Novel, unifying mechanism for aromatic primary-amines (therapeutics, carcinogens and toxins): electron transfer, reactive oxygen species, oxidative stress and metabolites
Peter
Kovacic
*a and
Ratnasamy
Somanathan
b
aDepartment of Chemistry and Biochemistry, San Diego State University, San Diego, CA 92182, USA
bCentro de Graduados e Investigación del Instituto Tecnológico de Tijuana, Apdo postal 1166, Tijuana, B.C., Mexico
First published on 17th December 2010
Abstract
Aromatic primary-amines (APAs) display physiological activity in various areas, including therapeutics, carcinogens and toxicants. More familiar examples include aniline and derivatives, naphthylamines, dapsone, sulfa drugs and procaineamide. Diverse mechanisms have been proposed for these agents. However, there has not been recognition for a unifying theme entailing electron transfer (ET), reactive oxygen species (ROS) and oxidative stress (OS). Prior reviews demonstrate that the ET-ROS-OS theme can be applied to therapeutics, carcinogens and toxicants. The preponderance of bioactive substances or their metabolites incorporate ET functionalities, which, we believe, play an important role in physiological responses. Among these, the focus in the present review is on APAs which generate ET hydroxylamine and nitroso metabolites which can generate ROS viaredox cycling. Evidence suggests involvement of the metabolites in the bioactivity. Further support is provided by AO protection of toxicity which supports involvement of ROS. Apparently, a multifaceted approach best pertains.
Introduction
Aromatic primary-amines (APAs) display physiological activity in various areas, including therapeutics, carcinogens and toxicants. More familiar examples include aniline and derivatives, naphthylamines, dapsone, sulfa drugs and procaineamide. Diverse mechanisms have been proposed for these agents. However, there has not been recognition for a unifying theme entailing electron transfer (ET), reactive oxygen species (ROS) and oxidative stress OS). Prior reviews demonstrate that the ET-ROS-OS theme can be applied to therapeutics,1–3 carcinogens4 and toxicants.5–18The preponderance of bioactive substances or their metabolites incorporate ET functionalities, which, we believe, play an important role in physiological responses. These main groups include quinones (or phenolic precursors), metal complexes (or complexors), aromatic nitro compounds (or reduced hydroxylamine and nitroso derivatives), and conjugated imines (or iminium species). In vivoredox cycling with oxygen can occur giving rise to OS through generation of ROS, such as hydrogen peroxide, hydroperoxides, alkyl peroxides, and diverse radicals (hydroxyl, alkoxyl, hydroperoxyl, and superoxide). In some cases, ET results in interference with normal electrical effects, e.g., in respiration or neurochemistry. Generally, active entities possessing ET groups display reduction potentials in the physiologically responsive range, i.e., more positive than −0.5 V. ET, ROS, and OS have been increasingly implicated in the mode of action of drugs and toxins (toxicants), e.g. anti-infective agents,1 anticancer drugs,2,3 carcinogens,4 reproductive toxins,5 nephrotoxins,6 hepatotoxins,7 cardiovascular toxins,8 nerve toxins,9 mitochondrial toxins,10 abused drugs,11 immunotoxins,12 pulmonary toxins,13 dermal toxins,14 ototoxins,15 eye toxins,16 thyroid toxins,17 and various other categories, including human illnesses.18a
There is a plethora of experimental evidence supporting the theoretical framework, including generation of the common ROS, lipid peroxidation, degradation products of oxidation, depletion of AOs, effect of exogenous AOs19DNA oxidation and cleavage products, as well as electrochemical data. This comprehensive, unifying mechanism is in keeping with the frequent observations that many ET substances display a variety of activities, e.g., multiple drug properties, as well as toxic effects.
Diverse mechanisms have been proposed for these agents. However, there has not been recognition for a unifying theme involving ET-ROS-OS. The unifying relationships lend credence to the proposed involvement of ET-ROS-OS in the physiological effects of the APAs class addressed in this review, and comprise an extension of the prior mechanistic framework. However, it should be emphasized that physiological activity is often complex and multifaceted, with various modes of action involved. A number of original references may be found in the reviews and articles cited; in many cases, references are representative.
