQ-A (1).
Results and discussion
The scaffold of TIQ-A (1) bears the minimal pharmacophoric feature that is mandatory for PARP-1 inhibition. This consists of an aromatic system with an amide group locked in an anti conformation. Consistent with this observation, a docking study of TIQ-A (1) into the catalytic site of human PARP-1 (pdb code: 1UK0) shows a good fit of the thieno[2,3-c]isoquinolin-5(4H)-one moiety within the binding cavity, forming hydrogen bond interactions with Gly863 as well as π–π stacking with the side chain of Tyr907 (Fig. 1).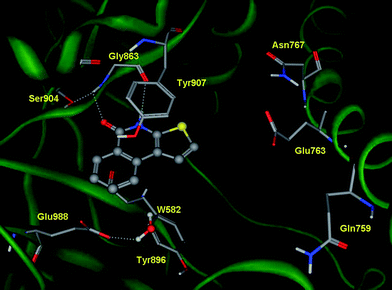 | ||
| Fig. 1 Binding mode of TIQ-A (1) into the catalytic site of human PARP-1 (pdb code: 1UK0), calculated from the docking study. | ||
Inspection of the docked pose pinpoints two specific regions of TIQ-A (1) amenable to chemical modifications: positions C2 and C9 of the thieno[2,3-c]isoquinolin-5(4H)-one moiety. While position C2 is located close to a negatively charged side chain of Glu763, C9 faces a polar region defined by the side chain of residue Glu988.
Conversely to position C2 that is so far unexplored, we have already reported that the insertion of a hydroxyl group in C9 was able to slightly enhance the potency of the parent compound. Thus, we envisaged the introduction of a dimethylaminomethyl group in C2 as suitable modification to reach Glu763 with favored electrostatic interactions, accomplishing the design of DAMTIQ (2) and HYDAMTIQ (3).
The synthetic route for the preparation of 1–3 is illustrated in Scheme 1. Thus, following an improved gram scale synthetic protocol, the palladium catalyzed coupling reaction of the commercially available 3-bromo-2-thiophencarboxylic acid (4) with the suitable arylboronic acid [phenylboronic acid (5) for 1, and 2, 2-methoxyphenylboronic acid (6) for 3] afforded the corresponding 3-aryl-2-thiophencarboxylic acid derivatives 7 and 8. Chlorination of compounds 7 and 8 with thionyl chloride in dry benzene, followed by treatment with sodium azide yielded the acylazide intermediates which, after thermolysis in boiling o-dichlorobenzene, gave the corresponding thieno[2,3-c]isoquinolin-5(4H)-one derivatives 1 and 9viaCurtius rearrangement.25 A Mannich-type reaction26 was then applied to derivatives 1 and 9 by reaction with N,N-dimethyl(methylene)ammonium chloride to give the corresponding 2-dimethylaminomethylthieno[2,3-c]isoquinolin-5(4H)-one hydrochloride derivatives 2 and 10 in high yields, respectively. Finally, reaction of 10 with boron tribromide in dichloromethane afforded the desired 9-hydroxyl derivative 3 as the hydrobromic salt. All structures were unambiguously assigned by using NMR spectroscopic techniques and the purity was assigned by RP-HPLC analysis (see ESI†).
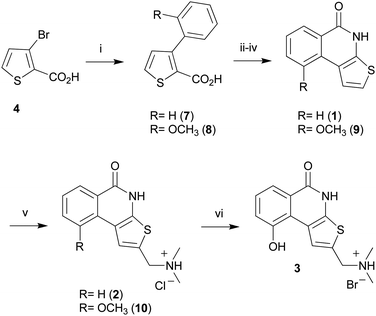 | ||
| Scheme 1 Reagents and conditions: (i) 5 (or 6), Pd(PPh3)4, EGDE, NaHCO3, reflux; (ii) SOCl2, C6H6, reflux; (iii) NaN3, THF, r.t.; (iv) C6H4Cl2, reflux; (v) dimethylformamide, N,N-dimethyl(methylene)ammonium chloride, CH3CN; (vi) BBr3, CH2Cl2, reflux. | ||
The compounds were evaluated as PARP-1 inhibitors in a fluorescence based multiwell assay,27 using human recombinant PARP-1 enzyme. Furthermore, in order to assess their selectivity against other members of poly(ADP-ribose)transferase superfamily, compounds 1–3 were also tested in PARP-2 and tankyrase-1 assays.28 The results are reported in Table 1.
