Activation energies for the decomposition of pharmaceuticals and their application to predicting hydrolytic stability in drug discovery†
Philip A.
MacFaul
*,
Linette
Ruston
and
J. Matthew
Wood
AstraZeneca, Mereside, Alderley Park, Macclesfield, Cheshire, SK10 4TG, UK. E-mail: Philip.MacFaul@astrazeneca.com; Tel: +44-1625-516080
First published on 17th December 2010
Abstract
The stability of drug-like molecules is one of the most important areas of the drug development process and Arrhenius studies can be used in order to predict the stability of compounds by performing forced degradation studies at elevated temperatures and then extrapolating the data to room temperature. Analysis of the activation energies calculated from 166 Arrhenius experiments performed on drug-like molecules in solution, using temperatures up to 90 °C, indicates that the mean activation energy for these degradations is 98.6 kJ/mol (23.6 kcal/mol). The impact on the predicted half-lives of employing different activation energies is discussed.
Major advances have been made in recent years to enhance the quality and throughput of in vitro adsorption, distribution, metabolism and excretion (ADME) assays to improve the drug discovery process and reduce attrition due to these factors.1 Assays that assess properties such as solubility, logD, plasma protein binding, clearance, cytochrome P450 inhibition and permeability are all now commonplace in drug discovery departments and used heavily to influence the design of compounds.2 Significant improvements have also been made in toxicity screening assays, for example studies are regularly conducted into the inhibition of ion channels such as hERG.1 However, one aspect of compound quality that does not always feature prominently in drug discovery programmes is hydrolytic stability.3,4 Stability studies are, of course, carried out in detail within development departments, where in-depth analysis of the active pharmaceutical ingredient (API) and drug product are carried out under forced and real-time conditions in both solid state and in solution, with the emphasis being on the impact of factors such temperature, humidity, oxygen, oxidants, excipients and light.5,6 Studies are carried out under conditions designed to explore hydrolytic, oxidative, thermal/humidity, and photolytic stress as described by the International Council of Harmonization (ICH)7 as degradation can lead to reduced efficacy and the potential for the generation of toxic impurities. Issues with instability that are uncovered during development can greatly add to the time and cost of the development process, hence it would seem appropriate to dedicate more time in discovery to stability studies that would reduce the risk of compounds with stability issues progressing into development.
Within drug discovery, stability studies are carried out under less formal conditions and often have different “drivers” for the study design than those encountered in development, and in contrast to development where the solid state stability is investigated, studies are carried out in solution.3,4 For assessment of compounds where concerns exist over the stability under biological assay conditions or in the gastro-intestinal tract, experiments can be conducted at room temperature or 37 °C as required, whereas for the assessment of the longer term stability of compounds in formulations that need to be stable for several days more forcing conditions are required. In this case, we at AstraZeneca, Alderley Park, incubate compounds at temperatures up to 90 °C for 18 h across the pH range from 1 to 10, and analyse regularly to allow kinetic detail to be captured. Carrying out hydrolytic stability studies under such forcing conditions allows a rapid turn-around of results, in comparison to real time or lower temperature studies, allowing information to be fed back to projects on a timescale that can have a real impact on compound design. If no degradation is observed under these conditions, it is likely that the compound is stable and possesses minimal risk going forward. If decomposition is observed at 90 °C and a rate measured, then that rate can be extrapolated to 25 °C by employing Arrhenius extrapolations. For an accurate assessment of hydrolytic stability, measurements are performed at additional temperature points and the data plotted according to the Arrhenius equation lnk = lnA – EA/RT where k = rate constant, A = pre-exponential factor, EA = activation energy, R = gas constant (8.314 J K−1 mol−1), and T = temperature in degrees Kelvin. An example of Arrhenius kinetic data measured at 90, 80 and 70 °C is shown in Fig. 1. This relationship then allows a rate constant at lower temperatures to be calculated and the risk for hydrolytic instability at the various pH's to be assessed.