Metabolism
Microsomal oxidases are capable of performing oxidation of APAs.20 The sequence of metabolic oxidation is shown in Scheme 1, involving the hydroxylamine and then the nitroso form. The two metabolites can operate in a redox cycle. The nitroso metabolite displays a favorable reduction potential. The ArNHO˙ radical appears to be involved. | ||
| Scheme 1 Oxidative metabolism of APAs. | ||
Anilines
It is well established that aniline metabolism entails oxidation to the hydroxylamine and nitroso derivatives.18b These can serve as a redox couple for generation of ROS. The nitroso form possesses favorable reduction potential for ET (Scheme 2). Damage to red blood cells by aniline appears to involve the hydroxylamine metabolite which undergoes oxidation to the nitroso derivative.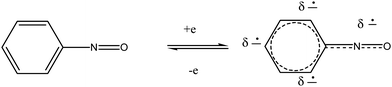 | ||
| Scheme 2 ET with nitrosobenzene. | ||
Alternatively oxidation can lead to ring hydroxylation which is discussed in various reports.18b,20–23 Further oxidation can generate iminoquinones or ET quinones that can generate ROS. Excess exposure to aniline produces hemolytic anemia and damage to the spleen, accompanied by splenic fibrosis with increased lipid peroxidation and oxidative protein damage.18b Damage to red blood cells by aniline appears to involve the hydroxylamine metabolite which is oxidized by oxyhemoglobin to give nitrosobenzene.
Carcinogens (APAs)
Aromatic primary-amines (APAs) derived from benzene, biphenyl, naphthalene and heterocycles are members of this class. A 2001 review proposes a general mode of action based on ET-ROS-OS, entailing the ArNHOH-ArNO couple.4 Twenty carcinogenic members of this category were analyzed. Of these, aniline, benzidine (Fig. 1) and others damaged DNA. N-Hydroxy metabolites of aminofluorene (Fig. 2) and aminobiphenyl created DNA adducts. DNA damage is often associated with ROS formation. Some studies suggest a role for ROS in cancer induction by this class. Increased 8-OHdG formation and corresponding CYP450 activity have been observed in urban bus drivers exposed to APAs. Catechins, an AO, proved beneficial. The usual activation of APAs to hydroxylamines is by cytochrome P-450.24 | ||
| Fig. 1 Benzidine. | ||
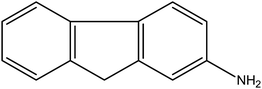 | ||
| Fig. 2 2-Aminofluorene. | ||
There are several recent articles related to bladder cancer and arylamines.25–33 A report also deals with 4-aminobiphenyl-DNA adducts in human breast cancer.34
Although 1-naphthylamine is not carcinogenic, the N-oxidized derivative, N-hydroxy-1-naphthylamine, is strongly tumorigenic.35 This review presents structure–activity relationships for many carcinogenic APAs of the main class. Heterocyclic amines are found in cooked foods. These compounds are analogous in aromatic character to benzenoid primary amines. Hence, it is reasonable to expect similar metabolism and biochemistry as reviewed as carcinogens.4Pyrolysis of food can create several types of heterocyclic amines (HCAs), many of which are suspected in carcinogenesis. They are generated primarily by reactions of amino acids in meats, and the amount produced is contingent upon the duration and temperature of cooking. The most prevalent HCAs in cooked foods are the imidazolinoxaline (A) and imidazopyridine (B) (Fig. 3). Exposure to A causes DNA damage and overexpression of oncogenes, and B induces guanine specific DNA adducts. Carcinogenic mechanisms involve ring epoxidation to form a phenol, or a multi-step process beginning with N-hydroxylation by CYP450 to create the RNHOH moiety which is esterified to RNHOCOR by N-acetyl transferase. Cleavage of the ester creates a nitrenium ion possessing a resonance hybrid with carbocation character. Electrophilic attack by N or C can then occur at nucleophilic sites on DNA to form adducts.
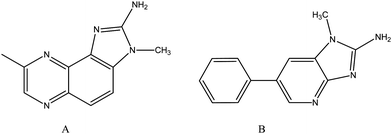 | ||
| Fig. 3 Cooked food carcinogens. | ||
Recently, other pathways have been offered for the metabolism and carcinogenicity, several of which include a role by ROS. The RNHOH metabolite of A, for instance has been implicated in the formation of ROS. It is hypothesized that RNHOH is converted to the RNHO˙ entity which can undergo redox cycling with O2 to form superoxide. Cu-OOH complex may be the principal mutagen. 8-OHdG, a common oxidative product, was detected. Catalase and bathcupronine (a Cu chelator) inhibited the process.
Endogenous and exogenous AOs can be helpful, e.g., GSH, caffeine, vitamin E, and certain dietary phenols. An alternative hypothesis suggests that tea constituents act as electrophile scavengers to ameliorate adduction. Genetic factors, including risk to those having certain phenotypes of CYP450 and NAT, have also been suggested.