As a general observation, compounds 1–3 show no selectivity for PARP-1 over PARP-2, whilst being inactive against tankyrase-1. Remarkably, the introduction of the dimethylaminomethyl group at C2 results in an overall increase of the potency of DAMTIQ (2), corroborating the design hypothesis. The activity is further boosted to the nanomolar range of potency in HYDAMTIQ (3), where the insertion of the hydroxyl group at C9 position is combined with the presence of the dimethylaminomethyl group at C2.
Additional pharmacological profiling of 3 revealed that it does not block hERG activity (14% inhibition at 10 μM), and does not show substantial activity against a diverse NovaScreen® panel of 62 promiscuous targets (all Ki's > 1 μM).
In order to explain the high potency of DAMTIQ (2) and HYDAMTIQ (3), the binding modes of these molecules were investigated using crystallization assays and docking studies. In particular, while HYDAMTIQ (3) was co-crystallized with human PARP-1, compound 2 was docked into the catalytic cleft of the resulting crystal upon removal of 3 and water molecules.
The thieno[2,3-c]isoquinolin-5(4H)-one scaffold of DAMTIQ (2) and HYDAMTIQ (3) shows a conserved pattern of interactions compared to the above reported pose of TIQ-A (1) (Fig. 2, 3). In addition, an electrostatic interaction between the positively charged nitrogen atom of the dimethylaminomethyl side chain and the carboxylate group of Glu763 can be observed, providing an explanation for the increased potency of these compounds.
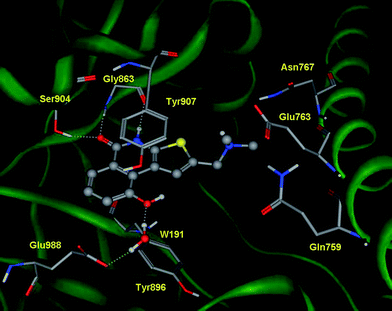 | ||
| Fig. 2 Crystal structure of human PARP-1 in complex with HYDAMTIQ (3). Key interactions involving residues as well as water molecules are shown with dashed lines. | ||
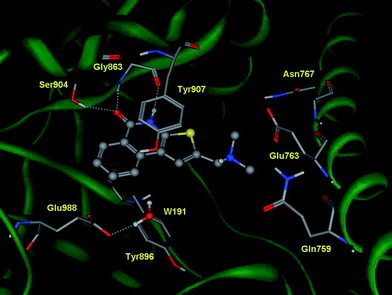 | ||
| Fig. 3 Docking experiments of DAMTIQ (2) into the catalytic site of human PARP-1. | ||
Furthermore, inspection of the crystal structure of PARP-1 in complex with HYDAMTIQ (3) reveals additional aspects of ligand binding that concern the water molecules and the ligand induced fit of the enzyme.
Within the binding cleft, nine water molecules are located in close proximity to HYDAMTIQ (3). Of these solvent molecules, water molecule W32 makes hydrogen bonds that link the hydroxyl group in C9 of HYDAMTIQ (3) with the side chain of Glu988. In accordance with previous docking studies, this latter water molecule should be considered as part of the hydration shell of polar inhibitors and, perhaps, involved in the catalytic mechanism of NAD hydrolysis by PARP-1.29
To further explore the role of water molecules in the binding of HYDAMTIQ (3) to PARP-1, a Watermap analysis was performed based on the crystal structure of the complex (Fig. 4).