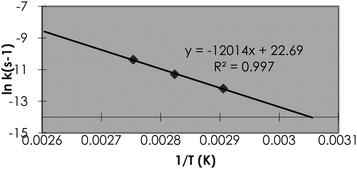 | ||
| Fig. 1 Arrhenius extrapolation for the decomposition of a drug molecule measured at 90, 80 and 70 °C. | ||
Fig. 2 illustrates the distribution of 166 activation energies measured for drug-like molecules which have obeyed the Arrhenius relationship using temperatures as high as 90 °C and cover a variety of chemistries and transformations. These studies were performed on a variety of molecules at various pH's and were chosen for Arrhenius studies based on the results obtained at the highest temperature that indicated instability of the compound at the particular pH. This Arrhenius data was gathered using least three different temperatures and a minimum of six time points were generated at each temperature. The typical initial concentration of the compounds was 10uM, and the kinetic data was generated from loss of the parent compound assessed by HPLC using UV detection. For all of the data shown in the table, a good fit to the Arrhenius relationship was maintained, and the least squares regression provided an R2 value of 0.95 or greater. In some cases, the degradation profiles differed between the maximum and minimum temperatures where secondary degradation products were observed at elevated temperatures. The use of 90 °C as the highest temperature for these types of studies, and the finding that the Arrhenius extrapolations are linear, is in contrast to a previous suggestion that these types of temperatures would be too high for general use in forced degradation studies.5 However, it should be noted that this recommendation of temperature is directed toward stress testing of solid material where there is a risk of phase changes. The data shows a mean EA value of 99 kJ/mol (23.6 kcal/mol), a median value of 95 kJ/mol (22.6 kcal/mol), a minimum value of 50 kJ/mol (11.9 kcal/mol) and a maximum of 198 kJ/mol (47.2 kcal/mol). This mean value is higher than that of 83.1 kJ/mol (19.8 kcal/mol) reported by Kennon,8 and is in agreement with Davis who suggested that 20 kcal/mol is a “quite conservative” estimate for transformations of drug-like molecules.5
 | ||
| Fig. 2 Distribution of activation energies (kJ/mol) observed for drug molecule degradations. | ||
In terms of extrapolating the measured data to lower temperatures, Table 1 indicates the relative differences in rate on increasing the temperature in 10 degree steps for various activation energies as described by Baertschi et al.5 although the range of activation energies has now been expanded using this current data set to further highlight the impact of greater activation energies. As can be seen, the lowest EA value of 50kJ/mol results in the least affect on the kinetics, with the rate increasing just less than a factor of two for every 10 degree rise in temperature. The average activation energy value of 99 kJ/mol, results in the rate increasing approximately three-fold for an increase in temperature of 10 degrees. Higher activation energies result in much more significant changes to the kinetics, with greater than five-fold increases in rate being observed for some compounds.
| Temperature change (°C) | Increase in relative rate of reaction | |||
|---|---|---|---|---|
| Ea = 50 kJ/mol | Ea = 99 kJ/mol | Ea = 150 kJ/mol | Ea = 200 kJ/mol | |
| 80–90 | 1.60 | 2.53 | 4.09 | 6.54 |
| 70–80 | 1.64 | 2.70 | 4.44 | 7.29 |
| 60–70 | 1.69 | 2.87 | 4.85 | 8.22 |
| 50–60 | 1.75 | 3.06 | 5.35 | 9.36 |
| 40–50 | 1.81 | 3.29 | 5.96 | 10.80 |
| 30–40 | 1.89 | 3.55 | 6.70 | 12.64 |
| 20–30 | 1.97 | 3.88 | 7.63 | 15.02 |
These extrapolations can be used in the absence of additional measurements that are required for Arrhenius calculations to predict rates at lower temperatures. Therefore, a generic rule-of-thumb extrapolation can be applied to rapidly assess the risk associated with a certain compound just using the data generated at 90 °C. In order to present a ‘worst-case’ scenario, a factor of two for the reduction in rate for every 10 degree reduction in temperature can be employed – the 2-for-10 extrapolation. This is an extremely conservative approach and regularly over-estimates the degree of degradation at lower temperatures. Alsante et al. also previously suggested using a conservative approach by employing an EA of 12 kcal/mol,9 however, others have recommended 17 kcal/mol and 19.8 kcal/mol.10,11 Despite the concerns of using such a conservative approach, this allows a rapid assessment of the risks for hydrolytic stability. As mentioned previously, there are no guidelines for stability studies within drug discovery, as it really depends on the question that is being addressed. Criteria such as less than 5% decomposition over 7 days could be employed for assessing suitability for formulations, and for this level of decomposition, this translates to a predicted half-life of around 100 days. For the purposes of risk assessment, this criterion could be employed to evaluate compound progression.