As is often the case, several modes of action may pertain. In this case, two routes have been documented for the APAs involving the hydroxylamine metabolite. Some data for ET-ROS-OS involvement are presented above. Also, peroxidase enzymes can oxidize the hydroxylamine from 2-naphthylamine to an oxy radical.18cAromatic amine N-cation radicals formed from APAs by peroxidases were able to oxidize GSH and NADH with formation of ROS (5). The prooxidant activity of the APAs increased as the redox potential, i.e., ease of oxidation, decreased, with o-anisidine and aminofluorene being the most effective in forming ROS. The other common pathway comprises esterification of the hydroxylamine followed by cleavage to a nitrenium ion whose resonance hybrid possesses carbocation character.4 Electrophilic attack can then occur at nucleophilic sites in DNA to form adducts. Alkylation has been related to ROS generation.
Dapsone
This drug, p,p′-diaminodiphenylsulfone (Fig. 4), displays various activities: antimalarial,1 antimicrobial, anti-inflammatory, pneumonia, HIV, dermatitis and leprosy.36 | ||
| Fig. 4 Dapsone. | ||
An appreciable body of evidence suggests that dapsone might well represent an additional example of a type which functions viaoxy radical generation.1 A number of reports deal with in vivo or in vitro conversion to the N-hydroxy derivative and possibly to the nitroso form. Several investigations demonstrate involvement of oxidative phenomena including generation of superoxide and hydrogen peroxide by the hydroxyamino and nitroso forms in vitro.
Further insight concerning mode of action is obtained from nine recent reports. ROS formation in keratinocytes was increased by the hydroxylamine metabolite, accompanied by a decrease in the AO GSH.37 In a related study similar results were obtained.38 Also, cytotoxicity and adduct formation by the hydroxylamine were reduced in the presence of ascorbic acid. The hydroxylamine generates ROS within erythrocytes under hemolytic conditions.39Protein adducts were detected in skin explants exposed to the hydroxylamine.40Dapsone is metabolized by peroxidases to nitroso derivatives in non-hemopoietic cells.41 The drug is metabolized to the hydroxylamine metabolite by CYP2C9,42 or by more than one CYP450 enzyme.43
Various reports deal with toxicity. The adverse reactions associated with administration of dapsone are believed to be caused by metabolism to the hydroxylamine.43 This metabolite is thought to be the cause of adverse effects to dapsone in HIV cases.44 Involvement of ET-ROS-OS is illustrated in Scheme 3.
 | ||
| Scheme 3 ET and ROS. Interaction and pathology. | ||
It should be noted that dapsone is structurally related to sulfa drugs, both of which incorporate anilines bearing a sulfonyl substituent.
Sulfa drugs
Various modes of action have been suggested for sulfa drugs (Fig. 5) as anti-infective agents. The most discussed is inhibition of folic acid synthesis, along with ionization of the amide. Our focus is on amino oxidation to hydroxylamines and nitroso metabolites which can redox cycle. Resulting formation of ROS can have beneficial effects or adverse reactions. There is evidence for production of potentially toxic metabolites of sulfadiazine.45 Binding and toxicity were decreased when microsomes were cytochrome P-450 inhibited, and by the addition of N-acetylcysteine or glutathione. The data suggest the production of toxic intermediates by oxidative metabolism of sulfadiazine which is detoxified by conjugation with glutathione. Covalent binding of such metabolites to cell macromolecules could lead to cell death and, to secondary hypersensitivity reactions. We suggest that the metabolites are probably the hydroxylamine and nitroso products. Among the most serious side effects of sulfonamides are hypersensitivity reactions, the pathogenesis of which has been suggested to be mediated by reactive metabolites.46 Covalent binding and toxicity are observed for reactive intermediates of sulfonamides generated by murine hepatic microsomal activating system. Hydroxylamine metabolites might be likely candidates for mediating such toxicity. The hydroxylamine of sulfadiazine and sulfamethoxazole displayed dose-related toxicity. Sulfadiazine hydroxylamine produced 82% cell death, whereas sulfamthoxazole hydroxylamine produced 62% cell death; the parent sulfonamides were not toxic to cells. The toxicity of sulfamethoxazole hydroxylamine was decreased by coincubation with glutathione or N-acetylcysteine. Hydroxyamine metabolites of the sulfonamides or their nitroso derivatives, normally detoxified by conjugation to glutathione, may be the proximate toxins mediating sulfonamide hypersensitivity. Idiosyncratic toxicity associated with sulfamethoxazole is thought to be a consequence of bioactivation to the hydroxylamine metabolite and further oxidation to the ultimate reactive metabolite, nitroso-sulfamethoxazole.47 These findings indicate that nitroso-sulfamethoxazole is highly immunogenic and may be responsible for the hypersensitivity reactions associated with sulfamethoxazole. | ||