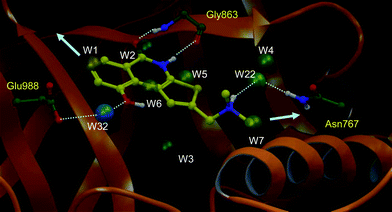 | ||
| Fig. 4 The Watermap profile is shown. Only thermodynamically interesting hydration sites (out of 34 water sites predicted by Watermap) are shown in spheres. Water numbering is based on the relative thermodynamic instability as predicted by Watermap (i.e. decreasing value of predicted ΔG). Water sites are labelled based on decreasing value of predicted ΔG and coloured from negative ΔH (blue) to positive ΔH (yellow). Hydrogen bonding interactions of HYDAMTIQ (3) with Gly863 and side chains of Glu988 and Asn767 are shown. Arrows point towards possible egress of unfavourable waters to the bulk of the solvent. | ||
Watermap is a novel tool which computes enthalpic (ΔH) and entropic (−TΔS) contributions of explicit water hydration sites and, thus, gives an estimate of the free energy (ΔG) of water molecules in the protein binding site.30,31
The thermodynamic profile gained from the Watermap analysis can be useful in understanding ligand binding affinity, structure–activity relationships, and in prospective compound design to improve potency and/or selectivity.32 Typically, the displacement of high energy water molecules into bulk solution by a ligand complementary to the protein active site is a major source of binding free energy gain. Indeed, water molecules are often entropically unfavourable due to restrained movements in the protein binding site. They may also have unfavourable enthalpy of binding due to their lack of ability to make hydrogen bonds with protein residues or nearby water molecules.30
The thermodynamic values of selected water molecules, as calculated by Watermap, are provided in Table 2. From a design perspective, water molecules showing a positive ΔG value may be displaced by ligand atoms with an expected gain in overall binding. Conversely, water molecules with favoured enthalpy of binding (ΔH negative) may be viewed as structural water molecules that provide additional anchoring points for ligand binding.
| Water | Occupancy | ΔG (kcal mol−1) | ΔH (kcal mol−1) | TΔS (kcal mol−1) | Closest atom (<1.5 Å) | Distance (Å) |
|---|---|---|---|---|---|---|
| W1 | 0.6 | 6.1 | 4.2 | 1.9 | C7 | 0.3 |
| W2 | 0.9 | 3.5 | 0.0 | 3.5 | C5 | 0.7 |
| W3 | 0.9 | 3.4 | 0.1 | 3.3 | HOH29 | 0.5 |
| W4 | 0.8 | 3.2 | 0.3 | 2.9 | HOH31 | 0.3 |
| W5 | 0.6 | 2.5 | 0.9 | 1.6 | C9b | 0.4 |
| W6 | 0.6 | 2.4 | 0.4 | 1.9 | S3 | 1.0 |
| W7 | 0.5 | 2.2 | 0.6 | 1.6 | C(NMe) | 0.4 |
| W8 | 0.6 | 2.1 | −0.3 | 2.4 | n/a | |
| W9 | 0.6 | 2.0 | 0.2 | 1.8 | O (OH) | 1.1 |
| W10 | 0.6 | 1.7 | −0.5 | 2.2 | C5a | 1.4 |
| W22 | 0.6 | 0.5 | −1.5 | 2.0 | N(NMe2) | 2.2 |
| W32 | 0.9 | −2.7 | −6.2 | −2.7 | HOH191 | 0.7 |
As a result, Watermap predicts a highly unstable water cluster (W1, W2, ΔG > 3.5 kcal mol−1) in the PARP-1 binding site. The thienoisoquinolinone core of HYDAMTIQ (3) displaces two of the most unfavourable water molecules (W1: ΔG = 6.1 kcal mol−1, W2: ΔG = 3.5 kcal mol−1). The least favourable water (W1) is displaced by the hydrophobic ring of HYDAMTIQ (3) whereas the amide carbonyl of HYDAMTIQ (3) replaces the H-bond interaction of W2 with the backbone amine of Gly863. In addition, Watermap predicts two other unstable water sites (W3: ΔG = 3.4 kcal mol−1 and, W4: ΔG = 3.2 kcal mol−1), which are present as structural water molecules in the crystal structure of PARP-1. These sites, however, are out of the plane of HYDAMTIQ (3) and cannot be approached by the current chemotypes. Water sites W5, W6 and W7 (ΔG = 2.47, 2.35 and 2.17 kcal mol−1, respectively) are in proximity to the ligand and, as a consequence, displaced by the ligand for free energy gain. In addition, Watermap analysis also predicts two water molecules that bridge the ligand to the enzyme. Specifically, the hydroxyl moiety of HYDAMTIQ (3) forms a water-mediated hydrogen bond with Glu988 via a highly enthalpic water molecule (W32, ΔH = −6.2, −TΔS = 3.5, ΔG = 2.0 kcal mol−1) that is also present in the crystal structure. In addition, the tertiary amine of HYDAMTIQ (3) may participate in a water-mediated hydrogen bonding with Asn767 (W22: ΔH = −1.5; −TΔS = 2.0; ΔG = 0.5 kcal mol−1) as shown in Fig. 4. Notably, W22 is not present in the crystal structure.