It is acknowledged that reactions in the solid state may well be more complex (and that activation energies may be different) than in solution, and forced degradation studies on the solid form are more appropriate in order to study and predict the stability of drug-like molecules. In terms of formulation stability, it is also noted that the physical stability of the API is key, where any phase changes could impact formulation performance.12 Nevertheless, it is hoped that the data presented here illustrates an updated view of the activation energies of the decomposition of such molecules in solution. The data also suggested that temperatures of up to 90 °C can be regularly employed to rapidly assess the risks associated with the kinetics of hydrolysis and potentially the hydrolytic pathways (keeping in mind the risk for different pathways at higher temperatures). Using the 2-for-10 rule of thumb for extrapolating to lower temperatures will over-estimate the risk associated with the predicted stability at, for example, room temperature, however, this approach enables compounds containing moieties that are susceptible to hydrolysis to be identified quickly during the drug discovery process and modifications to the design of the molecule made to enhance the properties of the candidate drug entering the development process.
Acknowledgements
Thanks go to Stuart Nicholson, Mark Timms, Kin Tam and Nicola Colclough for their contribution towards the experimental work that this analysis is based upon. One of us (P. M.) also thanks Steve Baertschi for his encouragement to carry out this analysis.Notes and references
- E. H. Kerns and L. Di, editors. Drug-like Properties: Concepts, Structure Design and Methods: from ADME to Toxicity Optimization, 2008, 1st ed., London, Academic Press Search PubMed.
- B. Testa B, S. D. Krämer, H. Wunderli-Allenspach and G. Folkers, editors. Pharmacokinectic Profiling in Drug Research: Biological, Physiochemical and Computational Strategies, 2006, Zurich, Verlag Helvetica Chimica Acta Search PubMed.
- L. Di and E. H. Kerns, Chem. Biodiversity, 2009, 6, 1875 CrossRef CAS.
- L. Di and E. H. Kerns, J. Biomol. Screening, 2006, 11, 40 CAS.
- S. W. Baertschi and P. J. Jansen in “Pharmaceutical Stress Testing: Predicting Drug Degradation”, 2005, New York, Informa Healthcare Search PubMed.
- J. T. Carstensen and C. T. Rhodes, editors. Drug Stability: Principles and Practices, 2007, 3rd ed., New York, Informa Search PubMed.
- International Conference on Harmonization, stability testing of new drug substances and products, Q1A(R2), 2003.
- L. Kennon, J. Pharm. Sci., 1964, 53, 815 CrossRef CAS.
- K. M. Alsante, R. C. Friedmann, T. D. Hatajik, L. L. Lohr, T. R. Sharp, K. D. Snyder and E. J. Szczesny, Sep. Sci. Tech., 2001, 3, 85 CAS.
- S. W. Baertschi, D. DeAntonis, A. McKeown, J. Bercu, S. Raillard and C. M. Riley in “Pharmaceutical Stress Testing: Predicting Drug Degradation”, 2nd Edition, Informa Healthcare, in press Search PubMed.
- U.S. Pharmacopeia General Chapter <1150> Pharmaceutical Stability; http://www.uspnf.com.
- M. Palucki, J. D. Higgins, E. Kwong and A. C. Templeton, J. Med. Chem., 2010, 53, 5897 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c0md00214c |
| This journal is © The Royal Society of Chemistry 2011 |