| Fig. 5 Sulfa drugs: a) sulfadiazine, b) sulfamethoxazole, c) sulfathiazole. | ||
The sulfonamides are antimicrobial agents which are commonly used in ambulatory therapy and have been widely used for the therapy of the infectious complications of immunosuppression and AIDS.48 The sulfonamides have been associated with a number of adverse effects, including utrical rashes, gastrointestinal discomfort, fixed drug eruptions and hypersensitivity reactions. The most serious reactions to the sulfonamides are hypersensitivity, which are multi-system processes characterized by fever and severe skin rashes, including erythema multiforme and toxic epidermal necrolysis as well as the frequent involvement of the liver, kidney, heart, lung and bone marrow. These reactions are very common among patients with AIDS who are treated with sulfonamides. A deficiency of GSH may thus explain the higher incidence of hypersensitivity reactions in patients with AIDS.47Sulfadiazine hydroxylamine has been postulated to be the mediator of the greatly increased rates of adverse reactions to the drug experienced by people with human immunodeficiency virus infection.49 Some of the adverse effects of arylamines are thought to be caused by the formation of the hydroxylamine metabolite that is further oxidized to the highly electrophilic nitroso metabolite, which covalently binds to cellular macromolecules, resulting in adverse reactions. Although sulfadiazine has been marketed since 1940's, very little is known about cytochrome P450 isoform involvement in hydroxylamine formation in humans. Sulfonamide hydroxylamine is cytotoxic and can be converted to an even more reactive species, the nitroso metabolite. Nitroso derivatives of sulfonamides bind to tissue proteins to form drug-protein complexes.50 The oxidation of sulfonamides to the hydroxylamine and nitroso, increases the immunogenicity and toxicity of the compounds. Antioxidants, such as glutathione and ascorbic acid, decrease the toxicity of these metabolites. Some of the research on hydroxylamine-nitroso metabolites point to formation of ROS and toxicity to body constituents.
The Introduction provides references on toxicity to ET-ROS-OS. Decreased toxicity by AOs provides evidence for ROS involvement. Also drug action, e.g., antibacterial, has been related to ROS, as in phagomimetic actions.1 It is common for drugs to operate via various mechanisms. A much-investigated one for sulfonamides involves inhibition of folic acid synthesis.51 The primary mode of action of the sulfonamides is competition with para-aminobenzoic acid for incorporation into folic acid. The sulfonamides impede this synthesis and are therefore toxic to those bacteria that synthesize their own folic acid. Mammals cannot synthesize this and related vitamins and depend on food sources for them; the sulfonamides are therefore not toxic to mammals in this regard. Sulfonamides compete in the step catalyzed by dihydropteroate synthase where condensation of para-aminobenzoic acid with hydroxymethyldi-hydropterin pyrophosphate takes place to form dihydropteroate. The 5-substituted-2,4-diaminopyrimidines, such as trimethoprim or pyrimethamine, block the production of tetrahydrofolic acid by inhibiting the enzyme dihydrofolate reductase. Thus, when they are used in combination, the sulfonamides and the 5-substituted-2,4-diaminopyrimidines block two consecutive steps in the biosynthesis of nucleic acids and proteins essential to many bacteria. In general, the more potent inhibitors of folate biosynthesis are the better bacterial growth inhibitors. Subsequently, evidence was obtained in incorporation of sulfonamides in the pteridine moiety to afford the metabolically inert 7,8-dihydropterin-sulfonamides which readily diffuse from the cell. Its poor lipid solubility would preclude efficient penetration across the essentially lipoidal bacterial membrane. The molecular form, having the much higher lipid solubility, will more readily cross the membrane. However, once within cell the drug would not be active unless it would ionize to some degree at physiological pH.52 It is now apparent that by varying the nature of the R substituent on the amide N,1 pKa and lipid solubility can be considerably varied. Thus, the heterocyclic rings found in that position on most clinically used sulfonamides have an electron-withdrawing effect. The resultant decreased electron density of the N1 atom weakens the N–H bond, thereby increasing the compound's acidity. The N-anion of the ionized amide possesses an electrostatic field. Recent reviews propose that these fields can play a role in physiological activity.53,54
Procaineamide