Collectively, the above water-mediated hydrogen bond network and the displacement of unfavourable water molecules by the thienoisoquinolinone core contribute to the overall boost in the potency of 3 over 1 and 2.
Comparing the binding cleft of the available crystal complexes of human PARP-1 with that of this study, some differences can only be observed on the side chain conformations of three residues: Gln759, Glu763 and Lys903 (Fig. 5). Accordingly, there are marginal ligand induced fit effects in the catalytic cleft of PARP-1 upon binding of HYDAMTIQ (3).
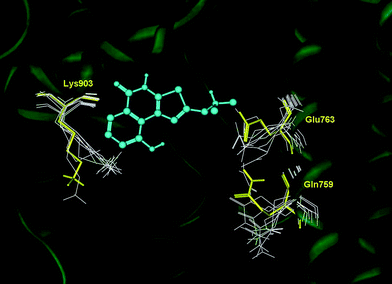 | ||
| Fig. 5 Superposition of the available complexes of PARP-1 (pdb codes: 1uk0, 1uk1, 2rcw, 2rd6, 3gjw, 3gn7 and 3l3m) with that resulting from co-crystallization experiments of HYDAMTIQ (3). Conformational changes of Gln759, Glu763 and Lys903 are highlighted in yellow (HYDAMTIQ, 3) and white (other ligands). | ||
HYDAMTIQ (3) was assessed for cellular activity in two in vitro functional assays. In the first, ATP level was measured in HEK293 cells exposed to the alkylating agent N-methyl-N′-nitro-N-nitrosoguanidine (MNNG) which causes DNA damage, PARP-1 activation and cellular ATP depletion. As reported in Fig. 6, 75 μM MNNG caused a significant ATP depletion. HYDAMTIQ (3) pre-treatment, completely prevented this depletion with an EC50 of 14 nM. Similar results, were obtained with a different PARP-1 inhibitor 6(5H)-phenanthridinone (PND) (EC50 = 945 nM, Fig. 6).
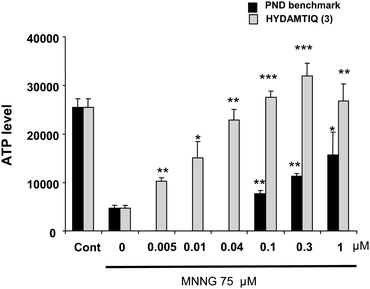 | ||
| Fig. 6 HYDAMTIQ (3) prevents the reduction of ATP levels in HEK293 cells exposed to 75 μM with an EC50 of 14 nM. Comparison with PND one of reference PARP-1 inhibitors (EC50 945 nM). Columns are ATP relative values, bars are SEM of at least 3 experiments in triplicate *p < 0.05, **p < 0.005, ***p < 0.0005 vs. MNNG alone (0). | ||
In a second model, primary cultures of mixed cortical cells exposed to 60 min oxygen glucose deprivation (OGD), HYDAMTIQ (3) was evaluated for its ability to attenuate neuronal cell death33 at 24 h post OGD.
Fig. 7 shows that HYDAMTIQ (3) significantly reduced the OGD-induced LDH release with an EC50 of 300 nM. Another PARP-1 inhibitor, namely PND, had an EC50 of 2373 nM in this assay.
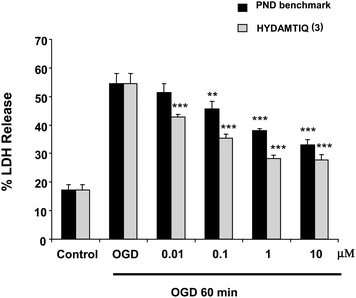 | ||
| Fig. 7 HYDAMTIQ (3) prevents neuronal cell death induced by 60 min of oxygen-glucose deprivation (OGD) in primary cultures of mixed cortical cells (EC50 300 nM). Columns are percentage LDH released in the incubation medium (100% LDH release was obtained by incubating sister cultures with 10 mM glutamate for 24 h). Comparison to one reference PARP-1 inhibitor PND (EC50 237 3nM) is shown. **p < 0.005, ***p < 0.0005 vs. OGD alone. | ||
On the basis of the excellent potency of HYDAMTIQ (3) at inhibiting PARP-1 and in protecting primary cultures of mixed cortical cells from the ischemic damage, the compound was next submitted to pharmacological evaluation in rats in vivo. Two different MCAO experiments were performed.|| In the first, the MCA was occluded for 2 h and the animals were evaluated 48 h later (tMCAO). In the second, the rats had the MCA occluded for 24 h (pMCAO).
When administered i.p. 3 times, starting 4 h after the occlusion (for 2 h) of the tMCAO, HYDAMTIQ (3) reduced brain infarct volumes by 17.5% at 0.1 mg kg−1, by 55% at 1 mg kg−1, and 62% at 10 mg kg−1, respectively (Fig. 8).
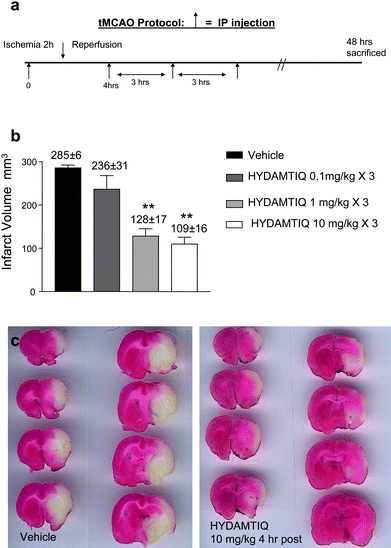 | ||
| Fig. 8 HYDAMTIQ (3) reduces stroke volumes in rats with tMCAO a) Experimental protocol. HYDAMTIQ was administered 3 times starting 4 h after carotid occlusion (see the arrows) b) Columns are infarct volumes (mm3). Vertical bars are SEM; at least 8 animals per group were evaluated. **P < 0.01 vs. controls (ANOVA followed by Mann-Whitney test). c) Morphological appearance of TTC stained brain coronal sections of a vehicle (left panel) and a HYDAMTIQ treated (right panel) rat brain obtained 48 h after artery occlusion. Note the significant reduction of the infarct (white) areas. | ||
Fig. 9 shows that it was possible to reduce brain infarct volumes also in the pMCAO (a more severe stroke model). In this case, however, HYDAMTIQ (3) (1 mg kg−1i.v.) was administered at 30 min, 3.5 and 6 h post pMCAO.
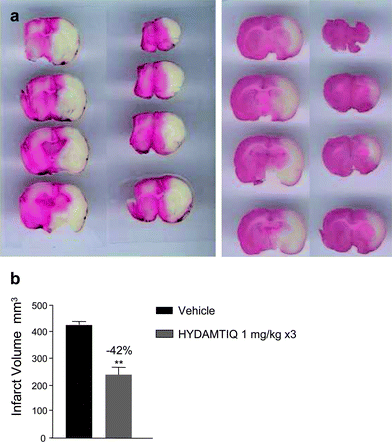 | ||
| Fig. 9 HYDAMTIQ (3) reduces stroke volumes in rats with pMCAO a) Morphological appearance of TTC stained brain coronal sections of a vehicle (left panel) and a HYDAMTIQ treated (right panel) rat brain obtained 24 h after artery occlusion. Note the significant reduction of the infarct (white) areas. HYDAMTIQ (3) was administered as 1 mg kg−1i.v. bolus, 30 min after artery occlusion, and at 3.5 and 8 h thereafter at the same dose but as i.p. injections. b) Columns are infarct volumes (mm3) and vertical bars are SEM; at least 8 animals per group were evaluated. **P < 0.01. | ||
Collectively, these findings qualified HYDAMTIQ (3) as a novel potent PARP-1 inhibitor with good in vivo anti-ischemic properties. As a consequence, compound 3 was next submitted to a preliminary pharmacokinetic characterization as well as an assessment of the compound's physicochemical properties. In vitro physicochemical profiling (Table 3) shows 3 to have good aqueous solubility (70 μg ml−1), good permeability in a BBB-PAMPA assay (Pe = 4.6 × 10−6 cm s−1), and minimal CYP inhibition.