This APA drug (Fig. 6) is mainly used for the treatment of cardiac arrhythmia. The mode of action apparently involves cardiac action potential, and conduction. Various reports deal with induction of a lupus-like conduction by procaineamide. The N-oxidized metabolites of the drug have been implicated as inciting agents in the autoimmune condition.55 The formation of significant amounts of the reactive hydroxylamine and nitroso metabolitesin vivo may have direct implications in the diverse and widespread symptomatology associated with drug-induced lupus. Further evidence suggests that the N-oxidized metabolites may be responsible for the lupus-like condition.56 The effects of the hydroxylamine metabolite may result from further oxidation to the nitroso form. Chronic use of the drug is associated with a higher incidence of lupus and agranulocytosis.57 The reactive hydroxylamine and nitroso metabolites covalently bind to protein and are toxic to lymphocytes. There is additional conformation of drug-induced lupus by the hydroxylamine and nitroso metabolites.58 The hydroxylamine and nitroso metabolites bind covalently to microsomal protein to a much greater extent that does the parent.59 The hydroxylamine is readily converted nonenzymatically to the nitroso form which is the reactive species for covalent binding. A similar investigation suggests that redox cycling of the oxidized metabolites contributes to cytotoxicity.60In vivooxidation of the drug apparently underlies induction of autoimmunity.61Drug metabolism can be accompanied by generation of ROS, increased lipid peroxidation and increased TBARS (thiobarbituric acid reactive substances). The intensified activity of AO enzymes is apparently a response to OS. Another article supports involvement of the N-oxidized metabolites.62 The study demonstrates that CYP206 is the major human cytochrome P450 isozyme involved in formation of the drug metabolites. The para-carbonyl group is electronically similar to the sulfonyl of dapsone and sulfa drugs in stabilizing delocalized radicals attracted by nitroso. | ||
| Fig. 6 Procaineamide structure. | ||
A recent report provides evidence for the ET-ROS-OS mechanism for lupus and related autoimmune diseases.63 OS arises from the immune system and other endogenous sources. The literature contains support for OS involvement of various drugs and other exogenous substances that produce the condition. Studies reveal prevention or amelioration by antioxidants.
APAs comprise one of the classes that induce the condition.63Metabolism entails oxidation to hydroxylamine, nitroso, and nitro derivatives, all of which potentially operate as ET agents. The first two can redox cycle to generate ROS. Evidence supports participation of the ArNHO˙ radical. The hydroxylamine can alkylate DNAvia the nitrenium ion, which can lead to OS. Also, nuclear hydroxylation forms phenolic precursors of ET quinones.
Other investigations are concerned with radical species, including ROS. Formation of protein free radicals may be mediated by radical metabolites of the drug.64Procaineamide may form pro-oxidant radicals when metabolized by peroxidases.65 The catalytic effectiveness was determined. Free radicals are proposed to play a crucial role in the adverse effects of the drug.66Selenium, acting as a potent AO, exerted protective effects. Oxidative metabolism results in enhanced production of ROS by macrophages, contributing to toxicity of lymphocytes.67
Unifying themes in nature:Focus on ET
In the natural domain, once a useful tool is found it is commonly utilized repeatedly. Examples in the structural category include amides (proteins), acetal (carbohydrates), esters (lipids), the isoprene unit (terpenes and natural rubber) and the steroid skeleton (hormones). A similar situation applies to reaction processes in which the following play repeated roles: electrochemistry, ET, ROS, redox cycling, enzymes and cell signaling.Of particular relevance in this review is application to ET. Until recently, the common ET functionalities were quinones (Scheme 4), metal complexes (Scheme 5) and aromatic nitro compounds. Precursors could also be involved, such as phenols for quinones or metabolites, such as hydroxylamine-nitroso (Scheme 2) from aromatic nitro compounds. Subsequently, other ET functionalities were recognized as playing widespread roles, e.g., iminiums (or imines)68 (Scheme 6) and α-dicarbonyls69–71 (Scheme 7). Minor types also exist, namely, nitrosonium-nitroxide couple72 (Scheme 8) and fullerenes (C60) (Scheme 9).73 Note that the APA class entailing oxidation to hydroxylamine and nitroso is the reverse of the aromatic nitro compounds in which reduction occurs. In the APA category, there has not been prior recognition of a unifying theme applied to various compounds in this category, in relation to the involvement of ET-ROS-OS and application to therapeutics, carcinogenes and toxicants. The mechanistic concept is supported by extensive literature including references in the Introduction. The unifying theme can be summarized in the following manner.
 | ||
| Scheme 4 Quinone radical anion | ||
 | ||
| Scheme 5 Metal(M) complex ET | ||
 | ||
| Scheme 6 Iminium ET | ||
 | ||
| Scheme 7 α-Dicarbonyl ET | ||
 | ||
| Scheme 8 ET by nitrosonium-nitroxide couple | ||
 | ||
| Scheme 9 Fullerene ET | ||
1. The bioactive APAs include simple anilines, carcinogens, dapsone, sulfa drugs and procaineamide. Undoubtedly, others will be added in the future.
2. All members of this class undergo enzyme catalyzed oxidation to hydroxylamines and nitroso compounds.
3. The metabolites generate ROS via ET involving redox cycling.
4. Various physiological responses are brought about by the ROS, including therapeutic actions, carcinogenesis and other toxic effects. Some of the beneficial results are phagomimetic,1 emulating the immune system. The adverse responses entail attack by ROS on normal cells.
Abbreviations
| APAs | aromatic primary-amines |
| ET | electron transfer |
| ROS | reactive oxygen species |
| OS | oxidative stress |
| AO | antioxidant |
Acknowledgements
Editorial assistance by Thelma Chavez is acknowledged.References
- P. Kovacic and L. E. Becvar, Curr. Pharm. Des., 2000, 6, 143–167 CAS.
- P. Kovacic and J. A. Osuna, Curr. Pharm. Des., 2000, 6, 277–309 CrossRef CAS.
- P. Kovacic, Med. Hypotheses, 2007, 69, 510–516 CrossRef CAS.
- P. Kovacic and J. D. Jacintho, Curr. Med. Chem., 2001, 8, 773–796 CAS.
- P. Kovacic and J. D. Jacintho, Curr. Med. Chem., 2001, 8, 863–892 CAS.
- P. Kovacic, A. Sacman and M. Wu-Weis, Curr. Med. Chem., 2002, 9, 823–847 CrossRef CAS.
- G. Poli, K. H. Cheeseman, M. U. Dianzani, T. F. Slater, in Free Radicals in the Pathogenesis of Liver Injury, Pergamon, New York, 1989, pp. 1–330 Search PubMed.
- P. Kovacic and L. A. Thurn, Curr. Vasc. Pharmacol., 2005, 3, 107–117 Search PubMed.
- P. Kovacic and R. Somanathan, Curr. Med. Chem.: Cent. Nerv. Syst. Agents, 2005, 249–258 Search PubMed.
- P. Kovacic, R. S. Pozos, R. Somanathan, R. Shangari and P. J. O'Brien, Curr. Med. Chem., 2005, 5, 2601–2623 CrossRef.
- P. Kovacic and A. L. Cooksy, Med. Hypotheses, 2005, 64, 366–367.
- P. Kovacic and R. Somanathan, J. Recept. Signal Transduction, 2003, 28, 323–346 Search PubMed.
- P. Kovacic, R. Somanathan, In Reviews of Environmental Contamination and Toxicology, ed. D. M. Whitacre, Springer, New York 2009, 201, pp. 41–69 Search PubMed.
- P. Kovacic, R. Somanathan, In Reviews of Environmental Contaminations and Toxicology, ed. D. M. Whitacre, Springer, New York, 2010, 203, pp. 119–138 Search PubMed.
- P. Kovacic and R. Somanathan, Med. Hypotheses, 2008, 70, 914–923 CrossRef CAS.
- P. Kovacic and R. Somanathan, Cell Membr. Free Radic. Res., 2008, 1, 56–69 Search PubMed.
- P. Kovacic and C. Edwards, J. Recept. Signal Transduction, 2010, 30, 133–142 Search PubMed.
- (a) B. Halliwell, J. M. C. Gutteridge, in Free Radicals in Biology and Medicine, Oxford University Press, New York, 1999, pp. 1–897 Search PubMed; (b) B. Halliwell, J. M. C. Gutteridge, in Free Radicals in Biology and Medicine, Oxford University Press, New York, 1999, pp. 571–572 Search PubMed; (c) B. Halliwell, J. M. C. Gutteridge, in Free Radicals in Biology and Medicine, Oxford University Press, New York, 1999, pp. 704 Search PubMed.
- P. Kovacic, R. Somanathan, in Frontiers in Antioxidants Research, ed. H. V. Panglossi, Nova Science Publishers, Hauppauge, NY, 2006. pp. 1–38 Search PubMed.
- H. A. Barton and M. A. Marletta, Toxicol. Lett., 1994, 70, 147–153 CrossRef CAS.
- Ray, H. Parikh, E. Hickery and D. Bagachi, Mol. Cell. Biochem., 2001, 218, 27–33 CrossRef.
- Seitz, U. A. Simanowski, B. Simon and B. Kommerell, Z. Gastroenterol., 1988, 26, 188–190 Search PubMed.
- P. A. Adams, C. Adams, M. C. Berman and D. A. Baldwin, J. Inorg. Biochem., 1988, 17, 261–267.