| a Kinetic aqueous solubility at pH 7.4, 23 °C, single determination. b Parallel Artificial Membrane Permeability Assay using a suitable membrane to mimic BBB barrier function, single determination. c Average of 2 experiments, St.Dev. ±10%. d Rat liver microsome half-life at 37 °C, single determination. e Human liver microsome half-life at 37 °C, single determination. f Rat hepatocyte clearance at 37 °C, single determination. g Human hepatocyte clearance at 37 °C, single determination. | |
|---|---|
| Aqueous Solubility (μg ml−1)a | 70.0 |
| BBB-PAMPA Pe (× 10−6 cm s−1)b | 4.6 |
| CYP3A4 IC50 (μM)c | <200 |
| CYP2D6 IC50 (μM)c | 170 |
| CYP2C9 IC50 (μM)c | <200 |
| RLM t1/2 (min)d | 3 |
| HLM t1/2 (min)e | 30 |
| RHEP CL (μl min−1/106cells)f | 26.4 |
| HHEP CL (μl min−1/106cells)g | 22.2 |
Rat liver microsomal stability for 3 indicates a short half-life (3 min), though rat hepatocyte stability shows moderate clearance (26.4 μL min−1/106cells). The human microsomal stability is improved compared to rat (t1/2: 30 min) and the hepatocyte stability is moderate (22.2 μL min−1/106cells).
Rat pharmacokinetic and brain exposure data show that 3 is cleared quickly on intravenous (i.v.) administration, albeit displaying good brain exposure (Table 4).
The i.v. clearance is high (69 ml min−1 kg−1) and the half-life is short (t1/2 = 0.8 h). This may explain why 3 shows efficacy when dosed at multiple time points in the pMCAO experiment. The volume of distribution is moderate indicating good partitioning of 3 into tissue. The brain to plasma portioning ratio for 3 is 0.26 showing brain exposure. The measured Cmax for 3 in brain tissue is 678 nM. Taking into account percentage of fraction unbound in the brain (13%), the Cmax of free 3 in brain is 87 nM which is 3-fold above it's PARP-1 IC50 (29 nM).
Conclusions
Herein we have reported the design, synthesis and biological appraisals of two follow-up compounds of TIQ-A (1): DAMTIQ (2) and HYDAMTIQ (3). The results, in particular, have shown that HYDAMTIQ (3) is a novel PARP-1 inhibitor exhibiting potency in the low nanomolar range, with no selectivity over PARP-2. Interestingly, a crystallization study of the complex between HYDAMTIQ (3) and PARP-1 has uncovered the presence of an additional hydrogen bond to the enzyme that is mediated by a water molecule, accounting for the enhanced potency of the compound with respect to DAMTIQ (2).Initial pharmacological appraisals of HYDAMTIQ (3) as a neuroprotective agent in models of brain ischemia show significant reduction of the infarct volume in both tMCAO and pMCAO. These findings, combined with the good pharmacokinetic and physicochemical properties of HYDAMTIQ (3), support the selection of this compound as a novel lead candidate to advance in clinical settings of brain ischemia.
Furthermore, in view of the therapeutic versatility of PARP-1 inhibition, our results prompt additional appraisals of compound 3 in other areas of drug discovery where the inhibition of the enzyme is sought as promising therapeutic approach.
References
- R. Alvarez-Gonzalez, Science's STKE, 2007, pe68, DOI:10.1126/stke.4152007pe68.
- P. A. Jeggo, Curr. Biol., 1998, 8, R49–51 CrossRef CAS.
- V. Schreiber, F. Dantzer, J. C. Ame and G. de Murcia, Nat. Rev. Mol. Cell Biol., 2006, 7, 517–528 CrossRef CAS.
- H. Juarez-Salinas, J. L. Sims and M. K. Jacobson, Nature, 1979, 282, 740–741 CrossRef CAS.
- R. C. Benjamin and D. M. Gill, J. Biol. Chem., 1980, 255, 10493–10501 CAS.
- B. W. Durkacz, O. Omidiji, D. A. Gray and S. Shall, Nature, 1980, 283, 593–596 CrossRef CAS.
- M. Rouleau, A. Patel, M. J. Hendzel, S. H. Kaufmann and G. G. Poirier, Nat. Rev. Cancer, 2010, 10, 293–301 CrossRef CAS.
- S. Shall and G. de Murcia, Mutat. Res., 2000, 460, 1–15 CAS.
- H. E. Bryant, N. Schultz, H. D. Thomas, K. M. Parker, D. Flower, E. Lopez, S. Kyle, M. Meuth, N. J. Curtin and T. Helleday, Nature, 2005, 434, 913–917 CrossRef CAS.
- H. Farmer, N. McCabe, C. J. Lord, A. N. Tutt, D. A. Johnson, T. B. Richardson, M. Santarosa, K. J. Dillon, I. Hickson, C. Knights, N. M. Martin, S. P. Jackson, G. C. Smith and A. Ashworth, Nature, 2005, 434, 917–921 CrossRef CAS.
- P. C. Fong, D. S. Boss, T. A. Yap, A. Tutt, P. Wu, M. Mergui-Roelvink, P. Mortimer, H. Swaisland, A. Lau, M. J. O'Connor, A. Ashworth, J. Carmichael, S. B. Kaye, J. H. Schellens and J. S. de Bono, N. Engl. J. Med., 2009, 361, 123–134 CrossRef CAS.
- A. Tutt, M. Robson, J. E. Garber, S. M. Domchek, M. W. Audeh, J. N. Weitzel, M. Friedlander, B. Arun, N. Loman, R. K. Schmutzler, A. Wardley, G. Mitchell, H. Earl, M. Wickens and J. Carmichael, Lancet, 2010, 376, 235–244 CrossRef CAS.
- M. W. Audeh, J. Carmichael, R. T. Penson, M. Friedlander, B. Powell, K. M. Bell-McGuinn, C. Scott, J. N. Weitzel, A. Oaknin, N. Loman, K. Lu, R. K. Schmutzler, U. Matulonis, M. Wickens and A. Tutt, Lancet, 2010, 376, 245–251 CrossRef CAS.
- F. Moroni, Curr. Opin. Pharmacol., 2008, 8, 96–103 CrossRef CAS.
- M. J. Eliasson, K. Sampei, A. S. Mandir, P. D. Hurn, R. J. Traystman, J. Bao, A. Pieper, Z. Q. Wang, T. M. Dawson, S. H. Snyder and V. L. Dawson, Nat. Med., 1997, 3, 1089–1095 CrossRef CAS.
- H. C. Ha and S. H. Snyder, Proc. Natl. Acad. Sci. U. S. A., 1999, 96, 13978–13982 CrossRef CAS.
- A. Chiarugi, Pharmacol. Res., 2005, 52, 15–24 CrossRef CAS.
- F. Moroni and A. Chiarugi, FEBS J., 2009, 276, 36–45 CrossRef CAS.
- D. V. Ferraris, J. Med. Chem., 2010, 53, 4561–4584 CrossRef CAS.
- R. Pellicciari, E. Camaioni, G. Costantino, M. Marinozzi, A. Macchiarulo, F. Moroni and B. Natalini, Farmaco, 2003, 58, 851–858 CrossRef CAS.
- A. Chiarugi, E. Meli, M. Calvani, R. Picca, R. Baronti, E. Camaioni, G. Costantino, M. Marinozzi, D. E. Pellegrini-Giampietro, R. Pellicciari and F. Moroni, J. Pharmacol. Exp. Ther., 2003, 305, 943–949 CrossRef CAS.