- D. Kim and F. P. Guengerich, Annu. Rev. Pharmacol., 2005, 45, 27–49 Search PubMed.
- G. M. Lower, T. Nilsson, C. E. Nelson, H. Wolf, T. E. Gamsky and G. T. Bryan, Int. J. Epidemiol., 2007, 36, 11–18 Search PubMed.
- S. Ohnishi, M. Murata and S. Kawanishi, Jpn. J. Cancer Res., 2002, 93, 736–743 CAS.
- K. Golka, A. Wiese, G. Assennato and H. Bolt, World J. Urol., 2004, 21, 382–391 CrossRef CAS.
- K. Naiman, H. Dračinská, M. Martínková, M. Šulc, M. Dračinskŷ, L. Kejíková, P. Hodek, J. Hudećek, J. Liberda, H. H. Schmeiser, E. Frei and M. Stiborová, Chem. Res. Toxicol., 2008, 21, 1610–1621 CrossRef CAS.
- T. Weiss, T. Bruing and H. M. Bolt, Crit. Rev. Toxicol., 2007, 37, 553–566 CrossRef CAS.
- M. Sarkar, R. Stabbert, R. D. Kinser, J. Oey, K. Rustemeier, K. Von Holt, G. Schepers, R. A. Walk and H. J. Roethig, Toxicol. Appl. Pharmacol., 2006, 213, 198–206 CrossRef CAS.
- M. Al-Zoughool and G. Talaska, J. Appl. Toxicol., 2006, 26, 524–532 CrossRef CAS.
- J. J. M. Van Hemelrijck, D. S. Michaud, G. N. Connolly and Z. Kabir, Cancer Epidemiol., Biomarkers Prev., 2009, 18, 1312–1320 Search PubMed.
- L. Airoldi, F. Orsi, C. Magagnotti, R. Coda, D. Randone, G. Casetta, M. Peluso, A. Hautefeuille, C. Malaveille and P. Vineis, Carcinogenesis, 2002, 23, 861–866 CrossRef CAS.
- B. Faraglia, S. Y. Chen, M. D. Gammon, Y. Zhang, S. L. Teitelbaum, A. L. Neugut, H. Ahsan, G. C. Gabowski, H. Hibshoosh, D. Lin, F. F. Kadlubar and R. M. Santella, Carcinogenesis, 2003, 24, 719–725 CrossRef CAS.
- R. Benigni, A. Giuliani, R. Franke and A. Gruska, Chem. Rev., 2000, 100, 3697–3714 CrossRef CAS.
- U. Paniker and N. Levine, Dermatol. Clin., 2001, 19, 79–86 CAS.
- F. D. Khan, S. Roychowdhury, R. Nemes, P. M. Woster and C. K. Svensson, Toxicology, 2005, 218, 90–99.
- P. M. Vyas, S. Roychowdhury, P. M. Woster and C. K. Svensson, Biochem. Pharmacol., 2005, 70, 275–286 CrossRef CAS.
- D. C. McMillan, C. L. Powell, Z. S. Bowman, J. D. Morrow and D. J. Jollow, Toxicological Sciences, 2005, 88, 274–283 Search PubMed.
- S. Roychowdhury, A. E. Cram, A. Aly and C. K. Svensson, Drug Metab. Dispos., 2007, 35, 1463–1465 CrossRef CAS.
- M. Besser, J. Vera, D. Chitnavis, C. Beatty and G. Vassiliou, International Journal of Laboratory Hematology, 2009, 31, 245–247 Search PubMed.
- J. M. Hutzler, M. J. Hauer and T. S. Tracy, Drug Metab. Dispos., 2001, 29, 1029–1034 CAS.
- H. J. Gill, M. D. Tingle and B. K. Park, Br. J. Clin. Pharmacol., 1995, 40, 531–538 CAS.
- H. R. Winter, C. B. Trapnell, J. T. Slattery, M. Jacobson, D. L. Greenspan, T. M. Hooton and J. D. Unadkat, Clin. Pharmacol. Ther., 2004, 76, 579–587 CrossRef CAS.
- N. H. Shear and S. P. Spielberg, Can. J. Physiol. Pharmacol., 1085, 63, 1370–1372 Search PubMed.
- M. J. Rieder, J. Uetrecht, N. H. Shear and S. P. Spielberg, J. Pharmacol. Exp. Therap., 1988, 244, 724–728 CAS.
- H. J. Gill, S. J. Hough, D. J. Naisbitt, J. L. Maggs, N. R. Kitteringham, M. Pirmohamed and B. K. Park, J. Pharmcol. Exp. Therap., 1997, 282, 795–801 Search PubMed.
- M. J. Rieder, R. Krause and I. A. Bird, Toxicology, 1995, 95, 141–146 CrossRef CAS.
- H. R. Winter and J. D. Unadkat, Drug Metab. Dispos., 2005, 33, 969–976 CrossRef CAS.
- L. A. Trepanier, Vet. Dermatol, 1999, 10, 241–248 CrossRef.
- W. A. Petri, in Goodman and Gilman's The Pharmacological Bases of Therapeutics, ed. J. G. Hardman, L. E. Limbard, A. Goodman Gilman, ed., 10th. ed., McGraw-Hill, NY, 2001, pp. 1171–1188 Search PubMed.
- A. Gringauz, in Introduction to Medicinal Chemistry, How drugs act and why, Wiley-VCH, NY, 1997, pp. 63–65 Search PubMed.
- P. Kovacic and M. E. Hall, J. Recept. Signal Transduction, 2010, 30, 1–9 Search PubMed.
- P. Kovacic and R. Somanathan, J. Recept. Signal Transduction, 2010, 30, 214–226 Search PubMed.
- J. F. Wheeler, L. E. Adams, A. B. Mongey, S. M. Roberts, W. R. Heineman and E. V. Hess, Drug Metab. Dispos., 1991, 19, 691–695 CAS.
- L. E. Adams, C. E. Sanders, R. A. Budinsky, R. Donavan-Brand, S. M. Roberts and E. V. Hess, J. Lab. Clin. Med., 1989, 113, 482–492 CAS.
- J. Uetrecht, N. Zahid and R. Rubin, Chem. Res. Toxicol., 1988, 1, 74–78 CrossRef CAS.
- C. Goebel, C. Vogel, M. Wulferink, S. Mittmann, B. Sachs, J. Abel, G. Degen, J. Uetrecht and E. Gleichmann, Chem. Res. Toxicol., 1999, 12, 488–500 CrossRef CAS.
- J. Uetrecht, J. Pharmacol. Exp. Ther., 1985, 232, 420–425 CAS.
- R. L. Rubin, J. Uetrecht and J. E. Jones, J. Pharmacol. Exp. Ther., 1987, 242, 833–841 CAS.
- K. Magner-Wróbel, M. Toborek, M. Drózdz and A. Danch, Pharmacol. Toxicol., 1993, 72, 94–97 CrossRef CAS.
- E. Lessard, A. Fortin, P. M. Bélanger, P. Beaune, B. A. Hamelin and J. Turgeon, Pharmacogenetics, 1997, 7, 381–390 CrossRef CAS.
- P. Kovacic and J. D. Jacintho, Mini-Rev. Med. Chem., 2003, 3, 568–575 CrossRef CAS.
- A. G. Siraki, L. J. Deterding, M. G. Bonini, J. Jiang, M. Ehrenshaft, K. B. Tomer and R. P. Mason, Chem. Res. Toxicol., 2008, 21, 1143–1153 CrossRef CAS.
- G. Galati, S. Tafazoli, O. Sabzevari, T. S. Chan and P. J. O'Brien, Chem.-Biol. Interact., 2002, 142, 25–41 CrossRef CAS.
- M. Toborek, K. Magner-Wróbel, M. Drózdz, A. Danch and E. Kopieczna-Grzebieniak, Arch. Toxicol., 1993, 67, 691–695 CrossRef CAS.
- L. E. Adams, S. M. Roberts, J. M. Carter, J. F. Wheeler, H. W. Zimmer, R. J. Donavan-Brand and E. V. Hess, Int. J. Immunopharmacol., 1990, 12, 809–819 CrossRef CAS.
- P. Kovacic and R. Somanathan, Curr. Bioactive Compds., 2010, 6, 44–59 Search PubMed.
- N. N. Niufar, F. L. Haycock, J. L. Wesemann, J. A. MacStay, V. L. Heasley and P. Kovacic, Rev. Mex. Chem. Soc. (J. Mex. Chem. Soc.), 2002, 46, 307–312 Search PubMed.
- P. Kovacic, Med. Hypotheses, 2007, 69, 1105–1110 CrossRef CAS.
- P. Kovacic and A. L. Cooksy, Rev. Environ. Contam. Toxicol., 2010, 204, 133–148 CrossRef CAS.
- P. Kovacic, Med. Hypotheses, 2005, 64, 350–356 CrossRef CAS.
- P. Kovacic and R. Somanathan, J. Nanosci. Nanotechnol., 2010, 10, 7919–7930 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2011 |