- M. Oumouna, R. Datta, K. Oumouna-Benachour, Y. Suzuki, C. Hans, K. Matthews, K. Fallon and H. Boulares, J. Immunol., 2006, 177, 6489–6496.
- A. S. Naura, C. P. Hans, M. Zerfaoui, D. You, S. A. Cormier, M. Oumouna and A. H. Boulares, Clin. Exp. Allergy, 2008, 38, 839–846 CrossRef CAS.
- C. P. Hans, M. Zerfaoui, A. S. Naura, D. Troxclair, J. P. Strong, K. Matrougui and A. H. Boulares, J. Pharmacol. Exp. Ther., 2009, 329, 150–158 CrossRef CAS.
- K. Ito, K. Yakushijin, S. Yoshina, A. Tanaka and K. Yamamoto, J. Heterocycl. Chem., 1978, 15, 301–305 CrossRef CAS.
- G. Kinast and L. F. Tietze, Angew. Chem., Int. Ed. Engl., 1976, 15, 239–240 CrossRef.
- K. S. Putt and P. J. Hergenrother, Anal. Biochem., 2004, 326, 78–86 CrossRef CAS.
- F. Moroni, L. Formentini, E. Gerace, E. Camaioni, D. E. Pellegrini-Giampietro, A. Chiarugi and R. Pellicciari, Br. J. Pharmacol., 2009, 157, 854–862 CrossRef CAS.
- D. Bellocchi, A. Macchiarulo, G. Costantino and R. Pellicciari, Bioorg. Med. Chem., 2005, 13, 1151–1157 CrossRef CAS.
- R. Abel, T. Young, R. Farid, B. J. Berne and R. A. Friesner, J. Am. Chem. Soc., 2008, 130, 2817–2831 CrossRef CAS.
- T. Beuming, R. Farid and W. Sherman, Protein Sci., 2009, 18, 1609–1619 CrossRef CAS.
- J. E. Chrencik, A. Patny, I. K. Leung, B. Korniski, T. L. Emmons, T. Hall, R. A. Weinberg, J. A. Gormley, J. M. Williams, J. E. Day, J. L. Hirsch, J. R. Kiefer, J. W. Leone, H. D. Fischer, C. D. Sommers, H. C. Huang, E. J. Jacobsen, R. E. Tenbrink, A. G. Tomasselli and T. E. Benson, J. Mol. Biol., 2010, 400, 413–433 CrossRef CAS.
- D. E. Pellegrini-Giampietro, F. Peruginelli, E. Meli, A. Cozzi, S. Albani-Torregrossa, R. Pellicciari and F. Moroni, Neuropharmacology, 1999, 38, 1607–1619 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental methods concerning chemistry (pp. S3–S7), biology and pharmacology (p. S7), crystallography (p. S11) as well as molecular modelling (p. S11) are detailed in the supplementary material. Figures on pp. S15–S20 contain the 1H-NMR and 13C-NMR spectra of DAMTIQ (2), HYDAMTIQ (3), and 10. Figures on pp. S21–S22 contain HPLC chromatograms of DAMTIQ (2) and HYDAMTIQ (3). See DOI: 10.1039/c1md00021g |
| ‡ Current address: Department of Medicinal Chemistry, Natural Product Center, University of Mississippi, Oxford, MS 38677, USA |
| § Current address: Ono Pharmaceuticals USA, Inc., 2000 Lenox Drive, Lawrenceville, NJ 08648, USA |
| ¶ Current address: GVK Biosciences Private Limited, Plot No. 28 A, IDA Nacharam Hyderabad – 500076, India |
| || All the experiments conducted in Italy were carried out according to the Italian guidelines for animal care (DL 116/92) in application of the European Communities Council Directive (86/609/EEC) and were formally approved by the Animal Care Committee of the Department of Pharmacology of the University of Florence. The experiments conducted at Pfizer (formerly Wyeth) in the United States were conducted according to protocols approved by the Institutional Animal Care and Use Committee. |
| This journal is © The Royal Society of Chemistry 2011 |

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
: